Effects of External Resistance, New Electrode Material, and Catholyte Type on the Energy Generation and Performance of Dual-Chamber Microbial Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
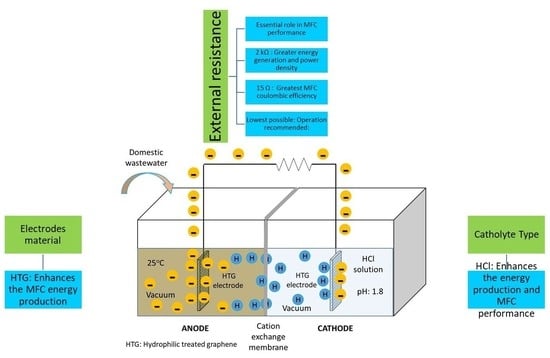
2.1. Experimental Setup
2.2. Procedures
2.2.1. Preparations for the Experiments
2.2.2. Biodegradation of Domestic Wastewater
- (a)
- Scenario 1 (HCl-G): cathode electrolyte: hydrochloric acid; anode and cathode electrode material: graphite.
- (b)
- Scenario 2 (HCl-HTG): cathode electrolyte: hydrochloric acid; anode and cathode electrode material: hydrophilically-treated graphene.
- (c)
- Scenario 3 (DW-G): cathode electrolyte: distilled water; anode and cathode electrode material: graphite.
- (d)
- Scenario 4 (DW-HTG): cathode electrolyte: distilled water; anode and cathode electrode material: hydrophilically-treated graphene.
2.2.3. Evaluation of the External Resistance
3. Results and Discussion
3.1. Biodegradation of Domestic Wastewater
3.2. Influence of the External Resistance on the MFC Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Liu, T.; Zhu, X.; Zhang, F.; Ye, D.; Liao, Q.; Li, Y. Boosting Power Density of Microbial Fuel Cells with 3D Nitrogen-Doped Graphene Aerogel Electrode. Adv. Sci. 2016, 3, 1600097. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Koók, L.; Nemestóthy, N.; Bélafi-Bakó, K.; Bakonyi, P. Treatment of dark fermentative H2 production effluents by microbial fuel cells: A tutorial review on promising operational strategies and practices. Int. J. Hydrogen Energy 2021, 46, 5556–5569. [Google Scholar] [CrossRef]
- Pushkar, P.; Mungray, A.K. Exploring the use of 3 dimensional low-cost sugar-urea carbon foam electrode in the benthic microbial fuel cell. Renew. Energy 2020, 147, 2032–2042. [Google Scholar] [CrossRef]
- Ren, H.; Pyo, S.; Lee, J.-I.; Park, T.-J.; Gittleson, F.S.; Leung, F.C.; Kim, J.; Taylor, A.D.; Lee, H.-S.; Chae, J. A high power density miniaturized microbial fuel cell having carbon nanotube anodes. J. Power Sources 2015, 273, 823–830. [Google Scholar] [CrossRef]
- Mohamed, H.O.; Sayed, E.T.; Obaid, M.; Choi, Y.-J.; Park, S.-G.; Al-Qaradawi, S.; Chae, K.-J. Transition metal nanoparticles doped carbon paper as a cost-effective anode in a microbial fuel cell powered by pure and mixed biocatalyst cultures. Int. J. Hydrogen Energy 2018, 43, 21560–21571. [Google Scholar] [CrossRef]
- Masoudi, M.; Rahimnejad, M.; Mashkour, M. Fabrication of anode electrode by a novel acrylic based graphite paint on stainless steel mesh and investigating biofilm effect on electrochemical behavior of anode in a single chamber microbial fuel cell. Electrochim. Acta 2020, 344, 136168. [Google Scholar] [CrossRef]
- Neto, S.A.; Reginatto, V.; De Andrade, A.R. Microbial Fuel Cells and Wastewater Treatment. In Electrochemical Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2018; pp. 305–331. [Google Scholar]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef] [PubMed]
- ElMekawy, A.; Hegab, H.M.; Losic, D.; Saint, C.P.; Pant, D. Applications of graphene in microbial fuel cells: The gap between promise and reality. Renew. Sustain. Energy Rev. 2017, 72, 1389–1403. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Mohamed, H.O.; Park, S.-G.; Al Mayyahi, R.B.; Aldhaifallah, M.; Rezk, H.; Ren, X.; Yu, H.; Chae, K.-J. Electrophoretically fabricated nickel/nickel oxides as cost effective nanocatalysts for the oxygen reduction reaction in air-cathode microbial fuel cell. Int. J. Hydrogen Energy 2020, 45, 5960–5970. [Google Scholar] [CrossRef]
