Recent Tissue Engineering Approaches to Mimicking the Extracellular Matrix Structure for Skin Regeneration
Abstract
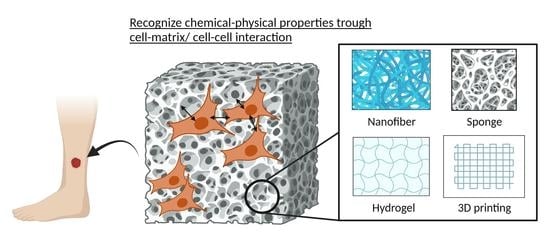
:1. Introduction
2. Skin Regeneration
2.1. Skin Structure
2.2. Wound-Healing Processes

3. Mimicking ECM Structure and Multilayer Nature of Skin
3.1. Nanofibers
3.2. Sponges
3.3. Hydrogels
3.4. Composite Materials
4. Incorporation of Drug Delivery System (DDS) Functionality
4.1. GFs
4.2. Cells
5. Mechanobiological Approaches
6. Dressings in Clinical Usage
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Baker, A.B. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front. Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound Dressings: Current Advances and Future Directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef] [Green Version]
- Laurano, R.; Boffito, M.; Ciardelli, G.; Chiono, V. Wound Dressing Products: A Translational Investigation from the Bench to the Market. Eng. Regen. 2022, 3, 182–200. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamalathevan, P.; Ooi, P.S.; Loo, Y.L. Silk-Based Biomaterials in Cutaneous Wound Healing: A Systematic Review. Adv. Ski. Wound Care 2018, 31, 565–573. [Google Scholar] [CrossRef]
- Babar, M.; Rajadas, J.; Hama, R.; Ulziibayar, A.; Reinhardt, J.W.; Watanabe, T.; Kelly, J.; Shinoka, T. Recent Developments in Biopolymer-Based Hydrogels for Tissue Engineering Applications. Biomolecules 2023, 13, 280. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-Based Hydrogels for Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2016, 66, 159–182. [Google Scholar] [CrossRef]
- Li, T.; Sun, M.; Wu, S. State-of-the-Art Review of Electrospun Gelatin-Based Nanofiber Dressings for Wound Healing Applications. Nanomaterials 2022, 12, 784. [Google Scholar] [CrossRef]
- Dickinson, L.E.; Gerecht, S. Engineered Biopolymeric Scaffolds for Chronic Wound Healing. Front. Physiol. 2016, 7, 341. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Feng, Y.; Wang, L.; Liu, D.; Qin, C.; Shi, Y. A Review of Preparation Methods of Porous Skin Tissue Engineering Scaffolds. Mater. Today Commun. 2022, 32, 104109. [Google Scholar] [CrossRef]
- Hernandez, J.L.; Woodrow, K.A. Medical Applications of Porous Biomaterials: Features of Porosity and Tissue-Specific Implications for Biocompatibility. Adv. Healthc. Mater. 2022, 11, 2102087. [Google Scholar] [CrossRef]
- ur Rehman Khan, A.; Morsi, Y.; Zhu, T.; Ahmad, A.; Xie, X.; Yu, F.; Mo, X. Electrospinning: An Emerging Technology to Construct Polymer-Based Nanofibrous Scaffolds for Diabetic Wound Healing. Front. Mater. Sci. 2021, 15, 10–35. [Google Scholar] [CrossRef]
- Sand, M.; Gambichler, T.; Sand, D.; Skrygan, M.; Altmeyer, P.; Bechara, F.G. MicroRNAs and the Skin: Tiny Players in the Body’s Largest Organ. J. Dermatol. Sci. 2009, 53, 169–175. [Google Scholar] [CrossRef]
- Guerra, A.; Belinha, J.; Jorge, R.N. Modelling Skin Wound Healing Angiogenesis: A Review. J. Theor. Biol. 2018, 459, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Jain, P.; Rauer, S.B.; Möller, M.; Singh, S. Mimicking the Natural Basement Membrane for Advanced Tissue Engineering. Biomacromolecules 2022, 23, 3081–3103. [Google Scholar] [CrossRef]
