Uptake Routes and Biodistribution of Polystyrene Nanoplastics on Zebrafish Larvae and Toxic Effects on Development
Abstract
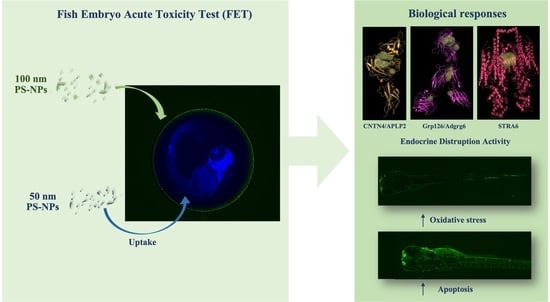
:1. Introduction
2. Materials and Methods
2.1. Nanoplastics
2.2. Zebrafish Embryo Toxicity Test (ZFET)
2.3. Localization of Nanoplastics
2.4. Cell Apoptosis Analysis
2.5. Oxidative Stress Analysis
2.6. Morphological Analysis by Scanning Electron Microscope (SEM)
2.7. Structure-Based Virtual Screening
2.8. Statistical Analysis
3. Results
3.1. Uptake and Biodistribution of Nanoplastics in Zebrafish Larvae
3.2. Survival, Hatch, and Development
3.3. Cell Apoptosis
3.4. Oxidative Stress
3.5. Virtual Screening
3.5.1. Receptor for Retinol Uptake Stra6 (STRA6)
3.5.2. Adhesion G Protein–Coupled Receptor G6 (Adgrg6 or GPR126)
3.5.3. Contactin 4 (CNTN4) and Amyloid Beta Precursor-like Protein 2 (APLP2) Complex
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahul Hamid, F.; Bhatti, M.S.; Anuar, N.; Anuar, N.; Mohan, P.; Periathamby, A. Worldwide distribution and abundance of microplastic: How dire is the situation? Waste Manag. Res. 2018, 36, 873–897. [Google Scholar] [CrossRef] [PubMed]
- Hammer, J.; Kraak, M.H.S.; Parsons, J.R. Plastics in the Marine Environment: The Dark Side of a Modern Gift. In Reviews of Environmental Contamination and Toxicology; Whitacre, D., Ed.; Springer: New York, NY, USA, 2012; Volume 220. [Google Scholar]
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Mariano, S.; Tacconi, S.; Fidaleo, M.; Rossi, M.; Dini, L. Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques. Front Toxicol. 2021, 3, 636640. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.J.; Smith, K.L., Jr. Plastics on the Sargasso sea surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef]
- Carlin, J.; Craig, C.; Little, S.; Donnelly, M.; Fox, D.; Zhai, L.; Walters, L. Microplastic accumulation in the gastrointestinal tracts in birds of prey in central Florida, USA. Environ. Pollut. 2020, 264, 114633. [Google Scholar] [CrossRef]
- Haave, M.; Gomiero, A.; Schönheit, J.; Nilsen, H.; Olsen, A.B. Documentation of Microplastics in Tissues of Wild Coastal Animals. Front. Environ. Sci. 2021, 9, 575058. [Google Scholar] [CrossRef]
- Soe, K.K.; Hajisamae, S.; Sompongchaiyakul, P.; Towatana, P.; Pradit, S. Feeding Habits and the Occurrence of Anthropogenic Debris in the Stomach Content of Marine Fish from Pattani Bay, Gulf of Thailand. Biology 2022, 11, 331. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Lu, L.; Zheng, M.; Zhang, X.; Tian, H.; Wang, E.; Ru, S. Polystyrene microplastics cause tissue damages, sex-specific reproductive disruption and transgenerational effects in marine medaka (Oryzias melastigma). Environ. Pollut. 2019, 254, 113024. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Bergami, E.; Pugnalini, S.; Vannuccini, M.L.; Manfra, L.; Faleri, C.; Savorelli, F.; Dawson, K.A.; Corsi, I. Long-term toxicity of surface-charged polystyrene nanoplastics to marine planktonic species Dunaliella tertiolecta and Artemia franciscana. Aquat. Toxicol. 2016, 189, 159–169. [Google Scholar] [CrossRef]
