Statistical Medium Optimization for the Production of Anti-Methicillin-Resistant Staphylococcus aureus Metabolites from a Coal-Mining-Soil-Derived Streptomyces rochei CMB47
Abstract
:1. Introduction
2. Materials and Methods
2.1. Actinobacterial Strain
2.2. Characterization and Identification of the Actinobacteria Strain
2.3. Genomic DNA Extraction, Amplification, Sequencing of the 16S rRNA Gene and Phylogenetic Analysis
2.4. Extracellular Enzyme Production Potential Evaluation
2.5. Screening the Culture Medium and Kinetics Study for the Anti-MRSA Metabolite Production
2.6. Strain Cultivation and Secondary Metabolite Extraction
2.6.1. Spore Suspension and Strain Cultivation
2.6.2. Crude Extract
2.7. Anti-MRSA Susceptibility Test of the Crude Extract
2.7.1. Well Diffusion Assay
2.7.2. Determination of Minimal Inhibitory Concentration (MIC) Values
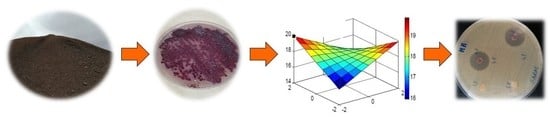
2.8. Experimental Design and Optimization of Anti-MRSA Activity
2.9. Fractionation of the Bioactive Crude Extract and Its Metabolite Profile by HPLC-DAD and LC–ESIMS Analyses
3. Results and Discussion
3.1. Isolation and Screening of Active Actinobacteria Strains
3.2. Polyphasic and Molecular Characterization of the Selected Active CMB47 Strain
3.3. Kinetic Study on the Production of Anti-MRSA Metabolites by Strain CMB47
3.4. Anti-MRSA Activity Evaluation of Crude Extracts
3.5. Central Composite Experimental Design Analysis
3.6. Secondary Metabolite Profiling by DAD-HPLC/ESI-MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation Publishes List of Bacteria for Which New Antibiotics are Urgently Needed. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed on 27 February 2022).
- GDB 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; De Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033–18045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.Y.; Caffrey, A. Thrombocytopenia with tedizolid and linezolid. Antimicrob. Agents Chemother. 2017, 62, e01453-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankenfeld, C.; Mittal, S.; Melendez, Y.; Mendez-Vigo, L.; Lamp, K.C.; Keller, K.N.; Bertolami, S.R. Daptomycin: A comparison of two intravenous formulations. Drug Des. Devel. Ther. 2018, 12, 1953–1958. [Google Scholar] [CrossRef] [Green Version]
- Long, S.W.; Olsen, R.J.; Mehta, S.C.; Palzkill, T.; Cernoch, P.L.; Perez, K.K.; Musick, W.L.; Rosato, A.E.; Musser, J.M. PBP2a mutations causing high-level ceftaroline resistance in clinical methicillin-resistant Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 2014, 58, 6668–6674. [Google Scholar] [CrossRef] [Green Version]
- Davies, J. Millennium bugs. Trends Cell Biol. 1999, 9, 2e5. [Google Scholar] [CrossRef]
- Watve, M.G.; Tickoo, R.; Jog, M.M.; Bhole, B.D. How many antibiotics are produced by the genus Streptomyces? Arch. Microbiol. 2001, 176, 386–390. [Google Scholar] [CrossRef]
- Berdy, J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinashi, H.; Mori, E.; Hatani, A. Isolation and characterization of linear plasmids from lankacidin-producing Streptomyces species. J. Antibiot. 1994, 47, 1447–1455. [Google Scholar] [CrossRef]
- Nindita, Y.; Cao, Z.; Fauzi, A.A.; Teshima, A.; Misaki, Y.; Muslimin, R.; Yang, Y.; Shiwa, Y.; Yoshikawa, H.; Tagami, M.; et al. The genome sequence of Streptomyces rochei 7434AN4, which carries a linear chromosome and three characteristic linear plasmids. Sci. Rep. 2019, 9, 10973–10983. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Li, Y.; Banakar, S.P.; Liu, L.; Shao, C.; Li, Z.; Wang, C. New metabolites from the co-culture of marine-derived actinomycete Streptomyces rochei MB037 and fungus Rhinocladiella similis 35. Front. Microbiol. 2019, 10, 915–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd-Elnaby, H.M.; Abo-Elala, G.M.; Abdel-Raouf, U.M.; Hamed, M.M. Antibacterial and anticancer activity of extracellular synthesized silver nanoparticles from marine Streptomyces rochei MHM13. Egypt. J. Aquat. Res. 2016, 42, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Elsilk, S.E.; Khalil, M.A.; Aboshady, T.A.; Alsalmi, F.A.; Ali, S.S. Streptomyces rochei MS-37 as a novel marine actinobacterium for green biosynthesis of silver nanoparticles and their biomedical applications. Molecules 2022, 27, 7296. [Google Scholar] [CrossRef]