- Robson, M.C.; Steed, D.L.; Franz, M.G. Wound Healing: Biologic Features and Approaches to Maximize Healing Trajectories. Curr. Probl. Surg. 2001, 38, 61–140. [Google Scholar] [CrossRef]
- Balasubramani, M.; Kumar, T.R.; Babu, M. Skin Substitutes: A Review. Burns 2001, 27, 534–544. [Google Scholar] [CrossRef]
- Ma, P.X. Biomimetic Materials for Tissue Engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [Green Version]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Sonnemann, K.J.; Bement, W.M. Wound Repair: Toward Understanding and Integration of Single-Cell and Multicellular Wound Responses. Annu. Rev. Cell Dev. Biol. 2011, 27, 237–263. [Google Scholar] [CrossRef] [Green Version]
- Young, A.; McNaught, C.E. The Physiology of Wound Healing. Surgery 2011, 29, 475–479. [Google Scholar] [CrossRef]
- Martin, P.; Leibovich, S.J. Inflammatory Cells during Wound Repair: The Good, the Bad and the Ugly. Trends Cell Biol. 2005, 15, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.J.; Martin, P. Wound Repair: A Showcase for Cell Plasticity and Migration. Curr. Opin. Cell Biol. 2016, 42, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerman, O.Z.; Galiano, R.D.; Armour, M.; Levine, J.P.; Gurtner, G.C. Cellular Dysfunction in the Diabetic Fibroblast: Impairment in Migration, Vascular Endothelial Growth Factor Production, and Response to Hypoxia. Am. J. Pathol. 2003, 162, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Stojadinovic, O.; Tomic-Canic, M. Role of Keratinocytes in Healing of Chronic Wounds. Surg. Technol. Int. 2008, 17, 105–112. [Google Scholar]
- Gorain, B.; Pandey, M.; Leng, N.H.; Yan, C.W.; Nie, K.W.; Kaur, S.J.; Marshall, V.; Sisinthy, S.P.; Panneerselvam, J.; Molugulu, N.; et al. Advanced Drug Delivery Systems Containing Herbal Components for Wound Healing. Int. J. Pharm. 2022, 617, 121617. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.G. Electrospun Medicated Nanofibers for Wound Healing: Review. Membranes 2021, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Truong, M.; Li, Y.V.; Wang, Z. Mechanical Considerations of Electrospun Scaffolds for Myocardial Tissue and Regenerative Engineering. Bioengineering 2020, 7, 122. [Google Scholar] [CrossRef]
- Daming, Z.; Jiang, C. Electrospinning of Three-Dimensional Nanofibrous Tubes with Controllable Architectures. Nano Lett. 2008, 8, 3283–3287. [Google Scholar] [CrossRef]
- Khattab, T.A.; Tolba, E.; Gaffer, H.; Kamel, S. Development of Electrospun Nanofibrous-Walled Tubes for Potential Production of Photoluminescent Endoscopes. Ind. Eng. Chem. Res. 2021, 60, 10044–10055. [Google Scholar] [CrossRef]
- Koyanagi, E.; Tara, S.; Sakata, C.; Shimada, K.; Kato, K.; Miyachi, H.; Tanaka, R.; Nakazawa, Y. A Novel Gradient and Multilayered Sheet with a Silk Fibroin/Polyvinyl Alcohol Core–Shell Structure for Bioabsorbable Arterial Grafts. J. Biomed. Mater. Res. Part A 2022, 110, 576–584. [Google Scholar] [CrossRef]