- Feng, H.; Liang, Y.; Guo, K.; Chen, W.; Shen, D.; Huang, L.; Zhou, Y.; Wang, M.; Long, Y. TiO2 nanotube arrays modified titanium: A stable, scalable, and cost-effective bioanode for microbial fuel cells. Environ. Sci. Technol. Lett. 2016, 3, 420–424. [Google Scholar] [CrossRef]
- Sabirov, I.; Enikeev, N.; Murashkin, M.Y.; Valiev, R.Z. Bulk Nanostructured Materials with Multifunctional Properties; Springer: Heidelberg, Germany, 2015. [Google Scholar]
- Baudler, A.; Schmidt, I.; Langner, M.; Greiner, A.; Schröder, U. Does it have to be carbon? Metal anodes in microbial fuel cells and related bioelectrochemical systems. Energy Environ. Sci. 2015, 8, 2048–2055. [Google Scholar] [CrossRef] [Green Version]
- Papiya, F.; Das, S.; Pattanayak, P.; Kundu, P.P. The fabrication of silane modified graphene oxide supported Ni–Co bimetallic electrocatalysts: A catalytic system for superior oxygen reduction in microbial fuel cells. Int. J. Hydrogen Energy 2019, 44, 25874–25893. [Google Scholar] [CrossRef]
- Santoro, C.; Serov, A.; Stariha, L.; Kodali, M.; Gordon, J.; Babanova, S.; Bretschger, O.; Artyushkova, K.; Atanassov, P. Iron based catalysts from novel low-cost organic precursors for enhanced oxygen reduction reaction in neutral media microbial fuel cells. Energy Environ. Sci. 2016, 9, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Rajesh, P.P.; Noori, T.; Ghangrekar, M.M. Improving Performance of Microbial Fuel Cell by Using Polyaniline-Coated Carbon–Felt Anode. J. Hazard. Toxic Radioact. Waste 2020, 24, 04020024. [Google Scholar] [CrossRef]
- Hu, M.; Li, X.; Xiong, J.; Zeng, L.; Huang, Y.; Wu, Y.; Cao, G.; Li, W. Nano-Fe3C@PGC as a novel low-cost anode electrocatalyst for superior performance microbial fuel cells. Biosens. Bioelectron. 2019, 142, 111594. [Google Scholar] [CrossRef]
- Daud, S.M.; Daud, W.R.W.; Abu Bakar, M.H.; Kim, B.H.; Somalu, M.R.; Muchtar, A.; Jahim, J.M.; Ali, S.A.M. Low-cost novel clay earthenware as separator in microbial electrochemical technology for power output improvement. Bioprocess Biosyst. Eng. 2020, 43, 1369–1379. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Majedi, F.S.; Kabiri, K.; Solati-Hashjin, M.; Moaddel, H. Novel nanocomposite proton exchange membranes based on Nafion® and AMPS-modified montmorillonite for fuel cell applications. J. Membr. Sci. 2010, 365, 286–293. [Google Scholar] [CrossRef]
- Das, I.; Das, S.; Sharma, S.; Ghangrekar, M. Ameliorated performance of a microbial fuel cell operated with an alkali pre-treated clayware ceramic membrane. Int. J. Hydrogen Energy 2020, 45, 16787–16798. [Google Scholar] [CrossRef]
- Cheraghipoor, M.; Mohebbi-Kalhori, D.; Noroozifar, M.; Maghsoodlou, M.T. Production of greener energy in microbial fuel cell with ceramic separator fabricated using native soils: Effect of lattice and porous SiO2. Fuel 2021, 284, 118938. [Google Scholar] [CrossRef]
- Raychaudhuri, A.; Sahoo, R.N.; Behera, M. Application of clayware ceramic separator modified with silica in microbial fuel cell for bioelectricity generation during rice mill wastewater treatment. Water Sci. Technol. 2021, 84, 66–76. [Google Scholar] [CrossRef]
