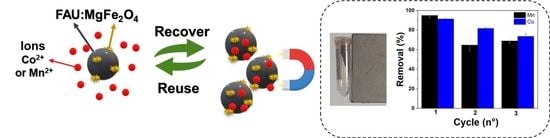
Magnetic Adsorbent Based on Faujasite Zeolite Decorated with Magnesium Ferrite Nanoparticles for Metal Ion Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. FAU Zeolite Synthesis
2.1.2. Magnesium Ferrite Synthesis
2.1.3. FAU:Ferrite Nanocomposite Synthesis—FAU:MgFe2O4(3:1)
2.2. Characterization Techniques
2.3. Adsorption Experiments
2.4. Magnetical Recovery
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paris, E.C.; Malafatti, J.O.D.; Moreira, A.J.; Santos, L.C.; Sciena, C.R.; Zenatti, A.; Escote, M.T.; Mastelaro, V.R.; Joya, M.R. CuO nanoparticles decorated on hydroxyapatite/ferrite magnetic support: Photocatalysis, cytotoxicity, and antimicrobial response. Environ. Sci. Pollut. Res. 2022, 29, 41505–41519. [Google Scholar] [CrossRef]
- Baines, C.; Lerebours, A.; Thomas, F.; Fort, J.; Kreitsberg, R.; Gentes, S.; Meitern, R.; Saks, L.; Ujvari, B.; Giraudeau, M. Linking pollution and cancer in aquatic environments: A review. Environ. Int. 2021, 149, 106391. [Google Scholar] [CrossRef] [PubMed]
- Malafatti, J.O.; Moreira, A.J.; Sciena, C.R.; Silva, T.E.; Freschi, G.P.; Pereira, E.C.; Paris, E.C. Prozac® removal promoted by HAP: Nb2O5 nanoparticles system: By-products, mechanism, and cytotoxicity assessment. J. Environ. Chem. Eng. 2021, 9, 104820. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Von Gunten, U.; Wehrli, B. Global water pollution and human health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Prabhu, P.P.; Prabhu, B. (Eds.) A review on removal of heavy metal ions from waste water using natural/modified bentonite. In Proceedings of the MATEC Web of Conferences, Osaka, Japan, 21–22 September 2018; EDP Sciences: Les Ulis, France, 2018. [Google Scholar]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Rincόn Joya, M.; Barba Ortega, J.; Malafatti, J.O.D.; Paris, E.C. Evaluation of photocatalytic activity in water pollutants and cytotoxic response of α-Fe2O3 nanoparticles. ACS Omega 2019, 4, 17477–17486. [Google Scholar] [CrossRef]
- Yousefi, Z.; Zazouli, M. Removal of heavy metals from solid wastes leachates coagulation-flocculation process. J. Appl. Sci. 2008, 8, 2142–2147. [Google Scholar] [CrossRef]
- Trivunac, K.; Stevanovic, S. Removal of heavy metal ions from water by complexation-assisted ultrafiltration. Chemosphere 2006, 64, 486–491. [Google Scholar] [CrossRef]
- Wu, R. Removal of heavy metal ions from industrial wastewater based on chemical precipitation method. Ekoloji 2019, 28, 2443–2452. [Google Scholar]
- Tran, T.-K.; Chiu, K.-F.; Lin, C.-Y.; Leu, H.-J. Electrochemical treatment of wastewater: Selectivity of the heavy metals removal process. Int. J. Hydrogen Energy 2017, 42, 27741–27748. [Google Scholar] [CrossRef]
- Lakherwal, D. Adsorption of heavy metals: A review. Int. J. Environ. Res. Dev. 2014, 4, 41–48. [Google Scholar]
- Salame, I.I.; Bandosz, T.J. Role of surface chemistry in adsorption of phenol on activated carbons. J. Colloid Interface Sci. 2003, 264, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.; Espinosa, J.; Gurgel, M.; Paris, E.; Leite, E.; Joya, M.; Pizani, P.; Varela, J.A.; Longo, E. Photoluminescence in disordered Sm-doped Pb Ti O 3: Experimental and theoretical approach. J. Appl. Phys. 2006, 100, 034917. [Google Scholar] [CrossRef]
- Chaouati, N.; Soualah, A.; Chater, M. Adsorption of phenol from aqueous solution onto zeolites Y modified by silylation. Comptes Rendus Chim. 2013, 16, 222–228. [Google Scholar] [CrossRef]