- Maibeche, R.; Boucherba, N.; Bendjeddou, K.; Prins, A.; Bouiche, C.; Hamma, S.; Benhoula, M.; Azzouz, Z.; Bettache, A.; Benallaoua, S.; et al. Peroxidase-producing actinobacteria from Algerian environments and insights from the genome sequence of peroxidase-producing Streptomyces sp. S19. Int. Microbiol. 2022, 25, 379–396. [Google Scholar] [CrossRef]
- Irdani, T.; Perito, B.; Mastromei, G. Characterization of a Streptomyces rochei endoglucanase. Ann. N. Y. Acad. Sci. 1996, 782, 173–181. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.; El-Shweihy, N.M. Bioprocess development for L-asparaginase production by Streptomyces rochei, purification and in-vitro efficacy against various human carcinoma cell lines. Sci. Rep. 2020, 10, 7942–7962. [Google Scholar] [CrossRef] [PubMed]
- Fielder, H.P.; Bruntner, C.; Bull, A.T. Marine actinomycetes as a source of novel secondary metabolites. Antonie Leeuwenhoek 2004, 87, 37–42. [Google Scholar] [CrossRef]
- Djinni, I.; Defant, A.; Kecha, M.; Mancini, I. Actinobacteria derived from Algerian ecosystems as a prominent source of antimicrobial molecules. Antibiotics 2019, 8, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machushynets, N.V.; Wu, C.; Elsayed, S.S.; Hankemeier, T.; Van Wezel, G.P. Discovery of novel glycerolated quinazolinones from Streptomyces sp. MBT27. J. Ind. Microbiol. Biotechnol. 2019, 46, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Djinni, I.; Defant, A.; Djoudi, W.; Chaabane Chaouch, F.; Souagui, S.; Kecha, M.; Mancini, I. Modeling improved production of the chemotherapeutic polypeptide actinomycin D by a novel Streptomyces sp. strain from a Saharan soil. Heliyon 2019, 5, e01695. [Google Scholar] [CrossRef] [Green Version]
- Djinni, I.; Djoudi, W. Streptomyces sp. WR1L1S8 a potent endophytic marine strain for heavy metal resistance and copper removal enhanced by RSM modeling. Acta Ecol. Sin. 2022, 42, 80–89. [Google Scholar] [CrossRef]
- Djinni, I.; Djoudi, W.; Harfi, N.; Stambouli, I.; Khamtache, S.; Makhlouf, D.; Yanat, B.; Souagui, S.; Kecha, M. Enhanced anti-E. coli ST131 metabolites production by a novel Streptomyces sp. CMB51 strain isolated from a coal mininig soil using statistical optimization. Geomicrobiol. J. 2022, 39, 39–53. [Google Scholar] [CrossRef]
- Kuster, E.; Williams, S.T. Selection of Media for Isolation of Streptomycetes. Nature 1964, 202, 928–929. [Google Scholar] [CrossRef] [PubMed]
- Shirling, E.B.; Gottlieb, D. Methods for characterization of Streptomyces species. Int. J. Syst. Bacteriol. 1966, 16, 313–340. [Google Scholar] [CrossRef] [Green Version]
- Williams, S.T.; Goodfellow, M.; Alderson, G. Genus Streptomyces Waksman and Henrici 1943. In Bergey’s Manual of Systematic Bacteriology; Williams, S.T., Sharpe, M.E., Holt, J.P., Eds.; Williams and Wilkins: Baltimore, MD, USA, 1989; Volume 4, pp. 2452–2492. [Google Scholar]
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.A.; Stanley, J.T.; Williams, S.T. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Williams and Wilkins: Baltimore, MD, USA, 1994. [Google Scholar]
- Daboor, S.M.; Haroon, A.M.; Neven, A.E.; Hanona, S.I. Heavy metal adsorption of Streptomyces chromofuscus. J. Clin. Med. 2014, 2, 431–437. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press, Cold Spring Harbor: New York, NY, USA, 1989; pp. 23–38. [Google Scholar]
- Gurtler, V.; Stanisich, V.A. New approches to typing and identification of bacteria using the 16S-23S rDNA spacer region. Microbiology 1996, 142, 3–16. [Google Scholar] [CrossRef] [Green Version]
- EzTaxon-e Server. Available online: http://eztaxon-e.ezbiocloud.net/ (accessed on 9 June 2019).
- European Bioinformatics Institute Server. Available online: http://www.ebi.ac.uk/Tools/msa/clustalw2/ (accessed on 9 June 2019).
