1. Introduction
Hypericum perforatum L. (St. John’s wort) is recognized as the most important medicinal plant all over the world. The
H. perforatum extracts represent a rich source of naphthodianthrones, acyl-phloroglucinols, flavonoids, hydroxycinnamic acids and xanthones that are responsible for antidepressant, antioxidant, antimicrobial, antiviral, anti-inflammatory and anticancer activities [
1]. The pharmaceutical industry is presently supplied with the upper flowering parts of wild-growing or cultivated plants for preparation of
H. perforatum commercial remedies [
2]. However, the obtainment of crude extracts with a stable quantity of bioactive compounds is still difficult due to the influence of various abiotic and biotic environmental factors on the phytochemical composition of field-grown plants [
3]. In addition, the heterogeneity of secondary metabolite contents is greatly influenced by the genotype, and developmental and physiological states, as well the harvesting period and processing of plant material [
4]. Therefore, the application of biotechnological tools for cultivation of
H. perforatum under controlled and aseptic conditions is a key prerequisite to obtain sustainable and high-quality biomass with standardized production of bioactive metabolites.
There is a plethora of studies reporting that
Hypericum cell, tissue and organ cultures represent an efficient system for continuous production of pharmacologically active compounds. Phytochemical analyses of
H. perforatum in vitro cultures have mainly been focused on shoots and plantlets as the richest sources of hypericins, hyperforins, phenolic acids and various groups of flavonoids [
5,
6,
7,
8]. The capability of undifferentiated callus and cell suspension cultures for production of these shoot-specific phenolic compounds has also been studied [
5,
6,
9]. On the other hand,
H. perforatum root cultures that have previously been considered as an unattractive model for commercial exploitation have now become a promising experimental system for accumulation of valuable metabolites [
10,
11,
12,
13]. The production of various primary and secondary metabolites has previously been reported in
H. perforatum root cultures elicited with chitosan [
12]. Similarly, Tocci et al. [
11] have observed that biomass production and xanthone accumulation in chitosan-elicited
H. perforatum root cultures are dependent on auxin concentration in the medium. In addition, several studies have pointed out that optimization of bioreactor technology is a key point for increased growth and secondary metabolite accumulation in
H. perforatum adventitious root cultures [
10,
14,
15]. Although significant product yield of
H. perforatum roots has been achieved through culture optimization, the obtainment of secondary-metabolite-enriched biomass needs further improvement for commercial scale-up production.
Agrobacterium-rhizogenes-mediated plant transformation represents a biotechnological approach that combines in vitro culture and “natural genetic engineering” technologies for the establishment of hairy roots (HR). The HR cultures have been proposed as a suitable system for large-scale biomass production due to their auxin-independent growth associated with strong biosynthetic capacity [
16]. Despite the recalcitrant nature of the genus
Hypericum to
Agrobacterium transformation, there are several reports of successful establishment of HR cultures [
17,
18,
19,
20].
Hypericum HR cultures have been the subject of extensive studies concerning their phenotype and regenerative potential, as well as secondary metabolite production [
19,
20,
21,
22,
23,
24,
25,
26]. From our recent studies,
H. perforatum HR cultures have been shown to represent an efficient source of phenolic acids, catechins, quercetin and kaempferol glycosides, as well as numerous xanthones with antioxidant, antimicrobial, antidepressant, neuroprotective and antidiabetic properties [
19,
24,
26]. However,
Hypericum HR were not always able to produce hypericins and hyperforins, which are usually accumulated in plant aerial parts [
19,
20,
26]. It has been shown that HR cultures exposed to light or photoperiod turn green due to development of chloroplasts that might contribute to the capability for de novo production of shoot-specific compounds [
27]. Results from our previous study showed that
H. perforatum HR are responsive to photoperiodic stimulus through de novo biosynthesis and accumulation of hydroxycinnamic acids, flavonols and xanthones [
28]. The data indicate that the biosynthetic potential of photoperiod-exposed HR has been activated due to stress-induced responses upon light exposure. The greening of HR could be considered as an unusual physiological phenomenon related to light-dependent production of reactive oxygen species (ROS). This oxidative burst triggers the non-enzymatic and enzymatic antioxidant machinery to combat the photooxidative stress in green HR [
29]. Altogether, these observations lay down the current hypothesis that exposure of
H. perforatum HR to light induces a complex array of plant defense responses through modulation of phenylpropanoid/naphthodianthrone metabolism and substantial modification of antioxidant status.