- Pecoraro, R.; Salvaggio, A.; Marino, F.; Caro, G.D.; Capparucci, F.; Lombardo, B.M.; Messina, G.; Scalisi, E.M.; Tummino, M.; Loreto, F.; et al. Metallic Nano-Composite Toxicity Evaluation by Zebrafish Embryo Toxicity Test with Identification of Specific Exposure Biomarkers. Curr. Protoc. Toxicol. 2017, 74, 1–14. [Google Scholar] [CrossRef]
- Chen, Q.; Gundlacj, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017, 584–585, 1022–1031. [Google Scholar] [CrossRef]
- Teng, M.; Zhao, X.; Wu, F.; Wang, C.; Wang, C.; White, J.C.; Zhao, W.; Zhou, L.; Yan, S.; Tian, S. Charge-specific adverse effects of polystyrene nanoplastics on zebrafish (Danio rerio) development and behavior. Environ. Int. 2022, 163, 107154. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Wu, Y.L.; Gong, Z.; Valiyaveettil, S. Toxicity of silver nanoparticles in zebrafish models. Nanotechonology 2008, 19, 255102. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test, OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Liu, H.; Nie, F.H.; Lin, H.Y.; Ma, Y.; Ju, X.H.; Chen, J.J.; Gooneratne, R. Developmental toxicity, EROD, and CYP1A mRNA expression in zebrafish embryos exposed to dioxin-like PCB126. Environ. Toxicol. 2016, 31, 201–210. [Google Scholar] [CrossRef]
- Duan, Z.H.; Duan, X.Y.; Zhao, S.; Wang, X.L.; Wang, J.; Liu, Y.B.; Peng, Y.W.; Gong, Z.Y.; Wang, L. Barrier function of zebrafish embryonic chorions against microplastics and nanoplastics and its impact on embryo development. J. Hazard. Mater. 2020, 395, 122621. [Google Scholar] [CrossRef]
- Sant, K.E.; Timme-Laragy, A.R. Zebrafish as a Model for Toxicological Perturbation of Yolk and Nutrition in the Early Embryo. Curr. Environ. Health Rep. 2018, 5, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Assémat, E.; Chatelet, F.; Chandellier, J.; Commo, F.; Cases, O.; Verroust, P.; Kozyraki, R. Overlapping expression patterns of the multiligand endocytic receptors cubilin and megalin in the CNS, sensory organs and developing epithelia of the rodent embryo. Gene. Expr. Patterns 2005, 6, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Monticelli, L. Modeling the effect of nano-sized polymer particles on the properties of lipid membranes. J. Phys. Condens. Matter. 2014, 26, 503101. [Google Scholar] [CrossRef]
- Fraher, D.; Sanigorski, A.; Mellett, N.A.; Meikle, P.J.; Sinclair, A.J.; Gibert, Y. Zebrafish embryonic Lipidomic analysis reveals that the yolk cell is metabolically active in Processing Lipid. Cell Rep. 2016, 14, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.F.; Galitan, L.; Cameron, J.; Goodwin, N.; Ramakrishnan, L. Delay of Initial Feeding of Zebrafish Larvae Until 8 Days Postfertilization Has No Impact on Survival or Growth Through the Juvenile Stage. Zebrafish 2018, 15, 515–518. [Google Scholar] [CrossRef]
- Van Pomeren, M.; Brun, N.R.; Peijnenburg, W.J.G.M.; Vijver, M.G. Exploring uptake and biodistribution of polystyrene (nano)particles in zebrafish embryos at different developmental stages. Aquat. Toxicol. 2017, 190, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Ochoa, E.; Byrd-Jacobs, C.A. The Olfactory System of Zebrafish as a Model for the Study of Neurotoxicity and Injury: Implications for Neuroplasticity and Disease. Int. J. Mol. Sci. 2019, 20, 1639. [Google Scholar] [CrossRef] [PubMed]
