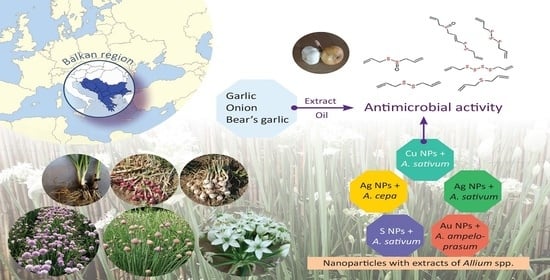
Allium Species in the Balkan Region—Major Metabolites, Antioxidant and Antimicrobial Properties
Abstract
:1. Introduction
2. Morphological Characteristics of Allium Species
3. Usage of Allium Species
4. Allium Species in the Balkan Region
4.1. A. cepa—Onion
4.2. A. sativum—Garlic
4.3. A. fistulosum—Welsh Onion or Japanese Bunching Onion
4.4. A. schoenoprasum—Chives
4.5. A. ampeloprasum—Leek
4.6. A. ursinum—Ramson, Wild Garlic, Bear’s Garlic
5. Major Metabolites of Common Allium Species
5.1. Organosulfur Compounds
5.2. Phenolic Compounds
5.3. Steroids
5.4. Fatty Acids
5.5. Terpenoids
6. Overview of Antioxidant Activity of Alliums Common in the Balkans
7. Exploration of Allium Metabolites for Antimicrobial Activity
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teshika, J.D.; Zakariyyah, A.M.; Zaynab, T.; Zengin, G.; Rengasamy, K.R.R.; Pandian, S.K.; Fawzi, M.M. Traditional and modern uses of onion bulb (Allium cepa L.): A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, S39–S70. [Google Scholar] [CrossRef]
- Block, E. Allium botany and cultivation, ancient and modern. In Garlic and Other Alliums: The Lore and The Science; RSC Publishing Cambridge: Cambridge London, UK, 2010; pp. 1–32. [Google Scholar]
- Stearn, W.T. European species of Allium and allied genera of Alliaceae: A synonymic enumeration. Ann. Musei Goulandris 1978, 4, 83–198. [Google Scholar]
- Fritsch, R.; Michael, K. Occurrence and taxonomic significance of cysteine sulphoxides in the genus Allium L. (Alliaceae). Phytochemistry 2006, 67, 1127–1135. [Google Scholar] [CrossRef]
- Brewster, J.L. The classification, origins, distribution and economic importance of the major vegetable crops. In Onions and Other Vegetable Alliums, 2nd ed.; Atherton, J., Rees, A., Eds.; Warwick HRL: Wellesbourne, Warwick, UK, 2008; Volume 15, pp. 1–26. [Google Scholar] [CrossRef]
- Pandey, A.; Rai, K.; Malav, P.; Subramani, R. Allium negianum (Amaryllidaceae): A new species under subg. Rhizirideum from Uttarakhand Himalaya, India. PhytoKeys 2021, 183, 77–93. [Google Scholar] [CrossRef]
- Fritsch, R.M.; Friesen, N. Evolution, domestication and taxonomy. In Allium Crop Science: Recent Advances; Rabinowitch, H.D., Currah, L., Eds.; CABI Publishing: Wallingfod, UK, 2002; pp. 5–27. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization Corporate Statistical Database. 2021. Available online: https://www.fao.org/faostat/en/#data/QC/visualize (accessed on 10 January 2023).
- Vuković, S.; Moravčević, D.; Gvozdanović-Varga, J.; Dojčinović, B.; Vujošević, A.; Pećinar, I.; Kilibarda, S.; Kostić, A.Ž. Elemental profile, general phytochemical composition and bioaccumulation abilities of selected Allium species biofortified with selenium under open field conditions. Plants 2023, 12, 349. [Google Scholar] [CrossRef]
- Fredotović, Ž.; Puizina, J. Edible Allium species: Chemical composition, biological activity and health effects. Ital. J. Food Sci. 2019, 31, 19–39. [Google Scholar]
- Benkeblia, N. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum). Food Sci. Technol. 2004, 37, 263–268. [Google Scholar] [CrossRef]
- Charles, D.J. Antioxidant Properties of Spices, Herbs and Other Sources; Springer: New York, NY, USA, 2013; pp. 225–230. [Google Scholar] [CrossRef]
- Kucekova, Z.; Mlcek, J.; Humpolicek, P.; Rop, O.; Valasek, P.; Saha, P. Phenolic compounds from Allium schoenoprasum, Tragopogon pratensis and Rumex acetosa and their antiproliferative effects. Molecules 2011, 16, 9207–9217. [Google Scholar] [CrossRef] [PubMed]
- Parvu, A.E.; Parvu, M.; Vlase, L.; Miclea, P.; Mot, A.C.; Silaghi-Dumitrescu, R. Anti-inflammatory effects of Allium schoenoprasum L. leaves. J. Physiol. Pharmacol. 2014, 65, 309–315. [Google Scholar] [PubMed]
- Denaro, M.; Smeriglio, A.; Barreca, D.; De Francesco, C.; Occhiuto, C.; Milano, G.; Trombetta, D. Antiviral Activity of Plants and Their Isolated Bioactive Compounds: An update. Phytother. Res. 2020, 34, 742–768. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Amodeo, V.; Statti, G.; Conforti, F. Biological properties and bioactive components of Allium cepa L.: Focus on potential benefits in the treatment of obesity and related comorbidities. Molecules 2019, 24, 119. [Google Scholar] [CrossRef] [Green Version]
- Popović–Dordevic, J.; Bokan, N.; Dramicanin, A.; Brceski, I.; Kostic, A. Content and weekly intake of essential and toxic elements in Serbian vegetables. J. Environ. Prot. Ecol. 2017, 18, 889–898. [Google Scholar]
- Popović-Djordjević, B.J.; Kostić, Ž.A.; Rajković, B.M.; Miljković, I.; Krstić, Đ.; Caruso, G.; Siavash-Moghaddam, S.; Brčeski, I. Organically vs. conventionally grown vegetables: Multi-elemental analysis and nutritional evaluation. Biol. Trace Elem. Res. 2022, 200, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Alimardanova, M.; Tlevlesova, D.A.; Simov, Z.; Dimitrov, D.; Matibayeva, A.I. Incorporating Allium odorum as a vegetable ingredient of processed cheeses. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 330–338. [Google Scholar]
- Voća, S.; Šic Žlabur, J.; Fabek Uher, S.; Peša, M.; Opacic, N.; Radman, S. Neglected potential of wild garlic (Allium ursinum L.)—Specialized metabolites content and antioxidant capacity of wild populations in relation to location and plant phenophase. Horticulturae 2022, 8, 24. [Google Scholar] [CrossRef]
- Kurnia, D.; Ajiati, D.; Heliawati, L.; Sumiarsa, D. Antioxidant properties and structure-antioxidant activity relationship of Allium species leaves. Molecules 2021, 26, 7175. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Wasef, L.G.; Elewa, Y.H.A.; Al-Sagan, A.A.; Abd El-Hack, M.E.; Taha, A.E.; Abd-Elhakim, Y.M.; Prasad Devkota, H. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [Green Version]
- Alam, A.; Al Arif Jahan, A.; Bari, M.S.; Khandokar, L.; Mahmud, M.H.; Junaid, M.; Chowdhury, M.S.; Khan, M.F.; Seidel, V.; Haque, M.A. Allium vegetables: Traditional uses, phytoconstituents, and beneficial effects in inflammation and cancer. Crit. Rev. Food Sci. Nutr. 2022, 1–35. [Google Scholar] [CrossRef]
- Mathew, B.C.; Biju, R.S. Neuroprotective effects of garlic- a review. Libyan J. Med. 2008, 3, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Patel, N.R.; Mohite, S.A.; Shaha, R.R. Formulation and evaluation of onion hair nourishing shampoo. J. Drug Deliv. Ther. 2018, 8, 335–337. [Google Scholar] [CrossRef]
- Aburjai, T.; Natsheh, F.M. Plants used in cosmetics. Phytother Res. 2003, 17, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Anačkov, G. Rod Allium L. 1754 (Amaryllidales, Alliaceae) u flori Vojvodine (Genus Allium L. 1754 (Amaryllidales, Alliaceae) in flora of the Vojvodina). Master’s Thesis, University of Novi Sad, Faculty of Sciences, Novi Sad, Serbia, 2003. (In Serbian). [Google Scholar]