- Feng, B.; Tu, H.; Yuan, H.; Peng, H.; Zhang, Y. Acetic-Acid-Mediated Miscibility toward Electrospinning Homogeneous Composite Nanofibers of GT/PCL. Biomacromolecules 2012, 13, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Haider, S.; Kang, I.K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Miguel, S.P.; Simões, D.; Moreira, A.F.; Sequeira, R.S.; Correia, I.J. Production and Characterization of Electrospun Silk Fibroin Based Asymmetric Membranes for Wound Dressing Applications. Int. J. Biol. Macromol. 2019, 121, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhang, Y.; Lim, C.T. Fabrication of Large Pores in Electrospun Nanofibrous Scaffolds for Cellular Infiltration: A Review. Tissue Eng. Part B Rev. 2011, 18, 77–87. [Google Scholar] [CrossRef]
- Ju, H.W.; Lee, O.J.; Lee, J.M.; Moon, B.M.; Park, H.J.; Park, Y.R.; Lee, M.C.; Kim, S.H.; Chao, J.R.; Ki, C.S.; et al. Wound Healing Effect of Electrospun Silk Fibroin Nanomatrix in Burn-Model. Int. J. Biol. Macromol. 2016, 85, 29–39. [Google Scholar] [CrossRef]
- Nam, J.; Huang, Y.; Agarwal, S.; Lannutti, J. Improved Cellular Infiltration in Electrospun Fiber via Engineered Porosity. Tissue Eng. 2007, 13, 2249–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalili, S.; Khorasani, S.N.; Razavi, S.M.; Hashemibeni, B.; Tamayol, A. Nanofibrous Scaffolds with Biomimetic Composition for Skin Regeneration. Appl. Biochem. Biotechnol. 2019, 187, 1193–1203. [Google Scholar] [CrossRef]
- Ahn, S.; Chantre, C.O.; Gannon, A.R.; Lind, J.U.; Campbell, P.H.; Grevesse, T.; Connor, B.B.O.; Kevin, P.; Parker, K. Soy Protein/Cellulose Nanofiber Scaffolds Mimicking Skin Extracellular Matrix for Enhanced Wound Healing. Adv. Healthc. Mater. 2019, 7, 1701175. [Google Scholar] [CrossRef]
- Lan, X.; Liu, Y.; Wang, Y.; Tian, F.; Miao, X.; Wang, H.; Tang, Y. Coaxial Electrospun PVA/PCL Nanofibers with Dual Release of Tea Polyphenols and ε-Poly (L-Lysine) as Antioxidant and Antibacterial Wound Dressing Materials. Int. J. Pharm. 2021, 601, 120525. [Google Scholar] [CrossRef] [PubMed]
- Keirouz, A.; Chung, M.; Kwon, J.; Fortunato, G.; Radacsi, N. 2D and 3D Electrospinning Technologies for the Fabrication of Nanofibrous Scaffolds for Skin Tissue Engineering: A Review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1626. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Narayanan, N.; Federici, E.; Yang, Z.; Zuo, X.; Gao, J.; Fang, F.; Deng, M.; Campanella, O.H.; Jones, O.G. Electrospinning Induced Orientation of Protein Fibrils. Biomacromolecules 2020, 21, 2772–2785. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Gong, X.; Jiao, W.; Li, Y.; Yin, X.; Si, Y.; Yu, J.; Ding, B. In-Situ Electrospinning of Thymol-Loaded Polyurethane Fibrous Membranes for Waterproof, Breathable, and Antibacterial Wound Dressing Application. J. Colloid Interface Sci. 2021, 592, 310–318. [Google Scholar] [CrossRef]
- Yan, X.; Yu, M.; Ramakrishna, S.; Russell, S.J.; Long, Y.Z. Advances in Portable Electrospinning Devices for in Situ Delivery of Personalized Wound Care. Nanoscale 2019, 11, 19166–19178. [Google Scholar] [CrossRef]
- Hady, T.F.; Hwang, B.; Pusic, A.D.; Waworuntu, R.L.; Mulligan, M.; Ratner, B.; Bryers, J.D. Uniform 40-Μm-Pore Diameter Precision Templated Scaffolds Promote a pro-Healing Host Response by Extracellular Vesicle Immune Communication. J. Tissue Eng. Regen. Med. 2021, 15, 24–36. [Google Scholar] [CrossRef]