- Umamaheswari, S.; Priyadarshinee, S.; Bhattacharjee, M.; Kadirvelu, K.; Ramesh, M. Exposure to polystyrene microplastics induced gene modulated biological responses in zebrafish (Danio rerio). Chemosphere 2021, 281, 128592. [Google Scholar] [CrossRef]
- Qiang, L.Y.; Cheng, J.P. Exposure to microplastics decreases swimming competence in larval zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2019, 176, 226–233. [Google Scholar] [CrossRef]
- Monneret, C. What is an endocrine disruptor? Comptes Rendus Biol. 2017, 340, 403–405. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, M.; Chen, X.; Yang, C.; Wu, L. Combined toxicity of microplastics and cadmium on the zebrafish embryos (Danio rerio). Sci.Total Environ. 2020, 743, 140638. [Google Scholar] [CrossRef]
- Solanki, A.K.; Kondkar, A.A.; Fogerty, J.; Su, Y.; Kim, S.-H.; Lipschutz, J.H.; Nihalani, D.; Perkins, B.D.; Lobo, G.P. A Functional Binding Domain in the Rbpr2 Receptor Is Required for Vitamin A Transport, Ocular Retinoid Homeostasis, and Photoreceptor Cell Survival in Zebrafish. Cells 2020, 9, 1099. [Google Scholar] [CrossRef]
- Baxendale, S.; Asad, A.; Shahidan, N.O.; Wiggin, G.R.; Whitfield, T.T. The adhesion GPCR Adgrg6 (Gpr126): Insights from the zebrafish model. Genesis 2021, 59, 23417. [Google Scholar] [CrossRef]
- Karuppan, S.J.; Vogt, A.; Fischer, Z.; Ladutska, A.; Swiastyn, J.; McGraw, H.F.; Bouyain, S. Members of the vertebrate contactin and amyloid precursor protein families interact through a conserved interface. J. Biol. Chem. 2022, 298, 101541. [Google Scholar] [CrossRef] [PubMed]
- Manuel, P.; Almeida, M.; Martins, M.; Oliveira, M. Effects of nanoplastics on zebrafish embryo-larval stages: A case study with polystyrene (PS) and polymethylmethacrylate (PMMA) particles. Environ. Res. 2022, 213, 113584. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contino, M.; Ferruggia, G.; Pecoraro, R.; Scalisi, E.M.; Cavallaro, G.; Bonaccorso, C.; Fortuna, C.G.; Salvaggio, A.; Capparucci, F.; Bottari, T.; et al. Uptake Routes and Biodistribution of Polystyrene Nanoplastics on Zebrafish Larvae and Toxic Effects on Development. Fishes 2023, 8, 168. https://doi.org/10.3390/fishes8030168
Contino M, Ferruggia G, Pecoraro R, Scalisi EM, Cavallaro G, Bonaccorso C, Fortuna CG, Salvaggio A, Capparucci F, Bottari T, et al. Uptake Routes and Biodistribution of Polystyrene Nanoplastics on Zebrafish Larvae and Toxic Effects on Development. Fishes. 2023; 8(3):168. https://doi.org/10.3390/fishes8030168
Chicago/Turabian StyleContino, Martina, Greta Ferruggia, Roberta Pecoraro, Elena Maria Scalisi, Gianfranco Cavallaro, Carmela Bonaccorso, Cosimo Gianluca Fortuna, Antonio Salvaggio, Fabiano Capparucci, Teresa Bottari, and et al. 2023. "Uptake Routes and Biodistribution of Polystyrene Nanoplastics on Zebrafish Larvae and Toxic Effects on Development" Fishes 8, no. 3: 168. https://doi.org/10.3390/fishes8030168