- Simin, N.; Mitić-Ćulafić, D.; Pavić, A.; Orcic, D.; Nemes, I.; Cetojevic-Simin, D.; Mimica-Dukić, N. An overview of the biological activities of less known wild onions (genus Allium sect Codonoprasum). Biol. Serbica 2019, 41, 57–62. [Google Scholar] [CrossRef]
- Božin, B. Biohemijska i farmakološka ispitivanja vrsta roda Allium L. (sect. Allium) (Biochemical and pharmacological investigations of species of the genus Allium L. (sect. Allium). Ph.D. Thesis, University of Novi Sad, Faculty of Sciences, Novi Sad, Serbia, 2009. (In Serbian). [Google Scholar]
- Keusgen, M.; Fritsch, R.M.; Hisoriev, H.; Kurbonova, P.A.; Khassanov, F.O. Wild Allium species (Alliaceae) used in folk medicine of Tajikistan and Uzbekistan. J. Ethnobiol. Ethnomed. 2006, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Kianian, F.; Marefati, N.; Boskabady, M.; Ghasemi, S.Z.; Boskabady, M.H. Pharmacological properties of Allium cepa, preclinical and clinical evidences: A review. Iran J. Pharm. Res. 2021, 20, 107–134. [Google Scholar] [CrossRef] [PubMed]
- Pareek, S.; Sagar, N.; Sharma, S.; Kumar, V. Onion (Allium cepa L.). In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Yahia, E.M., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2017; Volume 2, pp. 1145–1161. [Google Scholar]
- Zhao, X.X.; Lin, F.J.; Li, H.; Li, H.B.; Wu, D.T.; Geng, F.; Ma, W.; Wang, Y.; Miao, B.H.; Gan, R.Y. Recent advances in bioactive compounds, health functions, and safety concerns of onion (Allium cepa L.). Front Nutr. 2021, 8, 669805. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.J.; Uddin, T.M.; Matin Zidan, B.M.R.; Mitra, S.; Das, R.; Nainu, F.; Dhama, K.; Roy, A.; Hossain, M.J.; Khusro, A.; et al. Allium cepa: A treasure of bioactive phytochemicals with prospective health benefits. Evid Based Complement Alternat. Med. 2022, 2022, 4586318. [Google Scholar] [CrossRef]
- Worku, A.W.; Mehari, A.B. The significance of garlic (Allium sativum) on the livelihood of the local community. J. Food Ind. Microbiol. 2018, 4, 123. [Google Scholar] [CrossRef]
- Stavelikova, H. Morphological characteristics of garlic (Allium sativum L.) genetic resources collection—Information. Hort. Sci. 2008, 35, 130–135. [Google Scholar] [CrossRef] [Green Version]
- Bongiorno, P.; Fratellone, P.; Logiudice, P. Potential health benefits of garlic (Allium sativum): A narrative review. J. Complement. Integr. Med. 2008, 5, 1–24. [Google Scholar] [CrossRef]
- Labu, Z.; Rahman, M. Proven health benefits of garlic—A review. In Department of Pharmacy; World University of Bangladesh (WUB): Dhanmondi, Dhaka, Bangladesh, 2019; p. 1205. [Google Scholar]
- Barau, S.; Halimatu Sadiya, A.; Sani, H. Some medicinal plants with ameliorative potential on COVID-19 cytokine storm: A review. Equity J. Sci. Technol. 2021, 8, 48–54. [Google Scholar]
- Padula, G.; Xia, X.; Hołubowicz, R. Welsh onion (Allium fistulosum L.) seed physiology, breeding, production and trade. Plants 2022, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Ramakrishna, Y. Welsh onion (Allium fistulosum L.): A promising spicing-culinary herb of Mizoram. Indian J. Hill Farming 2017, 30, 201–208. [Google Scholar]
- Singh, V.; Chauhan, G.; Krishan, P.; Shri, R. Allium schoenoprasum L.: A review of phytochemistry, pharmacology and future directions. Nat. Prod. Res. 2018, 32, 2202–2216. [Google Scholar] [CrossRef] [PubMed]
- Algharib, A.M.; El-Gohary, A.E.; Hendawy, S.F.; Hussein, M.S. Response of chive (Allium schoenoprasum L.) plant to natural fertilizers. J. Ecol. Eng. 2021, 22, 200–208. [Google Scholar] [CrossRef]
- Eisazadeh, S.; Asadi Kapourchal, S.; Homaee, M.; Noorhosseini, S.A.; Damalas, C.A. Chive (Allium schoenoprasum L.) response as a phytoextraction plant in cadmium-contaminated soils. Environ. Sci. Pollut. Res. Int. 2019, 26, 152–160. [Google Scholar] [CrossRef]
- Swamy, K.R.M.; Veere Gowda, R. Leek and shallot. In Handbook of Herbs and Spices; Peter, K.V., Ed.; Woodhead Publishing: Cembridge, UK, 2006; Volume 3, pp. 365–389. [Google Scholar]
- Guenaoui, C.; Mang, S.; Figliuolo, G.; Neffati, M. Diversity in Allium ampeloprasum: From small and wild to large and cultivated. Genet. Resour. Crop Evol. 2012, 60, 97–114. [Google Scholar] [CrossRef]
- Sobolewska, D.; Podolak, I.; Makowska-Wąs, J. Allium ursinum: Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2015, 14, 81–97. [Google Scholar] [CrossRef] [Green Version]
- Cinkmanis, I.; Augšpole, I.; Sivicka, I.; Vucāne, S. Evaluation of the phenolic profile of Bear’s garlic (Allium ursinum L.) leaves. Proc. Latv. Acad. Sci. B Nat. Exact Appl. Sci. 2022, 76, 512–516. [Google Scholar] [CrossRef]
- Gomez-Casati, D.F.; Zanor, M.I.; Busi, M.V. Metabolomics in plants and humans: Applications in the prevention and diagnosis of diseases. Biomed Res. Int. 2013, 2013, 792527. [Google Scholar] [CrossRef] [Green Version]
- Bede, D.; Zaixiang, L. Dietary Polysaccharides from Allium species: A critical review in dietary polysaccharides from Allium Species: Extraction, characterization, bioactivity, and potential utilization. Acta Sci. Agric. 2020, 4, 98–112. [Google Scholar] [CrossRef]
- Block, E. The organosulfur chemistry of the genus Allium—Implications for the organic chemistry of sulfur. Angew. Chem. Int. Ed. Engl. 1992, 31, 1135–1178. [Google Scholar]
- Tapiero, H.; Towsend, D.M.; Tew, K.D. Organosulfur compounds from Alliaceae in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 183–193. [Google Scholar] [CrossRef]
- Kyung, K.H. Antimicrobial properties of allium species. Curr. Opin. Biotechnol. 2012, 23, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Lanzotti, V.; Bonanomi, G.; Scala, F. What makes Allium species effective against pathogenic microbes? Phytochem. Rev. 2013, 12, 751–772. [Google Scholar] [CrossRef]
- Lanzotti, V.; Scala, F.; Bonanomi, G. Compounds from Allium species with cytotoxic and antimicrobial activity. Phytochem. Rev. 2014, 13, 769–791. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumegwana, V.; Li, H.-B. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Zucca, P.; Orhan, I.E.; Azzini, E.; Adetunji, C.O.; Mohammed, S.A.; Banerjee, S.K.; Sharopov, F.; Rigano, D.; Sharifi-Rad, J.; et al. Allicin and health: A comprehensive review. Trends Food Sci Technol. 2019, 86, 502–516. [Google Scholar] [CrossRef]
- Goncharov, N.; Orekhov, A.N.; Voitenko, N.; Ukolov, A.; Jenkins, R.; Avdonin, P. Organosulfur compounds as nutraceuticals. In Nutraceuticals—Efficacy, Safety and Toxicity; Gupta, R.C., Ed.; Academic Press Elsevier Inc.: Cambridge, MA, USA, 2016; pp. 555–568. [Google Scholar] [CrossRef]
