Fluorescent Magnetic Mesoporous Nanoprobes for Biotechnological Enhancement Procedures in Gene Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
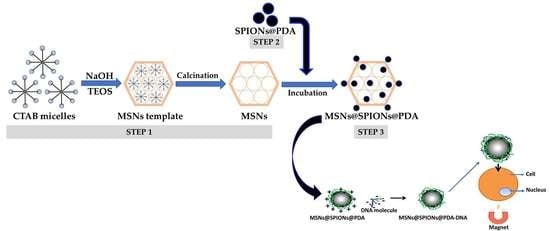
2.2. Synthesis of Fluorescent Magnetic MCM-41 Mesoporous Silica Nanostructures (MSNs@SPIONs@PDA)
2.3. Physicochemical Characterization
2.3.1. XRD-Structural Characterization
2.3.2. Surface Chemistry Characterization
2.3.3. Morphological Characterization
2.3.4. Hydrodynamic Particle Size and Zeta Potential measurements
2.3.5. Compositional Characterization
2.3.6. Magnetic Characterization
2.4. Biological Characterization
2.4.1. Cells Line
2.4.2. Trypsinization
2.4.3. Freezing
2.4.4. Obtaining Genetic Material
2.4.5. Passive Uptake
2.4.6. Magnetic Uptake
2.4.7. Transfection with Commercial Kit
2.4.8. Transfection with MSNs@SPIONs@PDA
2.4.9. Magnetofection with MSNs@SPIONs@PDA
2.4.10. Cell Uptake Visualization
2.4.11. MTT Assay
3. Results and Discussion
3.1. XRD Patterns
3.2. FTIR Spectra
3.3. TEM and HR-TEM Analysis
3.4. DLS Characterization
3.5. Magnetic Properties
3.6. Cellular Internalization: Magnetic Uptake vs. Passive Uptake
3.7. Assessment of Efficacy Transfection: Magnetofection vs. Classical Transfection
3.8. Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, S.K.; Menezes, M.E.; Bhatia, S.; Wang, X.-Y.; Emdad, L.; Sarkar, D.; Fisher, P.B. Gene Therapies for Cancer: Strategies, Challenges and Successes. J. Cell. Physiol. 2015, 230, 259–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, M.; Lee, L.K.C.; Peng, B.; Choi, C.H.J.; Tong, W.Y.; Voelcker, N.H. Delivering the Promise of Gene Therapy with Nanomedicines in Treating Central Nervous System Diseases. Adv. Sci. 2022, 9, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Sayed, N.; Allawadhi, P.; Khurana, A.; Singh, V.; Navik, U.; Pasumarthi, S.K.; Khurana, I.; Banothu, A.K.; Weiskirchen, R.; Bharani, K.K. Gene Therapy: Comprehensive Overview and Therapeutic Applications. Life Sci. 2022, 294, 120375. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Harnett, M.C.; Yan, T.H.; Georgas, E.; Qin, Y.X.; Zhou, H.C.; Wang, Y. Progress, Opportunities, and Challenges of Magneto-Plasmonic Nanoparticles under Remote Magnetic and Light Stimulation for Brain-Tissue and Cellular Regeneration. Nanomaterials 2022, 12, 2242. [Google Scholar] [CrossRef]
- Caffery, B.; Lee, J.S.; Alexander-Bryant, A.A. Vectors for Glioblastoma Gene Therapy: Viral & Non-Viral Delivery Strategies. Nanomaterials 2019, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Comanescu, C. Magnetic Nanoparticles: Current Advances in Nanomedicine, Drug Delivery and MRI. Chemistry 2022, 4, 872–930. [Google Scholar] [CrossRef]
- Tewari, A.K.; Upadhyay, S.C.; Kumar, M.; Pathak, K.; Kaushik, D.; Verma, R.; Bhatt, S.; Massoud, E.E.S.; Rahman, M.H.; Cavalu, S. Insights on Development Aspects of Polymeric Nanocarriers: The Translation from Bench to Clinic. Polymers 2022, 14, 3545. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Gao, J. Biocompatible Iron Oxide Nanoparticles for Targeted Cancer Gene Therapy: A Review. Nanomaterials 2022, 12, 3323. [Google Scholar] [CrossRef]
- Faneca, H. Non-Viral Gene Delivery Systems. Pharmaceutics 2021, 13, 3–6. [Google Scholar] [CrossRef]
- Zu, H.; Gao, D. Non-Viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef]