- Lopes, M.M.; Coutinho, T.C.; Malafatti, J.O.D.; Paris, E.C.; de Sousa, C.P.; Farinas, C.S. Immobilization of phytase on zeolite modified with iron (II) for use in the animal feed and food industry sectors. Process Biochem. 2021, 100, 260–271. [Google Scholar] [CrossRef]
- Taylor, W. The nature and properties of aluminosilicate framework structures. Proc. R. Soc. Lond. Ser. A Contain. Pap. A Math. Phys. Character 1934, 145, 80–103. [Google Scholar]
- Payra, P.; Dutta, P.K. Zeolites: A primer. In Handbook of Zeolite Science and Technology; CRC Press: Boca Raton, USA, 2003; pp. 1–24. [Google Scholar]
- Reinoso, D.; Adrover, M.; Pedernera, M. Green synthesis of nanocrystalline faujasite zeolite. Ultrason. Sonochem. 2018, 42, 303–309. [Google Scholar] [CrossRef]
- Liu, C.; Li, G.; Hensen, E.J.; Pidko, E.A. Relationship between acidity and catalytic reactivity of faujasite zeolite: A periodic DFT study. J. Catal. 2016, 344, 570–577. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Sing, K. Adsorption by clays, pillared clays, zeolites and aluminophosphates. In Adsorption by Powders and Porous Solids Principles Methodology and Applications; Academic Press: Amsterdam, The Netherlands, 2014; pp. 467–527. [Google Scholar]
- Jeffroy, M.; Boutin, A.; Fuchs, A.H. Understanding the equilibrium ion exchange properties in faujasite zeolite from Monte Carlo simulations. J. Phys. Chem. B 2011, 115, 15059–15066. [Google Scholar] [CrossRef]
- Coutinho, T.C.; Malafatti, J.O.; Paris, E.C.; Tardioli, P.W.; Farinas, C.S. Hydroxyapatite-CoFe2O4 magnetic nanoparticle composites for industrial enzyme immobilization, use, and recovery. ACS Appl. Nano Mater. 2020, 3, 12334–12345. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Alswat, A.A.; Salama, R.S. Gold-selenide quantum dots supported onto cesium ferrite nanocomposites for the efficient degradation of rhodamine B. Heliyon 2022, 8, e09652. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.C.; Petkowicz, D.I.; Smaniotto, A.; Pergher, S.B. Magnetic zeolites: A new adsorbent for removal of metallic contaminants from water. Water Res. 2004, 38, 3699–3704. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-L.; Liu, X.-W.; Fu, R.; Tan, Z.-Y. Magnetic P zeolites: Synthesis, characterization and the behavior in potassium extraction from seawater. Sep. Purif. Technol. 2008, 63, 92–100. [Google Scholar] [CrossRef]
- Supelano, G.; Cuaspud, J.G.; Moreno-Aldana, L.C.; Ortiz, C.; Trujillo, C.; Palacio, C.; Vargas, C.P.; Gómez, J.M. Synthesis of magnetic zeolites from recycled fly ash for adsorption of methylene blue. Fuel 2020, 263, 116800. [Google Scholar] [CrossRef]
- Loiola, A.R.; Bessa, R.A.; Oliveira, C.P.; Freitas, A.D.; Soares, S.A.; Bohn, F.; Pergher, S.B. Magnetic zeolite composites: Classification, synthesis routes, and technological applications. J. Magn. Magn. Mater. 2022, 560, 169651. [Google Scholar] [CrossRef]
- Fungaro, D.A.; Yamaura, M.; Graciano, J.E.A. Remoção de íons Zn2+, Cd2+ e Pb2+ de soluções aquosas usando compósito magnético de zeólita de cinzas de carvão. Química Nova 2010, 33, 1275–1278. [Google Scholar] [CrossRef]
- Xian, G.; Kong, S.; Li, Q.; Zhang, G.; Zhou, N.; Du, H.; Niu, L. Synthesis of spinel ferrite MFe2O4 (M = Co, Cu, Mn, and Zn) for persulfate activation to remove aqueous organics: Effects of M-site metal and synthetic method. Front. Chem. 2020, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, A.; Alshahrani, T.; Muhammad, G.; Alhossainy, M.; Laref, A.; Khan, A.R.; Ali, I.; Farid, H.M.T.; Ghrib, T.; Ejaz, S.R. Magnetic, dielectric and structural properties of spinel ferrites synthesized by sol-gel method. J. Mater. Res. Technol. 2021, 11, 158–169. [Google Scholar] [CrossRef]