- Chan, J.Y.-Y.; Yuen, A.C.-Y.; Chan, R.Y.-K.; Chan, S.-W. A review of the cardiovascular benefits and antioxidant properties of allicin. Phytother. Res. 2013, 27, 637–646. [Google Scholar] [CrossRef]
- Banerjee, S.K.; Mukherjee, P.K.; Maulik, S.K. Garlic as an antioxidant: The good, the bad and the ugly. Phytother. Res. 2003, 17, 97–106. [Google Scholar] [CrossRef]
- Schwartz, I.F.; Hershkovitz, R.; Iaina, A.; Gnessin, E.; Wollman, Y.; Chernichowski, T.; Blum, M.; Levo, Y.; Schwartz, D. Garlic attenuates nitric oxide production in rat cardiac myocytes through inhibition of inducible nitric oxide synthase and the arginine transporter CAT-2 (cationic amino acid transporter-2). Clin. Sci. 2002, 102, 487–493. [Google Scholar] [CrossRef]
- Ryu, J.H.; Park, H.-J.; Jeong, Y.-Y.; Han, S.; Shin, J.-H.; Lee, S.J.; Kang, M.J.; Sung, N.-J.; Kang, D. Aged red garlic extract suppresses Nitric oxide production in lipopolysaccharide treated RAW 264.7 macrophages through inhibition of NF-κB. J. Med. Food 2015, 18, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Martín-Cordero, C.; León-González, A.J.; Calderón-Montaño, J.M.; Burgos-Morón, E.; López-Lázaro, M. Pro-Oxidant Natural Products as Anticancer Agents. Curr. Drug Targets 2012, 13, 1006–1028. [Google Scholar] [CrossRef] [PubMed]
- Kodera, Y.; Suzuki, A.; Imada, O.; Kasuga, S.; Sumioka, I.; Kanezawa, A.; Taru, N.; Fujikawa, M.; Nagae, S.; Masamoto, K.; et al. Physical, chemical and biological properties of S-allylcysteine, an amino acid derived from garlic. J. Agric. Food Chem. 2002, 50, 622–632. [Google Scholar] [CrossRef]
- Colín-González, A.L.; Ali, S.F.; Túnez, I.; Santamaría, A. On the antioxidant, neuroprotective and anti-inflammatory properties of S-allyl cysteine: An update. Neurochem. Int. 2015, 89, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, T.; Darlin-Quine, S. Preventive effect of S-allyl cysteine sulphoxide (Aliin) on mitochondrial dysfunction in normal and isoproterenol induced cardiotoxicity in male Wistar rats: A histopathological study. Mol. Cell. Biochem. 2009, 328, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Koca, I.; Karadeniz, B.; Tekguler, B. Aroma components of green körmen (Allium scorodoprasum L. spp. rotondum) and garlic (Allium sativum) plants. Acta Hortic. 2016, 1143, 291–296. [Google Scholar] [CrossRef]
- Xu, S.; Simon Cho, B.H. Allyl mercaptan, a major metabolite of garlic compounds, reduces cellular cholesterol synthesis and its secretion in Hep-G2 cells. J. Nutr. Biochem. 1999, 10, 654–659. [Google Scholar] [CrossRef]
- Simon Cho, B.H.; Xu, S. Effects of allyl mercaptan and various allium-derivedcompounds on cholesterol synthesis and secretion in Hep-G2cells. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2000, 126, 195–201. [Google Scholar] [CrossRef]
- Nian, H.; Delage, B.; Pinto, J.T.; Dashwood, R.H. Allyl mercaptan, a garlic-derived organosulfur compound, inhibits histone deacetylase and enhances Sp3 binding on the P21WAF1 promoter. J. Carcinog. 2008, 29, 1816–1824. [Google Scholar] [CrossRef] [Green Version]
- Padiya, R.; Banerjee, S.K. Garlic as an anti-diabetic agent: Recent progress and patent reviews. Recent Pat. Food Nutr. Agric. 2013, 5, 105–127. [Google Scholar] [CrossRef]
- Puccineli, M.T.; Stan, S.D. Dietary bioactive Diallyl trisulfide in cancer prevention and treatment. Int. J. Mol. Sci. 2017, 18, 1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchiya, H.; Nagayama, M. Garlic allyl derivatives interact with membrane lipids to modify the membrane fluidity. J. Biomed. Sci. 2008, 15, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Lean, M.C.J.; McDonald, M.S.; Black, C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J. Agric. Food Chem. 1997, 45, 590–595. [Google Scholar] [CrossRef]
- Herrmann, K. Flavonoids and flavones in food plants; A review. J. Food Tech. 1976, 11, 433–448. [Google Scholar] [CrossRef]
- Hertog, G.L.; Hollman, P.C.H.; Katan, M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Bilyk, A.; Cooper, P.L.; Sapers, G.M. Varietal differences in distribution of quercetin and kaempferol in onion (Allium cepa L.) tissue. J. Agric. Food Chem. 1984, 32, 274–276. [Google Scholar] [CrossRef]
- Price, K.R.; Rhodes, M.J.C. Analysis of the major flavonol glycosides present in four varieties of onion (Allium cepa) and changes in composition resulting from autolysis. J. Sci. Food Agric. 1997, 74, 331–339. [Google Scholar] [CrossRef]
- Starke, H.; Herrmann, K. Flavonole und Flavone der Gemusearten. VII. Flavonole des Porrees, Schnittlauchs, und Knoblauchs. Z. Lebensm. Unters. Forch 1976, 161, 25–30. [Google Scholar] [CrossRef]
- Vlase, L.; Parvu, M.; Parvu, E.A.; Toiu, A. Phytochemical analysis of Allium fistulosum L. and A. ursinum L. Dig. J. Nanomater. Bios. 2013, 8, 457–467. [Google Scholar]
- Hollman, P.C.H.; Arts, I.C.W. Flavonols, flavones and flavanols–nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1083–1093. [Google Scholar] [CrossRef]
- Farag, M.A.; Ali, S.E.; Hodaya, R.H.; El-Seedi, H.R.; Sultani, H.N.; Laub, A.; Eissa, T.F.; Abou-Zaid, F.O.F.; Wessjohann, L.A. Phytochemical profiles and antimicrobial activities of Allium cepa Red cv. and A. sativum subjected to different drying methods: A comparative MS-based metabolomics. Molecules 2017, 22, 761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A. 2006, 1112, 3–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyle, S.P.; Dobson, V.L.; Duthie, S.J.; Kyle, J.A.; Collins, A.R. Absorption and DNA protective effects of flavonoid glycosides from an onion meal. Eur. J. Nutr. 2000, 39, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Packia Lekshmi, N.C.J.; Viveka, S.; Jeeva, S.; Raja Brindha, J. Phytochemicals in Allium species and its analytical methods—A review. Int. J. I. Pharm. Life Sci. 2015, 5, 38–58. [Google Scholar]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Gašić, U.M.; Nedić, N.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Polyphenolic profile and antioxidant properties of bee-collected pollen from sunflower (Helianthus annuus L.) plant. LWT-Food Sci. Technol. 2019, 112, 108244. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Mai, Y.; Li, H.; Wang, Z.; Xu, J.; He, X. Health benefits of the flavonoids from onion: Constituents and their pronounced antioxidant and anti-neuroinflammatory capacities. J. Agric. Food Chem. 2020, 68, 799–807. [Google Scholar] [CrossRef]
- Ciulla, M.; Marinelli, L.; Cacciatore, I.; Di Stefano, A. Role of dietary supplements in the management of Parkinson’s disease. Biomolecules 2019, 9, 271. [Google Scholar] [CrossRef] [Green Version]