- Hamimed, S.; Jabberi, M.; Chatti, A. Nanotechnology in Drug and Gene Delivery. Naunyn. Schmiedebergs. Arch. Pharmacol. 2022, 395, 769–787. [Google Scholar] [CrossRef]
- Bharti, S.; Anant, P.S.; Kumar, A. Nanotechnology in Stem Cell Research and Therapy. J. Nanoparticle Res. 2023, 25, 1–24. [Google Scholar] [CrossRef]
- Piñeiro, Y.; Gómez, M.G.; de Castro Alves, L.; Prieto, A.A.; Acevedo, P.G.; Gudiña, R.S.; Puig, J.; Teijeiro, C.; Vilar, S.Y.; Rivas, J. Hybrid Nanostructured Magnetite Nanoparticles: From Bio-Detection and Theragnostics to Regenerative Medicine. Magnetochemistry 2020, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Romano, M.; González Gómez, M.A.; Santonicola, P.; Aloi, N.; Offer, S.; Pantzke, J.; Raccosta, S.; Longo, V.; Surpi, A.; Alacqua, S.; et al. Synthesis and Characterization of a Biocompatible Nanoplatform Based on Silica-Embedded SPIONs Functionalized with Polydopamine. ACS Biomater. Sci. Eng. 2022, 9, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Popova, V.; Dmitrienko, E.; Chubarov, A. Magnetic Nanocomposites and Imprinted Polymers for Bio-Medical Applications of Nucleic Acids. Magnetochemistry 2022, 9, 12. [Google Scholar] [CrossRef]
- Siciliano, G.; Monteduro, A.G.; Turco, A.; Primiceri, E.; Rizzato, S.; Depalo, N.; Curri, M.L.; Maruccio, G. Polydopamine-Coated Magnetic Iron Oxide Nanoparticles: From Design to Applications. Nanomaterials 2022, 12, 1145. [Google Scholar] [CrossRef]
- Busa, P.; Koutavarapu, R.; Kuthati, Y. Polydopamine-Coated Copper-Substituted Mesoporous Silica Nanoparticles for Dual Cancer Therapy. Coatings 2022, 12, 60. [Google Scholar] [CrossRef]
- Chang, D.; Gao, Y.; Wang, L.; Liu, G.; Chen, Y.; Wang, T.; Tao, W.; Mei, L.; Huang, L.; Zeng, X. Polydopamine-Based Surface Modification of Mesoporous Silica Nanoparticles as PH-Sensitive Drug Delivery Vehicles for Cancer Therapy. J. Colloid Interface Sci. 2016, 463, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Sodagar-Taleghani, A.; Ebrahimnejad, P.; Pouya Hadipour Moghaddam, S.; Ebrahimnejad, F.; Asare-Addo, K.; Nokhodchi, A. A Review on the Latest Developments of Mesoporous Silica Nanoparticles as a Promising Platform for Diagnosis and Treatment of Cancer. Int. J. Pharm. 2022, 625, 122099. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.B.; Nayak, Y.; Garg, S.; Nayak, U.Y. Multifunctional Engineered Mesoporous Silica/Inorganic Material Hybrid Nanoparticles: Theranostic Perspectives. Coord. Chem. Rev. 2023, 478, 214977. [Google Scholar] [CrossRef]
- Blokpoel Ferreras, L.A.; Chan, S.Y.; Vazquez Reina, S.; Dixon, J.E. Rapidly Transducing and Spatially Localized Magnetofection Using Peptide-Mediated Non-Viral Gene Delivery Based on Iron Oxide Nanoparticles. ACS Appl. Nano Mater. 2021, 4, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Uthaman, S.; Muthiah, M.; Park, I.K.; Cho, C.S. Fabrication and Development of Magnetic Particles for Gene Therapy; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9780081005217. [Google Scholar]
- Sizikov, A.A.; Kharlamova, M.V.; Nikitin, M.P.; Nikitin, P.I.; Kolychev, E.L. Nonviral Locally Injected Magnetic Vectors for in Vivo Gene Delivery: A Review of Studies on Magnetofection. Nanomaterials 2021, 11, 1078. [Google Scholar] [CrossRef]
- Seco Gudiña, R.; Yáñez Vilar, S.; González Gómez, M.; Vargas Osorio, Z.; de la Fuente, M.; Piñeiro Redondo, Y.; López, R.; Rivas, J. Versatile Mesoporous Nanoparticles for Cell Applications. J. Nanosci. Nanotechnol. 2021, 21, 2824–2833. [Google Scholar] [CrossRef]