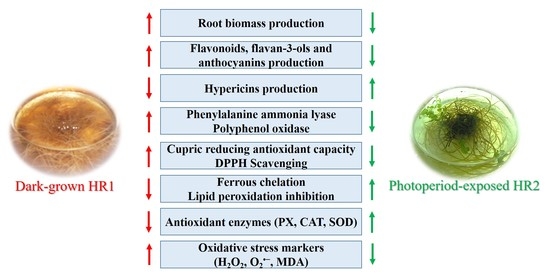
This is the first study to evaluate the antioxidant status of fifteen H. perforatum HR clones upon photooxidative stress. For this purpose, fifteen HR lines grown under dark and photoperiod conditions were analyzed according to the following topics:
- (1)
fresh weight, dry weight, fresh weight/dry weight ratio and dry weight yield;
- (2)
total phenolic, flavonoid, flavan-3-ol, anthocyanin and hypericin contents;
- (3)
phenylalanine ammonia lyase and polyphenol oxidase activities;
- (4)
cupric ion reducing antioxidant capacity, DPPH radical scavenging activity, ferrous chelating activity and lipid peroxidation inhibition;
- (5)
guaiacol peroxidase, catalase and superoxide dismutase activities;
- (6)
hydrogen peroxide, superoxide radical and malondialdehyde contents.
2. Materials and Methods
2.1. Establishment of Dark-Grown and Photoperiod-Exposed HR Clones
Solid-grown HR clones have been previously established by
A. rhizogenes A4-mediated transformation of
H. perforatum [
19,
25]. Fifteen HR clones derived from independent transformation events were denoted with capital letters (HR A-HR O). These HR lines were used here for establishment of dark-grown (HR1 A-HR1 O) and photoperiod-exposed (HR2 A-HR2 O) clones. For this purpose, 0.5 g of each solid-grown HR clone was inoculated into 100 mL liquid MS/B
5 medium in Erlenmeyer flasks (250 mL) and placed on a rotary shaker (100 rpm). The HR1 cultures were maintained in a culture room under darkness, while HR2 lines were exposed to photoperiod at 16 h light/8 h dark and irradiance of 50 μmol m
2 s
−1. A control experiment was set up with non-transformed roots cultured in liquid medium under darkness (NTR1) and photoperiod (NTR2).
2.2. Growth Characteristics
The HR1 and HR2 clones along with the corresponding control NTR1 and NTR2 roots were evaluated for fresh weight (FW), dry weight (DW), FW/DW ratio and dry weight yield (DWY). The HR (0.5 g) were cultured into liquid MS/B5 medium for one month and the FW was measured. For determination of DW, the HR were lyophilized under vacuum (0.22 mbar). The values for FW and DW of HR cultures were used for determination of FW/DW ratio and DWY (DWY = DW/FW × 100).
2.3. Phenolic Compound Contents
One-month-old HR1 and HR2 clones of
H. perforatum were harvested, frozen in liquid nitrogen, then lyophilized and stored at −80 °C, until phytochemical analyses. Phenolic compounds extraction and quantification were performed as previously reported [
25]. The phenolic compound contents in plant extracts included determination of total phenolics (TP), flavonoids (TF), flavan-3-ols (TFA), anthocyanins (TA) and hypericins (TH). Spectrophotometric analyses were performed on a SpectraMax 190 Microplate Reader (Molecular Devices Corp., Sunnyvale, CA, USA) supported with SoftMax Pro (v. 5.4.1) software.
The TP contents in HR were determined by mixing root extracts with Folin–Ciocalteu reagent and 0.7 M sodium carbonate [
30]. The samples were incubated at 50 °C for 15 min and then cooled at room temperature. The sample absorbance was measured at 765 nm and TP contents were expressed as milligrams of gallic acid (GA) equivalents per gram of dry weight (mg GA·g
−1 DW).
The TF contents in HR were determined using the assay described by Zhishen et al. [
31]. Root extract was mixed with 5% sodium nitrite, 10% aluminium chloride and 1 M sodium hydroxide. The sample absorbance was measured at 510 nm and TF contents were expressed as milligrams of catechin (C) equivalents per gram of dry weight (mg C·g
−1 DW).
The TFA contents in HR were estimated by 4-dimethylaminocinnamaldehyde (DMACA) assay [
32]. The 0.1% DMACA reagent was added to diluted root extracts. The sample absorbance was measured at 640 nm and TFA contents were expressed as milligrams of catechin (C) equivalents per gram of dry weight (mg C·g
−1 DW).
The TA contents in HR were determined by the pH-differential method [
33]. The root extracts were diluted with 0.025 M KCl (pH 1.0) and 0.4 M CH
3COONa (pH 4.5) buffers and incubated at room temperature for 20 min. The sample absorbance was measured at 510 nm and 700 nm. The molar extinction coefficient of cyanidin-3-glucoside (ε
535 = 26,900 L·mol
−1·cm
−1) was used for determination of TA contents expressed as milligrams of cyanidin-3-glucoside (CG) equivalents per gram of dry weight (mg CG·g
−1 DW).