- Masunga, N.; Mamba, B.B.; Getahun, Y.W.; El-Gendy, A.A.; Kefeni, K.K. Synthesis of single-phase superparamagnetic copper ferrite nanoparticles using an optimized coprecipitation method. Mater. Sci. Eng. B 2021, 272, 115368. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Nguyen, L.T.; Manh, N.C.; Quoc, D.N.; Quang, H.N.; Nguyen, H.T.; Nguyen, D.C.; Bach, L.G. A facile synthesis, characterization, and photocatalytic activity of magnesium ferrite nanoparticles via the solution combustion method. J. Chem. 2019, 2019, 3428681. [Google Scholar] [CrossRef]
- Fayazzadeh, S.; Khodaei, M.; Arani, M.; Mahdavi, S.; Nizamov, T.; Majouga, A. Magnetic properties and magnetic hyperthermia of cobalt ferrite nanoparticles synthesized by hydrothermal method. J. Supercond. Nov. Magn. 2020, 33, 2227–2233. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Han, G.; Zhang, L.; Huang, Y. Facile microwave-assisted synthesis of magnetic ferrite: Rapid interfacial reaction underlying intensifying mechanism. J. Clean. Prod. 2022, 361, 132181. [Google Scholar] [CrossRef]
- Ateia, E.E.; Ateia, M.A.; Fayed, M.G.; El-Hout, S.I.; Mohamed, S.G.; Arman, M. Synthesis of nanocubic lithium cobalt ferrite toward high-performance lithium-ion battery. Appl. Phys. A 2022, 128, 483. [Google Scholar] [CrossRef]
- Fang, X.; Ma, D.; Sun, B.; Xu, X.; Quan, W.; Xiao, Z.; Zhai, Y. A high-performance magnetic shield with MnZn ferrite and Mu-metal film combination for atomic sensors. Materials 2022, 15, 6680. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Singh, S.; Walia, R.; Kumar, V.; Verma, V. Modification in photovoltaic and photocatalytic properties of bismuth ferrites by tailoring band-gap and ferroelectric properties. J. Alloys Compd. 2022, 908, 164602. [Google Scholar] [CrossRef]
- Uddin, M.J.; Jeong, Y.-K. Application of magnesium ferrite nanomaterials for adsorptive removal of arsenic from water: Effects of Mg and Fe ratio. Chemosphere 2022, 307, 135817. [Google Scholar] [CrossRef]
- Reddy, S.; Swamy, B.K.; Chandra, U.; Mahathesha, K.; Sathisha, T.; Jayadevappa, H. Synthesis of MgFe2O4 nanoparticles and MgFe2O4 nanoparticles/CPE for electrochemical investigation of dopamine. Anal. Methods 2011, 3, 2792–2796. [Google Scholar] [CrossRef]
- Kurian, J.; Mathew, M.J. Structural, magnetic and mossbauer studies of magnesium ferrite nanoparticles prepared by hydrothermal method. Int. J. Nanosci. 2018, 17, 1760001. [Google Scholar] [CrossRef]
- Nonkumwong, J.; Pakawanit, P.; Wipatanawin, A.; Jantaratana, P.; Ananta, S.; Srisombat, L. Synthesis and cytotoxicity study of magnesium ferrite-gold core-shell nanoparticles. Mater. Sci. Eng. C 2016, 61, 123–132. [Google Scholar] [CrossRef]
- Peng, Z.-D.; Lin, X.-M.; Zhang, Y.-L.; Hu, Z.; Yang, X.-J.; Chen, C.-Y.; Chen, H.-Y.; Li, Y.-T.; Wang, J.-J. Removal of cadmium from wastewater by magnetic zeolite synthesized from natural, low-grade molybdenum. Sci. Total Environ. 2021, 772, 145355. [Google Scholar] [CrossRef]
- Neolaka, Y.A.; Lawa, Y.; Naat, J.; Riwu, A.A.; Mango, A.W.; Darmokoesoemo, H.; Widyaningrum, B.A.; Iqbal, M.; Kusuma, H.S. Efficiency of activated natural zeolite-based magnetic composite (ANZ-Fe3O4) as a novel adsorbent for removal of Cr (VI) from wastewater. J. Mater. Res. Technol. 2022, 18, 2896–2909. [Google Scholar] [CrossRef]