- González-Gómez, M.A.; Belderbos, S.; Yañez-Vilar, S.; Piñeiro, Y.; Cleeren, F.; Bormans, G.; Deroose, C.M.; Gsell, W.; Himmelreich, U.; Rivas, J. Development of Superparamagnetic Nanoparticles Coated with Polyacrylic Acid and Aluminum Hydroxide as an Efficient Contrast Agent for Multimodal Imaging. Nanomaterials 2019, 9, 1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, H.P.; Lim, Y.W.; Xiong, Z.; Martel, N.; Ferguson, C.; Ariotti, N.; Giacomotto, J.; Rae, J.; Floetenmeyer, M.; Moradi, S.V.; et al. Cavin4 Interacts with Bin1 to Promote T-Tubule Formation and Stability in Developing Skeletal Muscle. J. Cell Biol. 2021, 220, e201905065. [Google Scholar] [CrossRef]
- Sun, J.; Kim, D.H.; Guo, Y.; Teng, Z.; Li, Y.; Zheng, L.; Zhang, Z.; Larson, A.C.; Lu, G. A c(RGDfE) Conjugated Multi-Functional Nanomedicine Delivery System for Targeted Pancreatic Cancer Therapy. J. Mater. Chem. B 2015, 3, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Brasil, H.; de Carvalho, A.L.G.; Costa, F.F.; do Nascimento, L.A.S.; Mhadmhan, S.; Pineda, A.; Luque, R.; Valença, G.P. Preparation of Novel Mesoporous Ca/P MCM-41-Based Materials for Mechanochemical Diphenyl Sulfide Oxidation. Microporous Mesoporous Mater. 2020, 297, 110017. [Google Scholar] [CrossRef]
- Khaled, R.K.; Wahba, M.A.; Badry, M.D.; Zawrah, M.F.; Heikal, E.A. Highly Ordered Pure and Indium-Incorporated MCM-41 Mesoporous Adsorbents: Synthesis, Characterization and Evaluation for Dye Removal. J. Mater. Sci. 2022, 57, 4504–4527. [Google Scholar] [CrossRef]
- Coker, V.S.; Bell, A.M.T.; Pearce, C.I.; Patrick, R.A.D.; van der Laan, G.; Lloyd, J.R. Time-Resolved Synchrotron Powder X-Ray Diffraction Study of Magnetite Formation by the Fe(III)-Reducing Bacterium Geobacter Sulfurreducens. Am. Mineral. 2008, 93, 540–547. [Google Scholar] [CrossRef]
- Vargas-Osorio, Z.; González-Gómez, M.A.; Piñeiro, Y.; Vázquez-Vázquez, C.; Rodríguez-Abreu, C.; López-Quintela, M.A.; Rivas, J. Novel Synthetic Routes of Large-Pore Magnetic Mesoporous Nanocomposites (SBA-15/Fe3O4) as Potential Multifunctional Theranostic Nanodevices. J. Mater. Chem. B 2017, 5, 9395–9404. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.; Fan, F.; Zhang, Y.; Pei, Z.; Wang, W.; Pei, Y. A Facile Approach for Fabrication of Core-Shell Magnetic Molecularly Imprinted Nanospheres towards Hypericin. Polymers 2017, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Acevedo, P.G.; Gómez, M.A.G.; Prieto, A.; Alves, L.D.C.; Gudiña, R.S.; Piñeiro, Y.; Rivas, J. Fluorescent Single-Core and Multi-Core Nanoprobes as Cell Trackers and Magnetic Nanoheaters. Magnetochemistry 2022, 8, 83. [Google Scholar] [CrossRef]
- Stoia, M.; Istratie, R.; Păcurariu, C. Investigation of Magnetite Nanoparticles Stability in Air by Thermal Analysis and FTIR Spectroscopy. J. Therm. Anal. Calorim. 2016, 125, 1185–1198. [Google Scholar] [CrossRef]
- Shavel, A.; Rodríguez-González, B.; Spasova, M.; Farle, M.; Liz-Marzán, L.M. Synthesis and Characterization of Iron/Iron Oxide Core/Shell Nanocubes. Adv. Funct. Mater. 2007, 17, 3870–3876. [Google Scholar] [CrossRef]