- Terahara, N.; Yamaguchi, M.A.; Honda, T. Malonylated anthocyanins from bulbs of red onion, Allium cepa L. Biosci. Biotechnol. Biochem. 1994, 58, 1324–1325. [Google Scholar] [CrossRef]
- Fossen, T.; Andersen, O.M.; Ovstedal, D.O.; Pedersen, A.F.; Raknes, A. Characteristic anthocyanin pattern from onions and other Allium spp. J. Food Sci. 1996, 61, 703–706. [Google Scholar] [CrossRef]
- Donner, H.; Gao, L.; Mazza, G. Separation and characterization of simple and malonylated anthocyanins in red onions, Allium cepa L. Food Res. Int. 1997, 30, 637–643. [Google Scholar] [CrossRef]
- Fossen, T.; Slimestad, R.; Ovstedal, D.O.; Andersen, O.M. Covalent anthocyanin–flavonol complexes from flowers of chive, Allium schoenoprasum. Phytochemistry 2000, 54, 317–323. [Google Scholar] [CrossRef]
- Prakash, D.; Singh, B.N.; Upadhyay, G. Antioxidant and free radical scavenging activities of phenols from onion (Allium cepa). Food Chem. 2007, 102, 1389–1393. [Google Scholar] [CrossRef]
- Lin, X.F.; Min, W.; Luo, D. Anticarcinogenic effect of ferulic acid on ultraviolet-B irradiated human keratinocyte HaCaT cells. J. Med. Plants Res. 2010, 4, 1686–1694. [Google Scholar] [CrossRef]
- Baskaran, N.; Manoharan, S.; Balakrishnan, S.; Pugalendhi, P. Chemopreventive potential of ferulic acid in 7,12-dimethylbenz[a]anthracene-induced mammary carcinogenesis in Sprague—Dawley rats. Eur. J. Pharmacol. 2010, 637, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Vlase, L.; Parvu, M.; Parvu, E.A.; Toiu, A. Chemical constituents of three Allium species from Romania. Molecules 2012, 18, 114–127. [Google Scholar] [CrossRef] [Green Version]
- Djurdjevic, L.; Dinic, A.; Pavlovic, P.; Mitrovic, M.; Karadzic, B.; Tesevic, V. Allelopathic potential of Allium ursinum L. Biochem. Syst. Ecol. 2004, 32, 533–544. [Google Scholar] [CrossRef]
- Condrat, D.; Mosoarca, C.; Zamfir, A.D.; Crişan, F.; Szabo, M.R.; Lupea, A.X. Qualitative and quantitative analysis of gallic acid in Alchemilla vulgaris, Allium ursinum, Acorus calamus and Solidago virga-aurea by chip-electrospray ionization mass spectrometry and high performance liquid chromatography. Cent. Eur. J. Chem. 2010, 8, 530–535. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Kim, S.H.; Hagerman, A.E.; Lu, J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.Z.; Choi, S.U.; Bae, S.Y.; Pyo, S.; Lee, K.R. Immunobiological [correction of Immunobioloical] activity of a new benzyl benzoate from the aerial parts of Solidago virga-aurea var. gigantean. Arch. Pharm. Res. 2005, 28, 49–54. [Google Scholar] [CrossRef]
- Kim, H.; Han, T.H.; Lee, S.G. Anti-inflammatory activity of a water extract of Acorus calamus L. leaves on keratinocyte HaCaT cells. J. Ethnopharmacol. 2009, 122, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.-A. Plant sterols and the membrane environment. Trends Plant Sci. 1998, 3, 170–175. [Google Scholar] [CrossRef]
- Gunaherath, G.M.K.B.; Gunatilaka, A.A.L. Plant Steroids: Occurrence, biological significance and their analysis. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: New York, NY, USA, 2020; pp. 1–26. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Khanna, P.; Chowdhary, A. Phytosteroids from tissue culture of Allium cepa L. and Trachyspermum ammi S prague. J. Pharmacogn. Phytochem. 2013, 1, 42–48. [Google Scholar]
- Sabha, D.; Hiyasat, B.; Grötzinger, K.; Hennig, L.; Schlegel, F.; Mohr, F.-W.; Rauwald, H.W.; Dheinet, S. Allium ursinum L.: Bioassay-guided isolation and identification of a galactolipid and a phytosterol exerting antiaggregatory effects. Pharmacology 2012, 89, 260–269. [Google Scholar] [CrossRef]
- Cabral, C.E.; Klein, M.R.S.T. Phytosterols in the treatment of hypercholesterolemia and prevention of cardiovascular diseases. Arq. Bras. Cardiol. 2017, 109, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.J.H.; AbuMweis, S.S. Phytosterols as functional food ingredients: Linkages to cardiovascular disease and cancer. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 147–151. [Google Scholar] [CrossRef]
- Woyengo, T.A.; Ramprasath, V.R.; Jones, P.J.H. Anticancer effects of phytosterols. Eur. J. Clin. Nutr. 2009, 63, 813–820. [Google Scholar] [CrossRef] [Green Version]
- Sobolewska, D.; Janeczko, Z.; Kisiel, W.; Podolak, I.; Galanty, A.; Danuta Trojanowska, D. Steroidal glycosides from the underground parts of Allium ursinum L. and their cytostatic and antimicrobial activity. Acta Pol. Pharm. Drug Res. 2006, 6, 219–223. [Google Scholar]
- Vázquez, L.; Corzo-Martínez, M.; Arranz-Martínez, P.; Barroso, E.; Reglero, G.; Torres, C. Bioactive Lipids. In Bioactive Molecules in Food, Reference Series in Phytochemistry; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2019; pp. 467–527. [Google Scholar] [CrossRef]
- Billingsley, H.E.; Carbone, S.; Lavie, C.J. Dietary fats and chronic noncommunicable diseases. Nutrients 2018, 10, 1385. [Google Scholar] [CrossRef] [Green Version]
- Bjerregaard, P.; Pedersen, H.; Mulvad, G. The associations of a marine diet with plasma lipids, blood glucose, blood pressure and obesity among the Inuit in Greenland. Eur. J. Clin. Nutr. 2000, 54, 732–737. [Google Scholar] [CrossRef] [Green Version]
- Dyall, S.C. Methodological issues and inconsistencies in the field of omega-3 fatty acids research. Prostaglandins Leukot. Essent. Fatty Acids. 2011, 85, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Cameron-Smith, D.; Garg, M.; Sinclair, A.J. Docosapentaenoic acid (22: 5n-3): A review of its biological effects. Prog. Lipid Res. 2011, 50, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulcin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Martin-Arjol, I.; Bassas-Galia, M.; Bermudo, E.; Garcia, F.; Manresa, A. Identification of oxylipins with antifungal activity by LC-MS/MS from the supernatant of Pseudomonas 42A2. Chem. Phys. Lipids 2010, 163, 341–346. [Google Scholar] [CrossRef]
- Wiater, M.; Sobolewska, D.; Janeczko, Z. Fatty acids in lipid fraction from the undeground parts of Allium ursinum L. In Proceedings of the XVII Naukowy Zjazd Polskiego Towarzystwa Farmaceutycznego, Farmacja w perspektywie XXI w. Streszczenia, Kraków, Poland, 10–13 September 1998. [Google Scholar]
- Shirshova, T.I.; Beshlei, I.V.; Deryagina, V.P. Chemical composition of Allium schoenoprasum leaves and inhibitory effect of their extract on tumor growth in mice. Pharm. Chem J. 2013, 46, 672–675. [Google Scholar] [CrossRef]
- Kozioł, A.; Stryjewska, A.; Librowski, T.; Sałat, K.; Gaweł, M.; Monczewski, A.S.; Lochyński, S. An Overview of the pharmacological properties and potential applications of natural monoterpenes. Mini-Rev. Med. Chem. 2014, 14, 1156–1168. [Google Scholar] [CrossRef]