- Cyphert, E.L.; Bil, M.; von Recum, H.A.; Święszkowski, W. Repurposing Biodegradable Tissue Engineering Scaffolds for Localized Chemotherapeutic Delivery. J. Biomed. Mater. Res. Part A 2020, 108, 1144–1158. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Wang, Y.; Li, G.; Zheng, Z.; Kaplan, D.L.; Wang, X.; Wang, X. Flexible Water-Absorbing Silk-Fibroin Biomaterial Sponges with Unique Pore Structure for Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.T.; Jeong, J.Y.; Seo, Y.B.; Lee, O.J.; Ju, H.W.; Park, H.J.; Lee, Y.J.; Lee, J.S.; Kim, S.H.; Park, C.H. Fabrication and Characterization of the Porous Duck’s Feet Collagen Sponge for Wound Healing Applications. J. Biomater. Sci. Polym. Ed. 2017, 29, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Roh, D.H.; Kang, S.Y.; Kim, J.Y.; Kwon, Y.B.; Hae, Y.K.; Lee, K.G.; Park, Y.H.; Baek, R.M.; Heo, C.Y.; Choe, J.; et al. Wound Healing Effect of Silk Fibroin/Alginate-Blended Sponge in Full Thickness Skin Defect of Rat. J. Mater. Sci. Mater. Med. 2006, 17, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug Delivery Systems and Materials for Wound Healing Applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Wu, K.; Zhao, D.; Cui, H. Preparation and Evaluation of Heparinized Sponge Based on Collagen and Chitosan for Wound Healing. SAGE J. 2020, 35, 314–327. [Google Scholar] [CrossRef]
- Moay, Z.K.; Nguyen, L.T.H.; Hartrianti, P.; Lunny, D.P.; Leavesley, D.; Kok, Y.O.; Chong, S.J.; Chua, A.W.C.; Tee, S.I.; Ng, K.W. Keratin-alginate Sponges Support Healing of Partial-thickness Burns. Int. J. Mol. Sci. 2021, 22, 8594. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; Lan, Y.; Zuo, Q.; Li, C.; Zhang, Y.; Guo, R.; Xue, W. Acceleration of Skin Regeneration in Full-Thickness Burns by Incorporation of BFGF-Loaded Alginate Microspheres into a CMCS–PVA Hydrogel. J. Tissue Eng. Regen. Med. 2017, 11, 1562–1573. [Google Scholar] [CrossRef]
- Andrade del Olmo, J.; Alonso, J.M.; Sáez-Martínez, V.; Benito-Cid, S.; Moreno-Benítez, I.; Bengoa-Larrauri, M.; Pérez-González, R.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Self-Healing, Antibacterial and Anti-Inflammatory Chitosan-PEG Hydrogels for Ulcerated Skin Wound Healing and Drug Delivery. Biomater. Adv. 2022, 139, 212992. [Google Scholar] [CrossRef]
- Piao, Y.; You, H.; Xu, T.; Bei, H.P.; Piwko, I.Z.; Kwan, Y.Y.; Zhao, X. Biomedical Applications of Gelatin Methacryloyl Hydrogels. Eng. Regen. 2021, 2, 47–56. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H.; Tsai, C.W.; Tsai, N.Y.; Chiang, C.Y.; Lin, R.S.; Pereira, R.F.; Li, Y.C.E. An Injectable, Dual Crosslinkable Hybrid Pectin Methacrylate (PECMA)/Gelatin Methacryloyl (GelMA) Hydrogel for Skin Hemostasis Applications. Int. J. Biol. Macromol. 2021, 185, 441–450. [Google Scholar] [CrossRef]
- Huang, J.; Chen, L.; Gu, Z.; Wu, J. Red Jujube-Incorporated Gelatin Methacryloyl (GelMa) Hydrogels with Anti-Oxidation and Immunoregulation Activity for Wound Healing. J. Biomed. Nanotechnol. 2019, 15, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Acuña, R.; García, A.J. Synthetic Hydrogels Mimicking Basement Membrane Matrices to Promote Cell-Matrix Interactions. Matrix Biol. 2017, 57–58, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Correia, I.J. Electrospun Polycaprolactone/Aloe Vera_Chitosan Nanofibrous Asymmetric Membranes Aimed for Wound Healing Applications. Polymers 2017, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Morgado, P.I.; Miguel, S.P.; Correia, I.J.; Aguiar-Ricardo, A. Ibuprofen Loaded PVA/Chitosan Membranes: A Highly Efficient Strategy towards an Improved Skin Wound Healing. Carbohydr. Polym. 