- Souza, T.G.F.; Ciminelli, V.S.T.; Mohallem, N.D.S. A Comparison of TEM and DLS Methods to Characterize Size Distribution of Ceramic Nanoparticles. J. Phys. Conf. Ser. 2016, 733, 012039. [Google Scholar] [CrossRef] [Green Version]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Mazario, E.; Sánchez-Marcos, J.; Menéndez, N.; Herrasti, P.; García-Hernández, M.; Muñoz-Bonilla, A. One-Pot Electrochemical Synthesis of Polydopamine Coated Magnetite Nanoparticles. RSC Adv. 2014, 4, 48353–48361. [Google Scholar] [CrossRef]
- Binns, C. Tutorial Section on Nanomagnetism, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 6, ISBN 9780080983530. [Google Scholar]
- Kucheryavy, P.; He, J.; John, V.T.; Maharjan, P.; Spinu, L.; Goloverda, G.Z.; Kolesnichenko, V.L. Superparamagnetic Iron Oxide Nanoparticles with Variable Size and an Iron Oxidation State as Prospective Imaging Agents. Langmuir 2013, 29, 710–716. [Google Scholar] [CrossRef] [Green Version]
- Goya, G.F.; Berquó, T.S.; Fonseca, F.C.; Morales, M.P. Static and Dynamic Magnetic Properties of Spherical Magnetite Nanoparticles. J. Appl. Phys. 2003, 94, 3520–3528. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zou, B.; Rondinone, A.J.; Zhang, Z.J. Chemical Control of Superparamagnetic Properties of Magnesium and Cobalt Spinel Ferrite Nanoparticles through Atomic Level Magnetic Couplings. J. Am. Chem. Soc. 2000, 122, 6263–6267. [Google Scholar] [CrossRef]
- Graf, C.; Gao, Q.; Schütz, I.; Noufele, C.N.; Ruan, W.; Posselt, U.; Korotianskiy, E.; Nordmeyer, D.; Rancan, F.; Hadam, S.; et al. Surface Functionalization of Silica Nanoparticles Supports Colloidal Stability in Physiological Media and Facilitates Internalization in Cells. Langmuir 2012, 28, 7598–7613. [Google Scholar] [CrossRef]
- Sajib, M.; Albers, E.; Langeland, M.; Undeland, I. Understanding the Effect of Temperature and Time on Protein Degree of Hydrolysis and Lipid Oxidation during Ensilaging of Herring (Clupea Harengus) Filleting Co-Products. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Chrobak, E.; Boryczka, G.; Ewa, B. Betulin Acid Ester Derivatives Inhibit Cancer Cell Growth by Inducing Apoptosis through Caspase Cascade Activation: A Comprehensive In Vitro and In Silico Study. Int. J. Mol. Sci. 2023, 24, 196. [Google Scholar] [CrossRef]
- Vacas, A.; Sugden, C.; Velasco-Rodriguez, Ó.; Algarabel-Olona, M.; Peña-Guerrero, J.; Larrea, E.; Fernández-Rubio, C.; Nguewa, P.A. Construction of Two MCherry Plasmids (PXG-MCherry) for Transgenic Leishmania: Valuable Tools for Future Molecular Analysis. J. Parasitol. Res. 2017, 2017, 1964531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosnjak, M.; Kamensek, U.; Sersa, G.; Stolfa, D.; Lavrencak, J.; Cemazar, M. Inhibition of the Innate Immune Receptors for Foreign DNA Sensing Improves Transfection Efficiency of Gene Electrotransfer in Melanoma B16F10 Cells. J. Membr. Biol. 2018, 251, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, F.; Castaldo, R.; Latronico, T.; Lasala, P.; Gentile, G.; Lavorgna, M.; Striccoli, M.; Agostiano, A.; Comparelli, R.; Depalo, N.; et al. High Surface Area Mesoporous Silica Nanoparticles with Tunable Size in the Sub-Micrometer Regime: Insights on the Size and Porosity Control Mechanisms. Molecules 2021, 26, 4247. [Google Scholar] [CrossRef] [PubMed]
- Fisichella, M.; Dabboue, H.; Bhattacharyya, S.; Saboungi, M.L.; Salvetat, J.P.; Hevor, T.; Guerin, M. Mesoporous Silica Nanoparticles Enhance MTT Formazan Exocytosis in HeLa Cells and Astrocytes. Toxicol. Vitr. 2009, 23, 697–703. [Google Scholar] [CrossRef]