- Rajput, J.D.; Bagul, S.D.; Pete, U.D.; Zade, C.M.; Padhye, S.B.; Bendre, R.S. Perspectives on medicinal properties of natural phenolic monoterpenoids and their hybrids. Mol. Divers. 2017, 22, 225–245. [Google Scholar] [CrossRef]
- Olayemi, R.F. The role of monoterpenoids and sesqiterpenoids as defense chemicals in plants—A review. Niger. Res. J. Chem. Sci. 2017, 3, 1–15. [Google Scholar]
- Ajayi, G.O.; Akinsanya, M.A.; Agbabiaka, A.T.; Oyebanjo, K.S.; Hungbo, T.D.; Olagunju, J.A. D-Limonene: A major bioactive constituent in Allium fistulosum identified by GC-MS analysis. J. Phytopharm. 2019, 8, 257–259. [Google Scholar] [CrossRef]
- Gould, M.N. Cancer chemoprevention and therapy by monoterpenes. Environ. Health Perspect. 1997, 105, 977–979. [Google Scholar] [CrossRef]
- Santos, M.R.V.; Moreira, F.V.; Fraga, B.P.; de Souza, D.P.; Bonjardim, L.R.; Quintans-Junior, L.J. Cardiovascular effects of monoterpenes: A review. Rev. Bras. Farmacogn. 2011, 21, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Crowell, P.L.; Gould, M.N. Chemoprevention and therapy of cancer by D-limonene. Crit. Rev. Oncog. 1994, 5, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.S.; Özbek, H. Investigation of the Anti-inflammatory, Hypoglycemic Activity and Median Lethal Dose (LD50) Level of Limonene in Mice and Rats. Acta Pharm. Sci. 2018, 56, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.A.; Thompson, P.A.; Hakim, I.A.; Chow, H.H.S.; Thomson, C.A. D-Limonene: A bioactive food component from citrus and evidence for a potential role in breast cancer prevention and treatment. Oncol. Rev. 2011, 5, 31–42. [Google Scholar] [CrossRef]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrick, L. Gastroesophageal reflux disease (GERD): A review of conventional and alternative treatments. Altern. Med. Rev. 2011, 16, 116–133. [Google Scholar]
- Lachowicz, S.; Oszmiański, J.; Wiśniewski, R. Determination of triterpenoids, carotenoids, chlorophylls, and antioxidant capacity in Allium ursinum L. at different times of harvesting and anatomical parts. Eur. Food Res. Technol. 2018, 244, 1269–1280. [Google Scholar] [CrossRef] [Green Version]
- Namitha, K.K.; Negi, P.S. Chemistry and biotechnology of carotenoids. Crit. Rev. Food Sci. Nutr. 2010, 50, 728–760. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress-implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Ascenso, A.; Ribeiro, H.; Marques, H.C.; Oliveira, H.; Santos, C.; Simões, S. Chemoprevention of photocarcinogenesis by lycopene. Exp. Dermatol. 2014, 23, 874–878. [Google Scholar] [CrossRef]
- Woodside, J.V.; McGrath, A.J.; Lyner, N.; McKinley, M.C. Carotenoids and health in older people. Maturitas 2015, 80, 63–68. [Google Scholar] [CrossRef]
- Castro, V.; Carpena, M.; Fraga-Corral, M.; Lopez-Soria, A.; Garcia-Perez, P.; Barral-Martinez, M.; Perez-Gregorio, R.; Cao, H.; Simal-Gandara, J.; Prieto, A.M. Sulfur-containing compounds from plants. In Natural Secondary Metabolites; Carocho, M., Heleno, S.A., Barros, L., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Kim, G.-H.; Duan, Y.; Lee, S.-C.; Kim, H.-S. Assessment of antioxidant activity of garlic (Allium sativum L.) peels by various extraction solvents. J. Korean Oil Chem. Soc. 2016, 33, 204–212. [Google Scholar] [CrossRef]
- Lu, X.; Ross, C.F.; Powers, J.R.; Aston, D.E.; Rasco, B.A. Determination of total phenolic content and antioxidant activity of garlic (Allium sativum) and elephant garlic (Allium ampeloprasum) by attenuated total reflectance-Fourier transformed infrared spectroscopy. J. Agric. Food Chem. 2011, 59, 5215–5221. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.S.; Cha, H.S.; Lee, Y.S. Physicochemical and antioxidant properties of black garlic. Molecules 2014, 19, 16811–16823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeles, T.; Pérez-Aparicio, J.; Moreno Rojas, R.; Amo, M.T. Evolution of some physicochemical and antioxidant properties of black garlic whole bulbs and peeled cloves. Food Chem. 2015, 199, 135–139. [Google Scholar] [CrossRef]
- El-Hadidy, E.; Mossa, M.; Habashy, N. Effect of freezing on the pungency and antioxidants activity in leaves and bulbs of green onion in Giza 6 and Photon varieties. Ann. Agric. Sci. 2014, 59, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Ola-Mudathir, F.; Abdul-Wahab, A.; Moshood, A.; Obuotor, E.M. Comparative evaluation of antioxidant properties of methanol extracts of Allium cepa bulb, Allium cepa bulb peels and Allium fistulosum. Kragujevac J. Sci. 2018, 40, 131–141. [Google Scholar] [CrossRef]
- Lee, K.A.; Kim, K.-T.; Kim, H.J.; Chung, M.-S.; Chang, P.-S.; Park, H.; Pai, H.-D. Antioxidant activities of onion (Allium cepa L.) peel extracts produced by ethanol, hot water, and subcritical water extraction. Food Sci. Biotechnol. 2014, 23, 615–621. [Google Scholar] [CrossRef]
- Bernaert, N.; De Paepe, D.; Bouten, C.; Clercq, H.; Stewart, D.; Bockstaele, E.; Loose, M.; Droogenbroeck, B. Antioxidant capacity, total phenolic and ascorbate content as a function of the genetic diversity of leek (Allium ampeloprasum var. porrum). Food Chem. 2012, 134, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.C.; Jang, H.D.; Lin, W.D.; Duan, P.F. Antioxidant and antimicrobial activities of commercial rice wine extracts of Taiwanese Allium fistulosum. Food Chem. 2016, 190, 724–729. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Z.; Cui, R.; Su, L.; Sun, X.; Borras-Hidalgo, O.; Li, K.; Wei, J.; Yue, Q.; Zhao, L. Effects of nitrogen application on phytochemical component levels and anticancer and antioxidant activities of Allium fistulosum. PeerJ. 2021, 9, e11706. [Google Scholar] [CrossRef] [PubMed]
- Stajner, D.; Popović, B.M.; Calić-Dragosavac, D.; Malenčić, D.; Zdravković-Korać, S. Comparative study on Allium schoenoprasum cultivated plant and Allium schoenoprasum tissue culture organs antioxidant status. Phytother. Res. 2011, 25, 1618–1622. [Google Scholar] [CrossRef] [PubMed]
- Petkova, N.; Ivanov, I.; Topuzova, M.; Todorova, M.; Denev, P. Fructans and antioxidants in leaves of culinary herbs from Asteraceae and Amaryllidaceae families. Food Res. 2019, 3, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Sinaga, M.; Sudarmi, S.; Iksen, I.; Kevin, K.; Sari, M.P. Evaluation of total phenolic, flavonoid content, antioxidant and in vitro antilithogenesis activities of chives leaf (Allium schoenoprasum L.). Rasayan J. Chem. 2018, 11, 1604–1608. [Google Scholar] [CrossRef]