2017, 159, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kawazoe, N.; Yang, Y.; Chen, G. Preparation of Mesh-like Collagen Scaffolds for Tissue Engineering. Mater. Adv. 2022, 3, 1556–1564. [Google Scholar] [CrossRef]
- Sutrisno, L.; Chen, H.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. PLGA–Collagen–BPNS Bifunctional Composite Mesh for Photothermal Therapy of Melanoma and Skin Tissue Engineering. J. Mater. Chem. B 2022, 10, 204–213. [Google Scholar] [CrossRef]
- Vyas, C.; Ates, G.; Aslan, E.; Hart, J.; Huang, B.; Bartolo, P. Three-Dimensional Printing and Electrospinning Dual-Scale Polycaprolactone Scaffolds with Low-Density and Oriented Fibers to Promote Cell Alignment. 3D Print. Addit. Manuf. 2020, 7, 105–113. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, W.; Sun, M.; He, P.; Lv, H.; Wang, Q.; Zhang, S.; Wu, Q.; Ling, P.; Chen, S.; et al. Novel Bi-Layered Dressing Patches Constructed with Radially-Oriented Nanofibrous Pattern and Herbal Compound-Loaded Hydrogel for Accelerated Diabetic Wound Healing. Appl. Mater. Today 2022, 28, 101542. [Google Scholar] [CrossRef]
- Sun, L.; Li, L.; Wang, Y.; Li, M.; Xu, S.; Zhang, C. A Collagen-Based Bi-Layered Composite Dressing for Accelerated Wound Healing. J. Tissue Viability 2022, 31, 180–189. [Google Scholar] [CrossRef]
- Park, U.; Kim, K. Multiple Growth Factor Delivery for Skin Tissue Engineering Applications. Biotechnol. Bioprocess Eng. 2017, 22, 659–670. [Google Scholar] [CrossRef]
- Munisso, M.C.; Morimoto, N.; Notodihardjo, S.C.; Mitsui, T.; Kakudo, N.; Kusumoto, K. Collagen/Gelatin Sponges (CGSs) Provide Both Protection and Release of BFGF: An in Vitro Study. BioMed Res. Int. 2019, 2019, 4016351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Shi, D.; Han, Z.; Dong, Z.; Xie, Y.; Zhang, F.; Zeng, W.; Yi, Q. Heparinized Silk Fibroin Hydrogels Loading FGF1 Promote the Wound Healing in Rats with Full-Thickness Skin Excision. Biomed. Eng. Online 2019, 18, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hama, R.; Aytemiz, D.; Moseti, K.O.; Kameda, T.; Nakazawa, Y. Silk Fibroin Conjugated with Heparin Promotes Epithelialization and Wound Healing. Polymers 2022, 14, 3582. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, N.; Schirmer, L.; Atallah, P.; Wandel, E.; Ferrer, R.A.; Werner, C.; Simon, J.C.; Franz, S.; Freudenberg, U. Glycosaminoglycan-Based Hydrogels Capture Inflammatory Chemokines and Rescue Defective Wound Healing in Mice. Sci. Transl. Med. 2017, 9, eaai9044. [Google Scholar] [CrossRef]
- Lai, H.J.; Kuan, C.H.; Wu, H.C.; Tsai, J.C.; Chen, T.M.; Hsieh, D.J.; Wang, T.W. Tailored Design of Electrospun Composite Nanofibers with Staged Release of Multiple Angiogenic Growth Factors for Chronic Wound Healing. Acta Biomater. 2014, 10, 4156–4166. [Google Scholar] [CrossRef]