- Al Lawati, M.J.; Jafary, T.; Baawain, M.S.; Al-Mamun, A. A mini review on biofouling on air cathode of single chamber microbial fuel cell; prevention and mitigation strategies. Biocatal. Agric. Biotechnol. 2019, 22, 101370. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, Y.; Yang, F. Hydrogen production from microbial fuel cells-ammonia electrolysis cell coupled system fed with landfill leachate using Mo2C/N-doped graphene nanocomposite as HER catalyst. Electrochim. Acta 2019, 299, 672–681. [Google Scholar] [CrossRef]
- Florio, C.; Nastro, R.A.; Flagiello, F.; Minutillo, M.; Pirozzi, D.; Pasquale, V.; Ausiello, A.; Toscano, G.; Jannelli, E.; Dumontet, S. Biohydrogen production from solid phase-microbial fuel cell spent substrate: A preliminary study. J. Clean. Prod. 2019, 227, 506–511. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Z.; Su, X.; Zhao, P.; Zhou, J.; He, Q.; Ai, H. Cost-effective domestic wastewater treatment and bioenergy recovery in an immobilized microalgal-based photoautotrophic microbial fuel cell (PMFC). Chem. Eng. J. 2019, 372, 956–965. [Google Scholar] [CrossRef]
- Yadav, G.; Sharma, I.; Ghangrekar, M.; Sen, R. A live bio-cathode to enhance power output steered by bacteria-microalgae synergistic metabolism in microbial fuel cell. J. Power Sources 2020, 449, 227560. [Google Scholar] [CrossRef]
- Peixoto, L.; Parpot, P.; Martins, G. Assessment of Electron Transfer Mechanisms during a Long-Term Sediment Microbial Fuel Cell Operation. Energies 2019, 12, 481. [Google Scholar] [CrossRef] [Green Version]
- Catal, T.; Kul, A.; Atalay, V.E.; Bermek, H.; Ozilhan, S.; Tarhan, N. Efficacy of microbial fuel cells for sensing of cocaine metabolites in urine-based wastewater. J. Power Sources 2019, 414, 1–7. [Google Scholar] [CrossRef]
- Rossi, R.; Evans, P.J.; Logan, B.E. Impact of flow recirculation and anode dimensions on performance of a large scale microbial fuel cell. J. Power Sources 2018, 412, 294–300. [Google Scholar] [CrossRef]
- Zavala, M.L.; Delenne, P.R.T.; Peña, O.I.G. Improvement of Wastewater Treatment Performance and Power Generation in Microbial Fuel Cells by Enhancing Hydrolysis and Acidogenesis, and by Reducing Internal Losses. Energies 2018, 11, 2309. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Chata, G.; Zhang, Y.; Peng, Y.; Lu, J.E.; Wang, N.; Mercado, R.; Li, J.; Chen, S. Graphene oxide-supported zinc cobalt oxides as effective cathode catalysts for microbial fuel cell: High catalytic activity and inhibition of biofilm formation. Nano Energy 2019, 57, 811–819. [Google Scholar] [CrossRef]
- Boas, J.V.; Oliveira, V.; Marcon, L.; Simões, M.; Pinto, A. Optimization of a single chamber microbial fuel cell using Lactobacillus pentosus: Influence of design and operating parameters. Sci. Total Environ. 2019, 648, 263–270. [Google Scholar] [CrossRef]
- Zhao, W.; Ci, S. Nanomaterials As Electrode Materials of Microbial Electrolysis Cells for Hydrogen Generation. In Nanomaterials for the Removal of Pollutants and Resource Reutilization, Micro and Nano Technologies; Luo, X., Deng, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 213–242. [Google Scholar]
- Mohan, S.V.; Chiranjeevi, P.; Chandrasekhar, K.; Babu, P.S.; Sarkar, O. Acidogenic Biohydrogen Production From Wastewater. In Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2019; pp. 279–320. [Google Scholar]