- Sahnoun, D.; Megdiche, W.; Younes, I.; Majdi, H.; Mariem, S.; Mkadmini, K.; Ksouri, R.; Serairi Beji, R. Antioxidant activity and biochemical composition of fresh bulbs and leaves of wild garlic Allium ursinum. J. New Sci. 2017, 44, 2392–2399. [Google Scholar]
- Stanisavljević, N.; Soković Bajić, S.; Jovanović, Ž.; Matić, I.; Tolinački, M.; Popović, D.; Popović, N.; Terzić-Vidojević, A.; Golić, N.; Beškoski, V.; et al. Antioxidant and antiproliferative activity of Allium ursinum and their associated microbiota during simulated in vitro digestion in the presence of food matrix. Front. Microbiol. 2020, 11, 3043. [Google Scholar] [CrossRef]
- Gordanić, S.; Radanović, D.; Vuković, S.; Kolašinac, S.; Kilibarda, S.; Marković, T.; Moravčević, Đ.; Kostić, A.Ž. Phytochemical characterization and antioxidant potential of Allium ursinum L. cultivated on different soil types-a preliminary study. Emir. J. Food Agric. 2022, 34, 904–914. [Google Scholar] [CrossRef]
- Mihaylova, D.S.; Lante, A.; Tinello, F.; Krastanov, A.I. Study on the antioxidant and antimicrobial activities of Allium ursinum L. pressurised-liquid extract. Nat. Prod. Res. 2014, 28, 2000–2005. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Butt, M.S.; Khalid, N.; Sultan, S.; Raza, A.; Aleem, M.; Abbas, M. Garlic (Allium sativum): Diet based therapy of 21st century-a review. Asian Pac. J. Trop. Dis. 2015, 5, 271–278. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Netzel, G.; Chhim, P.; Netzel, M.E.; Sultanbawa, Y. Phytochemical characteristics and antimicrobial activity of Australian grown garlic (Allium sativum L.) cultivars. Foods 2019, 8, 358. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Liu, C.H.; Cai, J.; Zhang, W.; Qi, W.L.; Wang, Z.; Liu, Z.-B.; Yang, Y. Broad-spectrum antimicrobial activity, chemical composition and mechanism of action of garlic (Allium sativum) extracts. Food Control 2018, 86, 117–125. [Google Scholar] [CrossRef]
- Botas, J.; Fernandes, Â.; Barros, L.; Alves, M.J.; Carvalho, A.M.; Ferreira, I.C.F.R. A comparative study of black and white Allium sativum L.: Nutritional composition and bioactive properties. Molecules 2019, 24, 2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, C.L.; Dai, D.H.; Hu, W.L. Antimicrobial and antioxidant activities of the essential oil from onion (Allium cepa L.). Food Control 2013, 30, 48–53. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Lee, Y.R. Systematic study on active compounds as antibacterial and antibiofilm agent in aging onions. J. Food Drug Anal. 2018, 26, 518–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagar, N.A.; Pareek, S. Antimicrobial assessment of polyphenolic extracts from onion (Allium cepa L.) skin of fifteen cultivars by sonication-assisted extraction method. Heliyon. 2020, 6, eo5478. [Google Scholar] [CrossRef]
- Krstin, S.; Sobeh, M.; Braun, M.S.; Wink, M. Tulbaghia violacea and Allium ursinum extracts exhibit anti-parasitic and antimicrobial activities. Molecules 2018, 23, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlović, D.R.; Veljković, M.; Stojanović, N.M.; Gočmanac-Ignjatović, M.; Mihailov-Krstev, T.; Branković, S.; Sokolović, D.; Marčetić, M.; Radulović, N.; Radenković, M. Influence of different wild-garlic (Allium ursinum) extracts on the gastrointestinal system: Spasmolytic, antimicrobial and antioxidant properties. J. Pharm. Pharmacol. 2017, 69, 1208–1218. [Google Scholar] [CrossRef]
- Xu, X.Y.; Song, G.Q.; Yu, Y.Q.; Ma, H.Y.; Ma, L.; Jin, Y.N. Apoptosis and G2/M arrest induced by Allium ursinum (ramson) watery extract in an AGS gastric cancer cell line. OncoTargets Ther. 2013, 6, 779–783. [Google Scholar] [CrossRef] [Green Version]
- Synowiec, A.; Gniewosz, M.; Zieja, I.; Baczek, K.; Przybyl, J. Porównanie właściwości przeciwdrobnoustrojowych ekstraktów z czosnku niedźwiedziego (Allium ursinum). Zesz Probl Postępów Nauk Rol. 2010, 553, 203–209. [Google Scholar]
- Popova, A.; Mihaylova, D.; Alexieva, I. GC-MS chemical composition of volatile oil and mineral elementcontent of Allium ursinum and Nectaroscordum siculum. Pak. J. Bot. 2018, 50, 2351–2354. [Google Scholar]
- Radusin, T.; Torres-Giner, S.; Stupar, A.; Ristic, I.; Miletic, A.; Novakovic, A.; Lagaron, J.M. Preparation, characterization and antimicrobial properties of electrospun polylactide films containing Allium ursinum L. extract. Food Packag. Shelf Life 2019, 21, 100357. [Google Scholar] [CrossRef]
- Nakamoto, M.; Kunimura, K.; Suzuki, J.; Kodera, Y. Antimicrobial properties of hydrophobic compounds in garlic: Allicin, vinyldithiin, ajoene and diallyl polysulfides. Exp. Ther. Med. 2020, 19, 1550–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybak, M.E.; Calvey, E.M.; Harnly, J.M. Quantitative determination of allicin in garlic: Supercritical fluid extraction and standard addition of alliin. J. Agric. Food Chem. 2004, 52, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.; Hübbers, A.M.; Albrecht, F.; Leichert, L.I.O.; Slusarenko, A.J. Allicin, a natural antimicrobial defence substance from garlic, inhibits DNA gyrase activity in bacteria. Int. J. Med. Microbiol. 2020, 310, 151359. [Google Scholar] [CrossRef] [PubMed]
- Subbanna, S.; Ts, G.; Basalingappa, K.M. Biogenic nanoparticles from Allium sativum and its bioactives applications. Eur. J. Mol. Clin. Med. 2020, 7, 212–232. [Google Scholar]
- Naganawa, R.; Iwata, N.; Ishikawa, K.; Fukuda, H.; Fujino, T.; Suzuki, A. Inhibition of microbial growth by ajoene, a sulfur-containing compound derived from garlic. Appl. Environ. Microbiol. 1996, 62, 4238–4242. [Google Scholar] [CrossRef] [Green Version]
- Casella, S.; Leonardi, M.; Melai, B.; Fratini, F.; Pistelli, L. The role of diallyl sulfides and dipropyl sulfides in the in vitro antimicrobial activity of the essential oil of garlic, Allium sativum L., and leek, Allium porrum L. Phytother. Res. 2013, 27, 380–383. [Google Scholar] [CrossRef]
- Cavallito, C.J. Relationship of thiol structures to reaction with antibiotics. J. Biol. Chem. 1946, 164, 29–34. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [Green Version]
- Slusarenko, A.J.; Patel, A.; Portz, D. Control of plant diseases by natural products: Allicin from garlic as a case study. Eur. J. Plant Pathol. 2008, 121, 313–322. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Yang, J.; Lu, C.; Li, Y.; Luo, Y.; Cong, F.; Shi, R.; Wang, Z.; Chen, H.; et al. Allicin shows antifungal efficacy against Cryptococcus neoformans by blocking the fungal cell membrane. Front. Microbiol. 2022, 13, 1012516. [Google Scholar] [CrossRef] [PubMed]