- Choi, J.S.; Choi, S.H.; Yoo, H.S. Coaxial Electrospun Nanofibers for Treatment of Diabetic Ulcers with Binary Release of Multiple Growth Factors. J. Mater. Chem. 2011, 21, 5258–5267. [Google Scholar] [CrossRef]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; LeRoux, M.A. Concise Review: Role of Mesenchymal Stem Cells in Wound Repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef]
- Hocking, A.M.; Gibran, N.S. Mesenchymal Stem Cells: Paracrine Signaling and Differentiation during Cutaneous Wound Repair. Exp. Cell Res. 2010, 316, 2213–2219. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, S.; Pittenger, M.F. Human Mesenchymal Stem Cells Modulate Allogeneic Immune Cell Responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [Green Version]
- Mulder, G.D.; Vande Berg, J.S. Cellular Senescence and Matrix Metalloproteinase Activity in Chronic Wounds: Relevance to Debridement and New Technologies. J. Am. Podiatr. Med. Assoc. 2002, 92, 34–37. [Google Scholar] [CrossRef]
- Shafei, S.; Khanmohammadi, M.; Heidari, R.; Ghanbari, H.; Taghdiri Nooshabadi, V.; Farzamfar, S.; Akbariqomi, M.; Sanikhani, N.S.; Absalan, M.; Tavoosidana, G. Exosome Loaded Alginate Hydrogel Promotes Tissue Regeneration in Full-Thickness Skin Wounds: An in Vivo Study. J. Biomed. Mater. Res. Part A 2020, 108, 545–556. [Google Scholar] [CrossRef]
- Kuehlmann, B.; Bonham, C.A.; Zucal, I.; Prantl, L.; Gurtner, G.C. Mechanotransduction in Wound Healing and Fibrosis. J. Clin. Med. 2020, 9, 1423. [Google Scholar] [CrossRef]
- Oria, R.; Wiegand, T.; Escribano, J.; Elosegui-Artola, A.; Uriarte, J.J.; Moreno-Pulido, C.; Platzman, I.; Delcanale, P.; Albertazzi, L.; Navajas, D.; et al. Force Loading Explains Spatial Sensing of Ligands by Cells. Nature 2017, 552, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Hannan, R.T.; Peirce, S.M.; Barker, T.H. Fibroblasts: Diverse Cells Critical to Biomaterials Integration. ACS Biomater. Sci. Eng. 2018, 4, 1223–1232. [Google Scholar] [CrossRef]
- Chen, K.; Henn, D.; Januszyk, M.; Barrera, J.A.; Noishiki, C.; Bonham, C.A.; Griffin, M.; Tevlin, R.; Carlomagno, T.; Shannon, T.; et al. Disrupting Mechanotransduction Decreases Fibrosis and Contracture in Split-Thickness Skin Grafting. Sci. Transl. Med. 2022, 14, eabj9152. [Google Scholar] [CrossRef] [PubMed]
- Sima, L.E.; Buruiana, E.C.; Buruiana, T.; Matei, A.; Epurescu, G.; Zamfirescu, M.; Moldovan, A.; Petrescu, S.M.; Dinescu, M. Dermal Cells Distribution on Laser-Structured Ormosils. J. Tissue Eng. Regen. Med. 2013, 7, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Lanfer, B.; Seib, F.P.; Freudenberg, U.; Stamov, D.; Bley, T.; Bornhäuser, M.; Werner, C. The Growth and Differentiation of Mesenchymal Stem and Progenitor Cells Cultured on Aligned Collagen Matrices. Biomaterials 2009, 30, 5950–5958. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto, M.; Bellet, P.; Scapin, G.; Busetto, R.; Rampazzo, C.; Vitiello, L.; Shah, D.I.; Filippini, F. 3D Printed Graphene-PLA Scaffolds Promote Cell Alignment and Differentiation. Int. J. Mol. Sci. 2022, 23, 1736. [Google Scholar] [CrossRef]
- Xu, L.; Gao, S.; Zhou, R.; Zhou, F.; Qiao, Y.; Qiu, D. Bioactive Pore-Forming Bone Adhesives Facilitating Cell Ingrowth for Fracture Healing. Adv. Mater. 2020, 32, 1907491. [Google Scholar] [CrossRef]