The TH contents in HR were assayed using the protocol described in the study of Tusevski et al. [
25]. The hypericins were extracted from a powdered sample using 80% tetrahydrofuran at 65 °C. After centrifugation, the supernatant was lyophilized under vacuum and the dried extracts were dissolved in CH
3OH. The methanolic extracts were centrifuged (10 min at 12,000 rpm) and the absorbance of the supernatant was read at 590 nm. The TH contents were expressed as micrograms of hypericin (H) equivalents per gram of dry weight (µg H·g
−1 DW).
2.4. Phenylalanine Ammonia Lyase (PAL) and Polyphenol Oxydase (PPO) Activities
The extract for determination of phenylalanine ammonia lyase (PAL) and polyphenol oxidase (PPO) activities was prepared by homogenization of 0.3–0.4 g frozen root tissue in 1 mL suitable buffer. The homogenate was centrifuged at 13,000 rpm for 20 min at 4 °C and the supernatant was collected for determination of soluble protein content, as well as for PAL and PPO assays. The analysis of protein content in the enzyme extracts was performed with Bradford reagent. The reaction mixture consisting of the enzyme extract and Bradford reagent was incubated at room temperature for 10 min and the absorbance was measured at 595 nm. The total protein content in the enzyme extracts was calculated using bovine serum albumin as a standard.
The PAL assay in enzyme extracts was performed according to Gadzovska et al. [
6] with modifications reported by Tusevski et al. [
25]. The extraction of PAL enzyme from fresh HR tissue was done with 100 mM sodium borate buffer (pH 8.8). The mixture consisting of extraction buffer, enzyme extract and 20 mM L-phenylalanine was incubated at 40 °C. The increase of absorbance was monitored every 20 min for a period of 60 min at 290 nm. The molar extinction coefficient of trans-cinnamic acid (ε
290 = 9630 L·mol
−1·cm
−1) was used for calculation of PAL activity in pkat·mg
−1 proteins.
The PPO assay in enzyme extracts was performed according to the method of Das et al. [
34] with modifications described by Tusevski et al. [
25]. The extraction of PPO enzyme from fresh HR tissue was done with 50 mM potassium phosphate buffer (pH 7). The reaction mixture consisting of extraction buffer, enzyme extract and 40 mM pyrocatechol was incubated at room temperature. The increase of absorbance was monitored every 10 min for a period of 30 min at 390 nm. The molar extinction coefficient of 1,2-benzoquinone (ε
390 = 1417 L·mol
−1·cm
−1) was used for calculation of PPO activity in nkat·mg
−1 proteins.
2.5. Non-Enzymatic Antioxidant Capacity Assays
The antioxidant capacity of HR extracts was measured using the following methods: cupric ion reducing antioxidant capacity (CUPRAC), DPPH radical scavenging activity, ferrous chelating activity (FCA) and lipid peroxidation inhibition (LPI).
The CUPRAC assay was determined by the method of Apak et al. [
35] that included mixing of HR extract, 10 mM CuCl
2, 7.5 mM neocuproine and 1 M ammonium acetate buffer (pH 7). After incubation for 30 min at room temperature, the absorbance of the samples was read at 450 nm. The molar extinction coefficient of trolox (ε
535 = 1.67 × 10
4 L·mol
−1·cm
−1) was used for calculation of CUPRAC values expressed as micromoles of trolox (T) equivalents per gram of dry weight (μmol T·g
−1 DW).
The DPPH assay was performed by evaluation of HR extracts to scavenge 0.25 mM 2,2-diphenyl-1-picrylhydrazyl radical (DPPH
•) according to the method of Brand-Williams et al. [
36]. In a control sample, the extract was replaced with CH
3OH. After incubation at room temperature in the dark for 10 min, the decrease in absorbance of the samples was recorded at 518 nm. The DPPH radical scavenging activity was expressed as micromoles of trolox (T) equivalents per gram of dry weight (μM T·g
−1 DW).
The FCA was estimated by the method of Decker and Welch [
37] that included mixing HR extracts with 2 mM FeCl
2 and 5 mM ferrozine. The sample absorbance was measured at 562 nm, and FCA was expressed as milligrams of ethylenediaminetetraacetic acid (EDTA) equivalents per gram of dry weight (mg EDTA·g
−1 DW).
The LPI was determined according to the β-carotene bleaching method described by Sun and Ho [
38]. A linoleic acid-β-carotene emulsion was prepared by mixing 20 mg linoleic acid with 0.2 mg·mL
−1 β-carotene chloroformic solution and 200 mg Tween 40. Chloroform was evaporated under nitrogen flow for 10 min and the mixture was adjusted to a certain volume with distilled water. The mixture consisting of HR extracts and linoleic acid-β-carotene emulsion was incubated at 50 °C. In a control sample, the HR extract was replaced with CH
3OH. The absorbance was monitored in 15 min for a period of 45 min at 470 nm. The LPI was expressed as inhibition of β-carotene bleaching according to the following formula: LPI [%] = ((B − A)/B) × 100, where A is variation in absorbance of samples between 0 and 45 min and B is variation in absorbance of control between 0 and 45 min.