- Focke, M.; Feld, A.; Lichtenthaler, K. Allicin, a naturally occurring antibiotic from garlic, specifically inhibits acetyl-CoA synthetase. FEBS Lett. 1990, 261, 106–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsen, T.H.; Gennip, M.; Phipps, R.K.; Shanmugham, M.S.; Christensen, L.D.; Alhede, M.; Skindersoe, M.E.; Rasmussen, T.B.; Friedrich, K.; Uthe, F. Ajoene, a sulfur-rich molecule from garlic inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 2012, 56, 2314–2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemar, K.M.; Passa, O.; Aon, M.A.; Cortassa, S.; Müller, C.T.; Plummer, S.; O’Rourke, B.; Lloyd, D. Allyl alcohol and garlic (Allium sativum) extract produce oxidative stress in Candida albicans. Microbiology 2005, 151, 3257–3265. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.-S.; Lim, D.R.; Kyung, K.H. 3-(Allyltrisulfanyl)-2-aminopropanoic acid, a novel nonvolatile water-soluble antimicrobial sulfur compound in heated garlic. J. Med. Food. 2010, 13, 1247–1253. [Google Scholar] [CrossRef]
- Li, W.-R.; Zeng, T.-H.; Yao, J.-W.; Zhu, L.-P.; Zhang, Z.-Q.; Xie, X.-B.; Shi, Q.-S. Diallyl sulfide from garlic suppresses quorum-sensing systems of Pseudomonas aeruginosa and enhances biosynthesis of three B vitamins through its thioether group. Microb. Biotechnol. 2021, 14, 677–691. [Google Scholar] [CrossRef]
- Tang, Y.; Li, F.; Gu, D.; Wang, W.; Huang, J.; Jiao, X. Antimicrobial effect and the mechanism of diallyl trisulfide against Campylobacter jejuni. Antibiotics 2021, 10, 246. [Google Scholar] [CrossRef]
- Kehinde, A.; Taiwo, A. Review of toxicity of allicin from garlic. J. Pharm. Pharmacol. 2020, 4, 555647. [Google Scholar] [CrossRef]
- Lawal, B.; Shittu, O.K.; Oibiokpa, F.I.; Mohammed, H.; Umar, S.I.; Haruna, G.M. Antimicrobial evaluation, acute and sub-acute toxicity studies of Allium sativum. J. Acute Dis. 2016, 5, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Srećković, N.Z.; Nedić, Z.P.; Liberti, D.; Monti, D.M.; Mihailović, N.R.; Katanić Stanković, J.S.; Dimitrijević, S.; Mihailović, V.B. Application potential of biogenically synthesized silver nanoparticles using: Lythrum salicaria L. extracts as pharmaceuticals and catalysts for organic pollutant degradation. RSC Adv. 2021, 11, 35585–35599. [Google Scholar] [CrossRef]
- Velsankar, K.; Aswin Kumara, R.M.; Preethi, R.; Muthulakshmi, V.; Sudhahar, S. Green synthesis of CuO nanoparticles via Allium sativum extract and its characterizations on antimicrobial, antioxidant, antilarvicidal activities. J. Environ. Chem. Eng. 2020, 8, 104123. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Antimicrobial, antioxidant and antitumor activities of silver nanoparticles synthesized by Allium cepa extract: A green approach. J. Genet. Eng. Biotechnol. 2017, 15, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Girish, V.M.; Liang, H.; Aguilan, J.T.; Nosanchuk, J.D.; Friedman, J.M.; Nacharaju, P. Anti-biofilm activity of garlic extract loaded nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102009. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, W.A.; Azzazy, H.M.E.; El-Sherbiny, I.M. Honey/chitosan nanofiber wound dressing enriched with Allium sativum and Cleome droserifolia: Enhanced antimicrobial and wound healing activity. ACS Appl. Mater. Interfaces. 2016, 8, 6379–6390. [Google Scholar] [CrossRef]
- Sahni, G.; Panwar, A.; Kaur, B. Controlled green synthesis of silver nanoparticles by Allium cepa and Musa acuminata with strong antimicrobial activity. Int. Nano Lett. 2015, 5, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Bouqellah, N.A.; Mohamed, M.M.; Ibrahim, Y. Synthesis of eco-friendly silver nanoparticles using Allium sp. and their antimicrobial potential on selected vaginal bacteria. Saudi J. Biol. Sci. 2019, 26, 1789–1794. [Google Scholar] [CrossRef]
- Hatipoğlu, A. Rapid green synthesis of gold nanoparticles: Synthesis, characterization, and antimicrobial activities. Prog. Nutr. 2021, 23, e2021242. [Google Scholar] [CrossRef]
- Khairan, K.; Zahraturriaz; Jalil, Z. Green synthesis of sulphur nanoparticles using aqueous garlic extract (Allium sativum). Rasayan J. Chem. 2019, 12, 50–57. [Google Scholar] [CrossRef]











| Food Species | Ornamental Species | Weed Species | Species in the Balkan Region | Reference | |||
|---|---|---|---|---|---|---|---|
| A. cepa 1 | Onion, shallot 2 | A. cernuum 1 | Lady’s leek 2 | A. vinale 1 | Crow garlic 2 | A. cepa 1 | [2,3] |
| A. sativum | Garlic | A. longifolium | A. carinatum | Keeled garlic or witch’s garlic | A. sativum | [2,3] | |
| A. fistulosum | Welsh onion or Japanese bunching onion | A. moly | Lily leek | A. fistulosum | [2,3] | ||
| A. schoenoprasum | Chives | A. bisculum | A. schoenoprasum | [2,3] | |||
| A. ampeloprasum | Leek, kurrat, great-headed garlic, or pearl onions | A. neapolitanum | Naples garlic | A. ampeloprasum | [2,3] | ||
| A. tuberosum | Chinese chives | A. hollandicum | Dutch allium | A. ursinum | [2,3] | ||
| A. nutans | / | A. giganteum | Giant allium | [2,3] | |||
| A. odorum | Chinese chive or Chinese leek | A. cyaneum | [2,3] | ||||
| A. chinense | Rakkyo | A. rosenbachianum | [2,3] | ||||
| A. rotundum | / | A. ramosum | Garlic fragrant | [2,3] | |||
| A. ursinum | Ramson, Bear‘s garlic, or Wild garlic | A. cristophii | Star of Persia | [2,3] | |||
| A. oschaninii | French grey shallot | A. flavum | Yellow-flowered garlic | [2,3] | |||
| A. tricoccum | Ramp | [2,3] | |||||
| A. victorialis | Long-rooted onion or long-rooted garlic | [2,3] | |||||
| Species/Plant Parts | Solvent | Assays | Results 1 | Reference |
|---|---|---|---|---|
| A. sativum | ||||
| Garlic peel | 70 % methanol | * DPPH.(IC50, mg/mL) ABTS.+ (IC50, mg/mL) | 0.24 ± 0.00 0.51 ± 0.01 | [137] |
| 70% ethanol | DPPH. (IC50, mg/mL) ABTS.+ (IC50, mg/mL) | 0.20 ± 0.00 0.44 ± 0.01 | [137] | |
| Clove | 70 % methanol | TEAC (μmol Trolox/g FW) FRAP (μmol Trolox/g FW) DPPH. (μmol Trolox/g FW) | 57.86 ± 1.43 8.94 ± 0.31 7.60 ± 0.39 | [138] |
| deionized water | DPPH. (inhibition %) ABTS.+(mmol/L Trolox/g FW) | 4.65 92.43 | [139] | |
| Whole bulb | 50% ethanol | ABTS.+(mmol/L Trolox/kg FW) | 16.03 ± 0.41 | [140] |
| A. cepa | ||||
| Bulb | distilled water | DPPH. (Inhibition %) | 24.79–25.61 | [141] |
| 80% methanol | FRAP (mg AAE/g FW) TAC (mg AAE/g FW) DPPH. (IC50, mg/mL) | 5.37 ± 1.185 12.94 ± 1.944 0.24 ± 0.017 | [142] | |
| Leaves | distilled water | DPPH. (Inhibition %) | 14.76–15.86 | [141] |
| Bulb peel | 80% methanol | FRAP (mg AAE/g FW) TAC (mg AAE/g FW) DPPH. (IC50, mg/mL) | 8.59 ± 2.220 63.91 ± 1.312 0.19 ± 0.047 | [142] |
| 70% ethanol | DPPH. (Inhibition %) | 72.25 ± 2.74 | [143] | |
| hot water (80 °C) | DPPH. (Inhibition %) | 49.68±1.55 | [143] | |
| A. ampeloprasum | ||||