- Viswanathan, P.; Guvendiren, M.; Chua, W.; Telerman, S.B.; Liakath-Ali, K.; Burdick, J.A.; Watt, F.M. Mimicking the Topography of the Epidermal-Dermal Interface with Elastomer Substrates. Integr. Biol. 2016, 8, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobasseri, S.A.; Zijl, S.; Salameti, V.; Walko, G.; Stannard, A.; Garcia-Manyes, S.; Watt, F.M. Patterning of Human Epidermal Stem Cells on Undulating Elastomer Substrates Reflects Differences in Cell Stiffness. Acta Biomater. 2019, 87, 256–264. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511. [Google Scholar] [CrossRef] [Green Version]
- Hart, C.E.; Loewen-Rodriguez, A.; Lessem, J. Dermagraft: Use in the Treatment of Chronic Wounds. Adv. Wound Care 2012, 1, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchbinder, D.; Buchbinder, S.B. Wound healing: Adjuvant therapy and treatment adherence. Venous Ulcers 2007, 91–103. [Google Scholar] [CrossRef]
- Schiavon, M.; Francescon, M.; Drigo, D.; Salloum, G.; Baraziol, R.; Tesei, J.; Fraccalanza, E.; Barbone, F. The Use of Integra Dermal Regeneration Template Versus Flaps for Reconstruction of Full-Thickness Scalp Defects Involving the Calvaria: A Cost–Benefit Analysis. Aesthetic Plast. Surg. 2016, 40, 901–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loss, M.; Wedler, V.; Künzi, W.; Meuli-Simmen, C.; Meyer, V.E. Artificial Skin, Split-Thickness Autograft and Cultured Autologous Keratinocytes Combined to Treat a Severe Burn Injury of 93% of TBSA. Burns 2000, 26, 644–652. [Google Scholar] [CrossRef]
- Alrubaiy, L.; Al-Rubaiy, K.K. Skin Substitutes: A Brief Review of Types and Clinical Applications. Oman Med. J. 2009, 24, 4. [Google Scholar] [CrossRef]
- Reiffel, A.J.; Zheng, Y.; Henderson, P.W.; Millet, Y.H.; Bonassar, L.J.; Stroock, A.D.; Spector, J.A. The Acellular Dermal Replacement Scaffolds Strattice® and Integra®. Plast. Reconstr. Surg. 2011, 128, 37. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Sawaragi, E.; Sakamoto, M.; Nakano, T.; Yamanaka, H.; Tsuge, I.; Matsuno, K.; Tabata, Y.; Morimoto, N. Development of Gelatin Hydrogel Nonwoven Fabrics (Genocel®) as a Novel Skin Substitute in Murine Skin Defects. Regen. Ther. 2022, 21, 96–103. [Google Scholar] [CrossRef]
- Eo, S.; Kim, Y.; Cho, S. Vacuum-Assisted Closure Improves the Incorporation of Artificial Dermis in Soft Tissue Defects: Terudermis® and Pelnac®. Int. Wound J. 2011, 8, 261–267. [Google Scholar] [CrossRef]
- Okushi, T.; Yoshikawa, M.; Otori, N.; Matsuwaki, Y.; Asaka, D.; Nakayama, T.; Morimoto, T.; Moriyama, H. Evaluation of Symptoms and QOL with Calcium Alginate versus Chitin-Coated Gauze for Middle Meatus Packing after Endoscopic Sinus Surgery. Auris Nasus Larynx 2012, 39, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Kolokythas, P.; Aust, M.C.; Vogt, P.M.; Paulsen, F. [Dermal Subsitute with the Collagen-Elastin Matrix Matriderm in Burn Injuries: A Comprehensive Review]. Handchir. Mikrochir. Plast. Chir. 2008, 40, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Ryssel, H.; Germann, G.; Kloeters, O.; Gazyakan, E.; Radu, C.A. Dermal Substitution with Matriderm® in Burns on the Dorsum of the Hand. Burns 2010, 36, 1248–1253. [Google Scholar] [CrossRef]