2.6. Antioxidant Enzyme Activities
The activities of antioxidant enzymes guaiacol peroxidase (PX), catalase (CAT) and superoxide dismutase (SOD) were determined in enzyme extracts that were prepared by homogenization of frozen HR tissue with 50 mM potassium phosphate buffer (pH 7). Tissue homogenate was centrifuged at 13,000 rpm for 20 min at 4 °C and the supernatant was used for quantification of soluble proteins and antioxidant enzyme assays.
The PX activity was performed by mixing enzyme extract, 2% guaiacol and 0.3% hydrogen peroxide [
39]. The increase of absorbance was observed every minute for a period of 5 min at 470 nm. The molar extinction coefficient of tetraguaiacol (ε
470 = 26.6 L·mol
−1·cm
−1) was used for calculation of PX activity in nkat·mg
−1 proteins.
The method for determination of CAT activity [
40] was performed by addition of 0.1% hydrogen peroxide to diluted enzyme extract with 50 mM potassium phosphate buffer (pH 7). The absorbance decrease in the samples was monitored every 20 s for a period of 1 min at 240 nm. The molar extinction coefficient of hydrogen peroxide (ε
240 = 43.6 L·mol
−1·cm
−1) was used to express CAT activity in nkat·mg
−1 proteins.
The SOD activity was analyzed by preparing reaction mixtures consisting of enzyme extract, 50 mM extraction buffer (pH 7), 0.13 M methionine, 0.75 mM nitroblue tetrazolium and 0.02 mM riboflavin [
41]. The reaction mixtures were illuminated for 15 min (20-W fluorescent tubes), and the sample absorbance recorded at 560 nm was compared with that of the non-illuminated sample that served as a control. One unit (U) of SOD activity was defined as the amount of enzyme required to cause a 50% inhibition of the nitroblue tetrazolium photoreduction rate. The SOD activity was represented as U·mg
−1 protein.
2.7. Oxidative Stress Marker Contents
The HR extracts for determination of oxidative stress markers H2O2 and malondialdehyde (MDA) were prepared from fresh root material using 5% TCA, while the O2•− production rate was assessed in the same extracts used for antioxidant enzyme activities.
The intracellular H
2O
2 level in HR tissue was determined according to the method of Sergiev et al. [
42] that included mixing HR extracts, 10 mM potassium phosphate buffer (pH 7) and 1 M potassium iodide. The absorbance of the samples was measured at 390 nm, and the H
2O
2 content was expressed as micromoles of H
2O
2 equivalents per gram of fresh weight (μM H
2O
2·g
−1 FW).
The rate of O
2•− production was measured by preparing a reaction mixture containing HR extract, 50 mM potassium phosphate buffer (pH 7) and 10 mM hydroxylamine hydrochloride [
43]. After addition of Griess reagent to the mixture, the sample absorbance was read at 530 nm. The O
2•− production rate in HR was calculated using sodium nitrite as a standard and the results were expressed as nanomoles of generated O
2•− per min and gram of fresh weight (nM O
2•−·min
−1·g
−1 FW).
The MDA content was analyzed by the method of Health and Packer [
44]. The HR extracts mixed with 0.5% TBA in 20% TCA were heated at 95 °C for 30 min and then quickly cooled in ice. The sample absorbance was monitored at 532 and 600 nm. After subtracting the non-specific absorbance (600 nm), the MDA contents were calculated using the molar extinction coefficient of MDA (155 mM
−1·cm
−1). The MDA contents were expressed as nanomoles of MDA equivalents per gram of fresh weight (nM MDA·g
−1 FW).
2.8. Statistical Analysis
The experiments with dark-grown (HR1) and photoperiod-exposed (HR2) clones were repeated independently twice and the analyses were done in triplicate. All data were presented as an average value with standard deviation (± SD). The data were analyzed by one-way ANOVA (STATISTICA for Windows version 5.0; Tulsa, OK, USA) to detect significant differences between samples. A post hoc separation of means between different clones was performed by the Duncan’s multiple range test (p < 0.05). Significant differences at p < 0.05 between individual HR1 and HR2 clones were determined using the Student’s t-test. All parameters for phenolic compound contents and antioxidant status were subjected to principal component analysis (PCA) and hierarchical agglomerative clustering (HAC) using the statistical software XLSTAT 2014.5.03 (Addinsoft, NY, USA). The Pearson’s correlation coefficients for determination of the relationship between phenolic compound composition and antioxidant status were obtained by R software v. 4.2.1 (R Core Team, Vienna, Austria).