| Clove | 70 % methanol | TEAC (μmol Trolox/g FW) FRAP (μmol Trolox/g FW) DPPH. (μmol Trolox/g FW) | 47.50 ± 1.52 7.62 ± 0.64 6.95 ± 0.14 | [138] |
| Seed oil | hexane | ABTS.+ (µM TEAC/g oil) DPPH. (mM TEAC/g DW) | 136.30 ± 2.40 4.16 ± 0.12 | [138] |
| Pseudostem | 70% ethanol | DPPH. (μmol TE/g DW) FRAP (μmol FeSO4/g DW) | 2–11 3–18 | [144] |
| Leaves | 70% ethanol | DPPH. (μmol TE/g DW) FRAP (μmol FeSO4/g DW) | 5–14 14–37 | [144] |
| A. fistulosum | ||||
| Whole plant | 80% methanol | FRAP (mg AAE/g FW) TAC (mg AAE/g FW) DPPH. (IC50, mg/mL) | 3.38 ± 0.227 6.35 ± 1.698 0.58 ± 0.002 | [142] |
| Pseudostem | rice wine (34% alcohol) | DPPH. (IC50, mg/mL) TEAC (mmol TE/g extract) | 15.2 ± 0.2 15.1 ± 2.5 | [145] |
| 70% ethanol | ABTS.+ (Inhibition %) DPPH. (Inhibition %) | 57.44 ± 0.45 59.45 ± 0.24 | [146] | |
| A. schoenoprasum | ||||
| Root | 0.1 mol/L phosphate buffer (pH 7) | DPPH. (Inhibition %) FRAP (FRAP unit) | 24.33 ± 0.46 82.00 ± 7.00 | [147] |
| Leaves | 0.1 mol/L phosphate buffer (pH 7) | DPPH. (Inhibition %) FRAP (FRAP unit) | 13.714 ± 0.378 52.66 ± 6.34 | [147] |
| distilled water | DPPH. (Inhibition %) FRAP (mM TE/g DW) | 11.25 ± 0.50 5.40 ± 1.93 | [148] | |
| 70% ethanol | DPPH. (EC50, g/mg) TEAC (µg TE/g) | 6.72 ± 0.44 132.8 ± 23 | [14] | |
| ethanol | DPPH. (Inhibition %) | 61.08 | [149] | |
| ethyl acetate | DPPH. (Inhibition %) | 78.37 | [149] | |
| hexane | DPPH. (Inhibition %) | 49.46 | [149] | |
| Flower stalk | 0.1 mol/L phosphate buffer (pH 7) | DPPH. (Inhibition %) FRAP (FRAP unit) | 10.428 ± 0.330 25.66 ± 4.41 | [147] |
| A. ursinum | ||||
| Bulb | distilled water | DPPH. (Inhibition %) | 32.06–66.61 | [150] |
| Leaves | distilled water | DPPH. (Inhibition %) FRAP (mM TE/g DW) | 10.10 ± 0.10 42.11 ± 0.50 | [148] |
| 0.85% saline | DPPH. (µm TE/mL) | 0.45 ± 0.05 | [151] | |
| 80% acetone | CUPRAC (mg AAE/g FW) TAC (mg GAE/g FW) DPPH∙ (Inhibition %) FRP (mg AAE/g FW) | 2.75 ± 0.11 3.27 ± 0.19 27.69 ± 0.27 0.87 ± 0.00 | [152] | |
| Leaves and flowers | 85% ethanol | DPPH. (µM TE/g FW) ABTS∙+ (µM TE/g FW) FRAP (µM TE/g FW) | 0.25 ± 0.01 2.65 ± 0.04 2.02 ± 0.01 | [153] |
| Allium Organosulfur Compounds | Mechanism of Action | Reference |
|---|---|---|
| Allicin (diallyl-thiosulfinate) | Oxidation of cysteine in glutathione and proteins Inhibition of DNA gyrase in bacterial proteomes Influence on the biosynthesis of microbes’ ribonucleic acids and lipids inhibition of acetyl-CoA synthetase | [174] [169] [57] [177] |
| Ajoene | Controls the development of biofilms by inhibiting the QS-induced (quorum signaling) production of virulence factors | [178] |
| Allyl alcohol | Oxidized intracellularly to acrolein, which is a very powerful protein-alkylating agent and exhibits exceptional anti-yeast activity. Leads to oxidative stress such as NADH oxidation, glutathione depletion, and increase of reactive oxygen species. Alcohol dehydrogenases were identified as targets of allyl alcohol | [53] [179] |
| 3-(Allyltrisulfinyl)-2-aminopropanoic acid | Similar mechanism as allicin | [180] |
| Diallyl disulfide | Inhibition of transcription of most of the quorum-sensing (QS) system genes in P. aeruginosa (as lasR, rhlI/rhlR, and pqsABCDE/pqsR) which interferes with the production of the signal molecules C4-HSL (encoded by rhlI) and PQS (encoded by pqsABCDE). | [181] |
| Diallyl Trisulfide | Could destroy the bacterial cell membrane and decrease the activity of the bacterial membrane transporter system. | [182] |
| Type of Nanoparticles (NPs) 1 | Test Microorganisms | Zone of Inhibition (mm) | MIC 2 (mg/mL) | Reference |
|---|---|---|---|---|
| Ag NPs synthesized using A. cepa extract | Bacillus subtilis ATCC 663315 | 25 | 0.312 | [187] |
| Bacillus subtilis NCTC 1040010 | 27 | 0.156 | ||
| Bacillus cereus ATCC 1457916 | 22 | 0.625 | ||
| Bacillus licheniformis ABRII611 | 25 | 0.312 | ||
| Bacillus sp. 2BSG-PDA-1610 | 19 | 0.625 | ||
| Bacillus sp. DV2-3711 | 22 | 0.312 | ||
| Staphylococcus aureus NCTC 744715 | 23 | 0.312 | ||
| Streptococcus mutans ATCC 365415 | 20 | 0.312 | ||
| Escherichia coli NCTC 1041811 | 24 | 0.312 | ||
| Klebsiella pneumoniae ATCC 10031 | 11 | 2.5 | ||
| Salmonella typhimurium NCIMB 933114 | 20 | 0.312 | ||
| Pseudomonas aeruginosa ATCC 1014515 | 29 | 0.156 | ||
| Proteus vulgaris ATCC 27973 | 15 | 0.625 | ||
| Serratia marcescens ATCC 25179 | 12 | 0.625 | ||
| Candida albicans | 14 | 0.625 | ||
| Ag NPs synthesized using A. cepa | Escherichia coli MTCC No. 729 | 0.016 | [190] | |
| Bacillus subtilis MTCC No. 736 | 0.016 | |||
| Pseudomonas aeruginosa MTCC No. 4637 | 0.016 | |||
| Fusarium oxysporum MTCC No. 3656 | 0.032 | |||
| Ag NPs synthesized using A. cepa and A. sativa | Against selected vaginal bacteria | Growth inhibition | [191] | |
| Streptococcus pneumoniae | ||||
| Pseudomonas aeruginosa | ||||
| Au NPs synthesized using A. ampeloprasum | Staphylococcus aureus ATCC 29213 | 0.0612 | [192] | |
| Bacillus subtilis ATCC 11774 | 0.25 | |||
| Escherichia coli ATCC2 5922 | 0.5 | |||
| Pseudomonas aeruginosa ATCC 27833 | 1 | |||
| Candida albicans | 0.125 | |||
| Cu NPs synthesized using A. sativum | Escherichia coli | 3.9 (50 µg/mL) | [186] | |
| Staphylococcus. aureus | 2.8 (50 µg/mL) | |||
| Bacillus subtilis | 3.35 (50 µg/mL) | |||
| Streptococcus pyogenes | 3.05 (50 µg/mL) | |||
| Pseudomonas aeruginosa | 3.75 (50 µg/mL) | |||
| Klebsiella pneumonia | 3.5 (50 µg/mL) | |||
| Candida albicans | 2.95 (50 µg/mL) | |||
| Aspergillus flavus | 2.35 (50 µg/mL) | |||
| Aspergillus fumigates | 2.70 (50 µg/mL) | |||
| Aspergillus niger | 2.15 (50 µg/mL) | |||
| S NPs synthesized using A. sativum | Candida albicans | No inhibition effect | [193] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuković, S.; Popović-Djordjević, J.B.; Kostić, A.Ž.; Pantelić, N.D.; Srećković, N.; Akram, M.; Laila, U.; Katanić Stanković, J.S. Allium Species in the Balkan Region—Major Metabolites, Antioxidant and Antimicrobial Properties. Horticulturae 2023, 9, 408. https://doi.org/10.3390/horticulturae9030408
Vuković S, Popović-Djordjević JB, Kostić AŽ, Pantelić ND, Srećković N, Akram M, Laila U, Katanić Stanković JS. Allium Species in the Balkan Region—Major Metabolites, Antioxidant and Antimicrobial Properties. Horticulturae. 2023; 9(3):408. https://doi.org/10.3390/horticulturae9030408
Chicago/Turabian StyleVuković, Sandra, Jelena B. Popović-Djordjević, Aleksandar Ž. Kostić, Nebojša Dj. Pantelić, Nikola Srećković, Muhammad Akram, Umme Laila, and Jelena S. Katanić Stanković. 2023. "Allium Species in the Balkan Region—Major Metabolites, Antioxidant and Antimicrobial Properties" Horticulturae 9, no. 3: 408. https://doi.org/10.3390/horticulturae9030408