- Mthombeni, N.H.; Onyango, M.S.; Aoyi, O. Adsorption of hexavalent chromium onto magnetic natural zeolite-polymer composite. J. Taiwan Inst. Chem. Eng. 2015, 50, 242–251. [Google Scholar] [CrossRef]
- Omer, M.I.; Elbadawi, A.; Yassin, O. Synthesis and structural properties of MgFe2O4 ferrite nano-particles. J. Appl. Ind. Sci. 2013, 1, 20–23. [Google Scholar]
- Yamaura, M.; Fungaro, D.A. Synthesis and characterization of magnetic adsorbent prepared by magnetite nanoparticles and zeolite from coal fly ash. J. Mater. Sci. 2013, 48, 5093–5101. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Talebipour, S.; Neyestani, M.R.; Ranjan, S.; Dasgupta, N. Graphene oxide MgFe2O4 nanocomposites for Cr (VI) remediation: A comparative modeling study. Nanotechnol. Environ. Eng. 2018, 3, 10. [Google Scholar] [CrossRef]
- Pedrosa, S.; Martins, S., Jr.; Souza, R.; Dantas, J.; Souza, C.; Rebouças, G.; De Araújo, J.; Dantas, A.L.; Carriço, A. Dipolar effects on the magnetic phases of superparamagnetic clusters. J. Appl. Phys. 2018, 123, 233902. [Google Scholar] [CrossRef]
- Paris, E.C.; Malafatti, J.O.; Musetti, H.C.; Manzoli, A.; Zenatti, A.; Escote, M.T. Faujasite zeolite decorated with cobalt ferrite nanoparticles for improving removal and reuse in Pb2+ ions adsorption. Chin. J. Chem. Eng. 2020, 28, 1884–1890. [Google Scholar] [CrossRef]
- Sheykhan, M.; Mohammadnejad, H.; Akbari, J.; Heydari, A. Superparamagnetic magnesium ferrite nanoparticles: A magnetically reusable and clean heterogeneous catalyst. Tetrahedron Lett. 2012, 53, 2959–2964. [Google Scholar] [CrossRef]
- Bamzai, K.; Kour, G.; Kaur, B.; Kulkarni, S. Effect of cation distribution on structural and magnetic properties of Dy substituted magnesium ferrite. J. Magn. Magn. Mater. 2013, 327, 159–166. [Google Scholar] [CrossRef]
- Feng, Y.; Li, S.; Zheng, Y.; Yi, Z.; He, Y.; Xu, Y. Preparation and characterization of MgFe2O4 nanocrystallites via PVA sol-gel route. J. Alloys Compd. 2017, 699, 521–525. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, Y.; Wang, J.; Chen, Q. Synthesis of MgFe2O4 nanocrystallites under mild conditions. Mater. Chem. Phys. 2006, 97, 394–397. [Google Scholar] [CrossRef]
- Da Dalt, S.; Bergmann, C. Thermal, Structural and Magnetic Behavior of the MgFe2O4 Nanostructured. In Proceedings of the IBEROMET XIX CONAMET/SAM, Viña del Mar, Chile, 2–5 November 2010; Redalyc: Ciudad de México, Mexico, 2010; pp. 1–7. [Google Scholar]
- Naaz, F.; Dubey, H.K.; Kumari, C.; Lahiri, P. Structural and magnetic properties of MgFe2O4 nanopowder synthesized via co-precipitation route. SN Appl. Sci. 2020, 2, 808. [Google Scholar] [CrossRef]
- Paris, E.C.; Malafatti, J.O.D.; Sciena, C.R.; Junior, L.F.N.; Zenatti, A.; Escote, M.T.; Moreira, A.J.; Freschi, G.P.G. Nb2O5 nanoparticles decorated with magnetic ferrites for wastewater photocatalytic remediation. Environ. Sci. Pollut. Res. 2021, 28, 23731–23741. [Google Scholar] [CrossRef] [PubMed]
- Su, N.R.; Lv, P.; Li, M.; Zhang, X.; Li, M.; Niu, J. Fabrication of MgFe2O4–ZnO heterojunction photocatalysts for application of organic pollutants. Mater. Lett. 2014, 122, 201–204. [Google Scholar] [CrossRef]
- Bose, S.; Tripathy, B.K.; Debnath, A.; Kumar, M. Boosted sono-oxidative catalytic degradation of Brilliant green dye by magnetic MgFe2O4 catalyst: Degradation mechanism, assessment of bio-toxicity and cost analysis. Ultrason. Sonochem. 2021, 75, 105592. [Google Scholar] [CrossRef] [PubMed]