- Schneider, J.; Biedermann, T.; Widmer, D.; Montano, I.; Meuli, M.; Reichmann, E.; Schiestl, C. Matriderm® versus Integra®: A Comparative Experimental Study. Burns 2009, 35, 51–57. [Google Scholar] [CrossRef]
- Morimoto, N.; Kakudo, N.; Valentin Notodihardjo, P.; Suzuki, S.; Kusumoto, K. Comparison of Neovascularization in Dermal Substitutes Seeded with Autologous Fibroblasts or Impregnated with BFGF Applied to Diabetic Foot Ulcers Using Laser Doppler Imaging. J. Artif. Organs 2014, 17, 352–357. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Chouhan, D.; Mandal, B.B. Silk Biomaterials in Wound Healing and Skin Regeneration Therapeutics: From Bench to Bedside. Acta Biomater. 2020, 103, 24–51. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, P.; Zhou, F.; Zhao, Y.; Ren, L.; Yuan, X. Antimicrobial Eugenol-Loaded Electrospun Membranes of Poly(ε-Caprolactone)/Gelatin Incorporated with REDV for Vascular Graft Applications. Colloids Surf. B Biointerfaces 2018, 162, 335–344. [Google Scholar] [CrossRef]
- Croitoru, A.M.; Ficai, D.; Ficai, A.; Mihailescu, N.; Andronescu, E.; Turculet, C.F. Nanostructured Fibers Containing Natural or Synthetic Bioactive Compounds in Wound Dressing Applications. Materials 2020, 13, 2407. [Google Scholar] [CrossRef]





| Dressing | Types | Form | Major Components | Ref. |
|---|---|---|---|---|
| DermagraftTM | Dermal substitute | Hybrid | Polyglactin mesh, human dermal fibroblast | [94] |
| ApligrafTM | Epidermal and dermal skin substitutes | Bilayer | Cell | [95] |
| IntegraTM | Artificial skin | Hybrid | Bovine tendon collagen /glycosaminoglycan, silicone layer | [96,97] |
| AlloDerm® | Acellular dermal matrix | Decellularized tissue | Human dermis | [98] |
| Stratice™ | Acellular dermal matrix | Decellularized tissue | Porcine dermis | [99] |
| SurgiMend® | Acellular dermal matrix | Decellularized tissue | Bovine dermis | [99] |
| Transcyte® | Tissue-engineered skin substitute | Hybrid | Nylon mesh, silastic layer, human fibroblast | [98] |
| Genocel® | Tissue-engineered skin substitute | Nanofiber (Solution-blow) | Gelatin | [100] |
| Pelnac® | Artificial dermis | Hybrid | Silicone layer, collagen sponge | [101] |
| Beschitin-F® | Skin substitute | Hybrid | Chitin-coated gauze | [102] |
| Matriderm® | Dermal substitute | Three-dimensional matrix | Bovine type I collagen, elastin | [103,104,105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hama, R.; Reinhardt, J.W.; Ulziibayar, A.; Watanabe, T.; Kelly, J.; Shinoka, T. Recent Tissue Engineering Approaches to Mimicking the Extracellular Matrix Structure for Skin Regeneration. Biomimetics 2023, 8, 130. https://doi.org/10.3390/biomimetics8010130
Hama R, Reinhardt JW, Ulziibayar A, Watanabe T, Kelly J, Shinoka T. Recent Tissue Engineering Approaches to Mimicking the Extracellular Matrix Structure for Skin Regeneration. Biomimetics. 2023; 8(1):130. https://doi.org/10.3390/biomimetics8010130
Chicago/Turabian StyleHama, Rikako, James W. Reinhardt, Anudari Ulziibayar, Tatsuya Watanabe, John Kelly, and Toshiharu Shinoka. 2023. "Recent Tissue Engineering Approaches to Mimicking the Extracellular Matrix Structure for Skin Regeneration" Biomimetics 8, no. 1: 130. https://doi.org/10.3390/biomimetics8010130