- Wu, H.; Wei, M.; Xu, Y.; Li, Y.; Zhai, X.; Su, P.; Ma, Q.; Zhang, H. PDA-Based Drug Delivery Nanosystems: A Potential Approach for Glioma Treatment. Int. J. Nanomedicine 2022, 17, 3751–3775. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Pan, Y.; Chen, S.; Fan, X.; Li, X.; Chen, G.; Ma, Y.; Cai, Y.; Zhang, J.; et al. Polycatechol-Derived Mesoporous Polydopamine Nanoparticles for Combined ROS Scavenging and Gene Interference Therapy in Inflammatory Bowel Disease. ACS Appl. Mater. Interfaces 2022, 14, 19975–19987. [Google Scholar] [CrossRef]
- Vargas-Osorio, Z.; da Silva-Candal, A.; Piñeiro, Y.; Iglesias-Rey, R.; Sobrino, T.; Campos, F.; Castillo, J.; Rivas, J. Multifunctional Superparamagnetic Stiff Nanoreservoirs for Blood Brain Barrier Applications. Nanomaterials 2019, 9, 449. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Osorio, Z.; Luzardo-Álvarez, A.; Piñeiro, Y.; Vázquez-Vázquez, C.; Gómez-Amoza, J.L.; Blanco-Méndez, J.; Otero Espinar, F.J.; Rivas, J. Three-Dimensional Hybrid Mesoporous Scaffolds for Simvastatin Sustained Delivery with In Vitro Cell Compatibility. ACS Omega 2019, 4, 5496–5508. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Osorio, Z.; Klotschan, A.; Arango-Ospina, M.; Piñeiro, Y.; Liverani, L.; Rivas, J.; Michálek, M.; Galusek, D.; Boccaccini, A.R. Effect of Glycerol and H3PO4 on the Bioactivity and Degradability of Rod-like SBA-15 Particles with Active Surface for Bone Tissue Engineering Applications. Microporous Mesoporous Mater. 2022, 329, 111543. [Google Scholar] [CrossRef]
- Pérez Sayans, M.; Rivas Mundiña, B.; Chamorro Petronacci, C.M.; García García, A.; Gómez García, F.J.; Crecente Campo, J.; Yañez Vilar, S.; Piñeiro Redondo, Y.; Rivas, J.; López Jornet, P. Effect of Mesoporous Silica and Its Combination with Hydroxyapatite on the Regeneration of Rabbit’s Bone Defects: A Pilot Study. Biomed. Mater. Eng. 2021, 32, 281–294. [Google Scholar] [CrossRef] [PubMed]










| Sample | DH (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| MSNs | 100.3 ± 30.5 | 0.2 | −52.5 |
| SPIONs@PDA | 77.34 ± 28.2 | 0.2 | 24.4 |
| MSNs@SPIONs@PDA | 111.7 ± 47.7 | 0.3 | −33.2 |
| Transfection Method | Incubation Time (min) |
|---|---|
| Passive | 240 |
| Magnetic | 15 |
| Commercial K2® kit | 960 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Gómez, M.A.; Seco-Gudiña, R.; García-Acevedo, P.; Arnosa-Prieto, Á.; de Castro-Alves, L.; Piñeiro, Y.; Rivas, J. Fluorescent Magnetic Mesoporous Nanoprobes for Biotechnological Enhancement Procedures in Gene Therapy. Magnetochemistry 2023, 9, 67. https://doi.org/10.3390/magnetochemistry9030067
González-Gómez MA, Seco-Gudiña R, García-Acevedo P, Arnosa-Prieto Á, de Castro-Alves L, Piñeiro Y, Rivas J. Fluorescent Magnetic Mesoporous Nanoprobes for Biotechnological Enhancement Procedures in Gene Therapy. Magnetochemistry. 2023; 9(3):67. https://doi.org/10.3390/magnetochemistry9030067
Chicago/Turabian StyleGonzález-Gómez, Manuel A., Román Seco-Gudiña, Pelayo García-Acevedo, Ángela Arnosa-Prieto, Lisandra de Castro-Alves, Yolanda Piñeiro, and José Rivas. 2023. "Fluorescent Magnetic Mesoporous Nanoprobes for Biotechnological Enhancement Procedures in Gene Therapy" Magnetochemistry 9, no. 3: 67. https://doi.org/10.3390/magnetochemistry9030067