- Haritha, R.; Siva Kumar, K.; Jagan Mohan, Y.S.Y.V.; Romana, T. Amylolytic and proteolytic Actinobacteria isolated from marine sediments of Bay of Bengal. Int. J. Microbiol. Res. 2010, 1, 37–44. [Google Scholar]
- Rathnan, R.K.; Ambili, M. Cellulase enzyme production by Streptomyces sp. using fruit waste as substrate. Aust. J. Basic Appl. Sci. 2011, 5, 1114–1118. [Google Scholar]
- Sierra, G. A simple method for the detection of lipolytic activity of microorganisms and some observations on the influence of the contact between cells and fatty substrates. Antonie Leeuwenhoek 1957, 23, 15–22. [Google Scholar] [CrossRef]
- Sambasiva Rao, K.R.S.; Tripathy, N.K.; Mahalaxmi, Y.; Prakasham, R.S. Laccase and peroxidase free tyrosinase production by isolated microbial strain. J. Microbiol. Biotechnol. 2012, 22, 207–214. [Google Scholar] [CrossRef] [Green Version]
- DeVos, P.; Garrity, G.M.; Jones, D.; Krieg, N.R.; Ludwig, W.; Rainey, F.A.; Schleifer, K.H.; Whitman, W.B. Bergey’s Manual of Systematic Bacteriology, 2nd ed.; The Firmicute; Springer: New York, NY, USA, 2009; Volume 3. [Google Scholar]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics, 2nd ed.; John Innes Foundation: Norwich, UK, 2000. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 9th ed.; CLSI Document M07-A9; Clinical and Laboratory Standards Institute, CLSI: Louis, MS, USA, 2012; Volume 32, pp. 1–68. [Google Scholar]
- Box, G.E.P.; Hunter, W.G.; Hunter, J.S. Statistics for Experimenters; Edition Wiley Interscience: New York, NY, USA, 1978. [Google Scholar]
- Sado, G.; Sado, M.C. Les Plans D’expériences de L’expérimentation à L’assurance Qualités; AFNOR Technique: Paris, France, 1991. [Google Scholar]
- Goupy, J. Plans d’expériences Pour Surfaces de Réponse; Dunod: Paris, France, 1999. [Google Scholar]
- Basilio, A.; Gonzalez, I.; Vicente, M.; Gorrochategui, J.; Cabello, A.; Gonzalez, A.; Genilloud, O. Patterns of antimicrobial activities from soil actinomycetes isolated under different conditions of pH and salinity. J. Appl. Microbiol. 2003, 95, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Singh, T.A.; Bharat, B.; Bhasin, S.; Modi, H.A. Approach towards different fermentative techniques for the production of bioactive actinobacterial melanin. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 695–700. [Google Scholar] [CrossRef]
- Singh, R.; Dubey, A.K. Isolation and characterization of a new endophytic Actinobacterium Streptomyces californicus strain ADR1 as a promising source of anti-bacterial, anti-biofilm and antioxidant metabolites. Microorganisms 2020, 8, 929. [Google Scholar] [CrossRef] [PubMed]
- Djinni, I.; Djoudi, W.; Souagui, S.; Rabia, F.; Rahmouni, S.; Mancini, I.; Kecha, M. Streptomyces thermoviolaceus SRC3 strain as a novel source of the antibiotic adjuvant streptazolin: A statistical approach toward the optimized production. J. Microbiol. Meth. 2018, 148, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, C.N.; Tandel, K.; Grover, N.; Bhatt, P.; Sahni, A.K.; Sen, S.; Prahraj, A.K. In vitro vancomycin susceptibility amongst methicillin resistant Staphylococcus aureus. Med. J. Armed Forces India. 2014, 70, 215–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, T.; Matsumoto, A.; Takahashi, Y.; Tomoda, H.; Omura, S. Phenatic acids A and B, new potentiators of antifungal miconazole activity produced by Streptomyces sp. K03-0132. J. Antibiot. 2005, 58, 252–259. [Google Scholar] [CrossRef] [Green Version]




| Factors | Levels | ||||
|---|---|---|---|---|---|
| −α (−2) | −1 | 0 | +1 | +α (+2) | |
| Starch (g/L) | 2 | 6 | 8 | 14 | 18 |
| NaNO3 (g/L) | 1 | 2 | 3 | 4 | 5 |
| Incubation Time (days) | 3 | 5 | 7 | 9 | 11 |
| pH | 3 | 5 | 7 | 9 | 11 |
| Regression variance | 4.17 |
| Residual variance | 1.33 |
| Estimated F value | 3.13 |
| Tabulated F value | 2.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djinni, I.; Djoudi, W.; Boumezoued, C.; Barchiche, H.; Souagui, S.; Kecha, M.; Mancini, I. Statistical Medium Optimization for the Production of Anti-Methicillin-Resistant Staphylococcus aureus Metabolites from a Coal-Mining-Soil-Derived Streptomyces rochei CMB47. Fermentation 2023, 9, 381. https://doi.org/10.3390/fermentation9040381
Djinni I, Djoudi W, Boumezoued C, Barchiche H, Souagui S, Kecha M, Mancini I. Statistical Medium Optimization for the Production of Anti-Methicillin-Resistant Staphylococcus aureus Metabolites from a Coal-Mining-Soil-Derived Streptomyces rochei CMB47. Fermentation. 2023; 9(4):381. https://doi.org/10.3390/fermentation9040381
Chicago/Turabian StyleDjinni, Ibtissem, Warda Djoudi, Chahinaz Boumezoued, Halima Barchiche, Samiha Souagui, Mouloud Kecha, and Ines Mancini. 2023. "Statistical Medium Optimization for the Production of Anti-Methicillin-Resistant Staphylococcus aureus Metabolites from a Coal-Mining-Soil-Derived Streptomyces rochei CMB47" Fermentation 9, no. 4: 381. https://doi.org/10.3390/fermentation9040381