- Hengstler, J.G.; Bolm-Audorff, U.; Faldum, A.; Janssen, K.; Reifenrath, M.; Götte, W.; Jung, D.; Mayer-Popken, O.; Fuchs, J.; Gebhard, S. Occupational exposure to heavy metals: DNA damage induction and DNA repair inhibition prove co-exposures to cadmium, cobalt and lead as more dangerous than hitherto expected. Carcinogenesis 2003, 24, 63–73. [Google Scholar] [CrossRef]
- Scharf, B.; Clement, C.C.; Zolla, V.; Perino, G.; Yan, B.; Elci, S.G.; Purdue, E.; Goldring, S.; Macaluso, F.; Cobelli, N. Molecular analysis of chromium and cobalt-related toxicity. Sci. Rep. 2014, 4, 5729. [Google Scholar] [CrossRef]
- Fernandes, A.; Almeida, C.; Menezes, C.; Debacher, N.; Sierra, M. Removal of methylene blue from aqueous solution by peat. J. Hazard. Mater. 2007, 144, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, G.; Luo, Q.; Li, X.; Wang, Z. The thermodynamics and kinetics for the removal of copper and nickel ions by the zeolite Y synthesized from fly ash. Mater. Res. Express 2018, 6, 025001. [Google Scholar] [CrossRef]
- Simancas, R.; Chokkalingam, A.; Elangovan, S.P.; Liu, Z.; Sano, T.; Iyoki, K.; Wakihara, T.; Okubo, T. Recent progress in the improvement of hydrothermal stability of zeolites. Chem. Sci. 2021, 12, 7677–7695. [Google Scholar] [CrossRef]
- Heard, C.J.; Grajciar, L.; Uhlík, F.; Shamzhy, M.; Opanasenko, M.; Čejka, J.; Nachtigall, P. Zeolite (in) stability under aqueous or steaming conditions. Adv. Mater. 2020, 32, 2003264. [Google Scholar] [CrossRef] [PubMed]
- Condomitti, U.; Zuin, A.; Silveira, A.T.; Araki, K.; Toma, H.E. Direct use of superparamagnetic nanoparticles as electrode modifiers for the analysis of mercury ions from aqueous solution and crude petroleum samples. J. Electroanal. Chem. 2011, 661, 72–76. [Google Scholar] [CrossRef]
- Liosis, C.; Papadopoulou, A.; Karvelas, E.; Karakasidis, T.E.; Sarris, I.E. Heavy metal adsorption using magnetic nanoparticles for water purification: A critical review. Materials 2021, 14, 7500. [Google Scholar] [CrossRef] [PubMed]










| Sample | SExt (m2 g−1) | SSABET (m2 g−1) | VMicro (cm3 g−1) |
|---|---|---|---|
| FAU | 390 | 650 | 0.30 |
| MgFe2O4 | 14 | 19 | 0.051 |
| FAU:MgFe2O4 | 34 | 400 | 0.20 |
| Model | Parameter | Value | |
|---|---|---|---|
| Co (a) | Mn (b) | ||
| R2 | 0.99941 | 0.99998 | |
| Pseudo-First Order | k1 (min−1) | 7.89 × 103 | 8.80 × 102 |
| qe | 3.85 | 3.54 | |
| R2 | 0.99941 | 0.99997 | |
| Pseudo-Second Order | k2 (g mg−1 min−1) | 1.02 × 1020 | 1.08 × 1019 |
| qe | 3.85 | 3.54 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meirelles, M.R.; Malafatti, J.O.D.; Escote, M.T.; Pinto, A.H.; Paris, E.C. Magnetic Adsorbent Based on Faujasite Zeolite Decorated with Magnesium Ferrite Nanoparticles for Metal Ion Removal. Magnetochemistry 2023, 9, 136. https://doi.org/10.3390/magnetochemistry9050136
Meirelles MR, Malafatti JOD, Escote MT, Pinto AH, Paris EC. Magnetic Adsorbent Based on Faujasite Zeolite Decorated with Magnesium Ferrite Nanoparticles for Metal Ion Removal. Magnetochemistry. 2023; 9(5):136. https://doi.org/10.3390/magnetochemistry9050136
Chicago/Turabian StyleMeirelles, Mariana Rodrigues, João Otávio Donizette Malafatti, Márcia Tsuyama Escote, Alexandre Henrique Pinto, and Elaine Cristina Paris. 2023. "Magnetic Adsorbent Based on Faujasite Zeolite Decorated with Magnesium Ferrite Nanoparticles for Metal Ion Removal" Magnetochemistry 9, no. 5: 136. https://doi.org/10.3390/magnetochemistry9050136