- Capodaglio, A.G.; Cecconet, D.; Molognoni, D. An Integrated Mathematical Model of Microbial Fuel Cell Processes: Bioelectrochemical and Microbiologic Aspects. Processes 2017, 5, 73. [Google Scholar] [CrossRef] [Green Version]
- Koók, L.; Nemestóthy, N.; Bélafi-Bakó, K.; Bakonyi, P. Investigating the specific role of external load on the performance versus stability trade-off in microbial fuel cells. Bioresour. Technol. 2020, 309, 123313. [Google Scholar] [CrossRef]
- Aelterman, P.; Versichele, M.; Marzorati, M.; Boon, N.; Verstraete, W. Loading rate and external resistance control the electricity generation of microbial fuel cells with different three-dimensional anodes. Bioresour. Technol. 2008, 99, 8895–8902. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.; Li, J.; Liao, Q.; Ye, D. Biofilm formation and electricity generation of a microbial fuel cell started up under different external resistances. J. Power Sources 2011, 196, 6029–6035. [Google Scholar] [CrossRef]
- Katuri, K.P.; Scott, K.; Head, I.M.; Picioreanu, C.; Curtis, T.P. Microbial fuel cells meet with external resistance. Bioresour. Technol. 2011, 102, 2758–2766. [Google Scholar] [CrossRef]
- Pasternak, G.; Greenman, J.; Ieropoulos, I. Dynamic evolution of anodic biofilm when maturing under different external resis-tive loads in microbial fuel cells, Electrochemical perspective. J. Power Sources 2018, 400, 392–401. [Google Scholar] [CrossRef]
- Woodward, L.; Tartakovsky, B.; Perrier, M.; Srinivasan, B. Maximizing power production in a stack of microbial fuel cells us-ing multiunit optimization method. Biotechnol. Prog. 2009, 25, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Walter, W.G. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Jadhav, G.; Ghangrekar, M. Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour. Technol. 2009, 100, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]








| Resistance (Ω) | HTG | Graphite | ||
|---|---|---|---|---|
| HCl | DW | HCl | DW | |
| 15 | 6.26 | 9.94 | 2.32 | 0.07 |
| 680 | 5.24 | 6.96 | 2.07 | 0.07 |
| 2 k | 4.74 | 4.47 | 1.77 | 0.07 |
| 10 k | 2.08 | 1.36 | 1.05 | 0.06 |
| 15 k | 1.58 | 0.97 | 0.82 | 0.06 |
| 22 k | 1.15 | 0.67 | 0.63 | 0.05 |
| 33 k | 0.82 | 0.46 | 0.47 | 0.07 |
| 47 k | 0.59 | 0.33 | 0.35 | 0.05 |
| 67 k | 0.42 | 0.23 | 0.26 | 0.03 |
| 220 k | 0.12 | 0.07 | 0.05 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Zavala, M.Á.; Cámara Gutiérrez, I.C. Effects of External Resistance, New Electrode Material, and Catholyte Type on the Energy Generation and Performance of Dual-Chamber Microbial Fuel Cells. Fermentation 2023, 9, 344. https://doi.org/10.3390/fermentation9040344
López Zavala MÁ, Cámara Gutiérrez IC. Effects of External Resistance, New Electrode Material, and Catholyte Type on the Energy Generation and Performance of Dual-Chamber Microbial Fuel Cells. Fermentation. 2023; 9(4):344. https://doi.org/10.3390/fermentation9040344
Chicago/Turabian StyleLópez Zavala, Miguel Ángel, and Iris Cassandra Cámara Gutiérrez. 2023. "Effects of External Resistance, New Electrode Material, and Catholyte Type on the Energy Generation and Performance of Dual-Chamber Microbial Fuel Cells" Fermentation 9, no. 4: 344. https://doi.org/10.3390/fermentation9040344