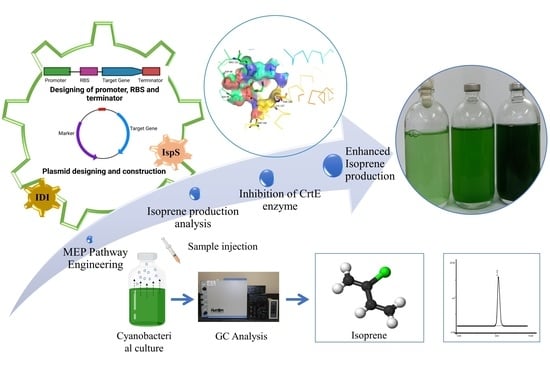
Geranyl Diphosphate Synthase (CrtE) Inhibition Using Alendronate Enhances Isoprene Production in Recombinant Synechococcus elongatus UTEX 2973: A Step towards Isoprene Biorefinery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Preparation of Plasmid Constructs
2.3. Conjugal Transfer of Plasmid into UTEX 2973
2.4. Genomic DNA Isolation and PCR Analysis of Recombinant Strains of UTEX 2973
2.5. Growth Profile and Dry Cell Weight Determination
2.6. Expression Analysis of IspS and the IDI Gene in Recombinant UTEX 2973 Strains through Semi-Quantitative RT-PCR
2.7. Analysis of Protein by SDS-PAGE
2.8. Geranyl Diphosphate Synthase (CrtE) Inhibition Studies: Structure Preparation, Molecular Docking Simulations, and Analysis
2.9. Isoprene Production Conditions and Quantification
3. Results and Discussions
3.1. Construction of Plasmids and Recombinant UTEX 2973 Strains
3.2. Expression of IspS and IDI Transgenes and the Growth Profile of Recombinant UTEX 2973 Strains
3.3. In Silico Studies on CrtE Enzyme Inhibition by Alendronate
3.4. Isoprene Production by Recombinant UTEX 2973 Strains and the Effect of Alendronate on Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, I.; Rautela, A.; Kumar, S. Approaches in the Photosynthetic Production of Sustainable Fuels by Cyanobacteria Using Tools of Synthetic Biology. World. J. Microbiol. Biotechnol. 2021, 37, 201. [Google Scholar] [CrossRef]
- Andrews, F.; Faulkner, M.; Toogood, H.S.; Scrutton, N.S. Combinatorial Use of Environmental Stresses and Genetic Engineering to Increase Ethanol Titres in Cyanobacteria. Biotechnol. Biofuels 2021, 14, 240. [Google Scholar] [CrossRef]
- Melis, A. Photosynthesis-to-Fuels: From Sunlight to Hydrogen, Isoprene, and Botryococcene Production. Energy Environ. Sci. 2012, 5, 5531–5539. [Google Scholar] [CrossRef]
- Lindberg, P.; Park, S.; Melis, A. Engineering a Platform for Photosynthetic Isoprene Production in Cyanobacteria, Using Synechocystis as the Model Organism. Metab. Eng. 2010, 12, 70–79. [Google Scholar] [CrossRef]
- Halfmann, C.; Gu, L.; Gibbons, W.; Zhou, R. Genetically Engineering Cyanobacteria to Convert CO2, Water, and Light into the Long-Chain Hydrocarbon Farnesene. Appl. Microbiol. Biotechnol. 2014, 98, 9869–9877. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.H.; Su, H.Y.; Chow, T.J.; Lee, T.-M.; Cheng, W.-H.; Chang, J.-S.; Chen, H.J. Engineering Cyanobacteria with Enhanced Growth in Simulated Flue Gases for High-Yield Bioethanol Production. Biochem. Eng. J. 2021, 165, 107823. [Google Scholar] [CrossRef]
- Kobayashi, S.; Atsumi, S.; Ikebukuro, K.; Sode, K.; Asano, R. Light-Induced Production of Isobutanol and 3-Methyl-1-Butanol by Metabolically Engineered Cyanobacteria. Microb. Cell. Fact. 2022, 21, 7. [Google Scholar] [CrossRef]
- Lin, P.-C.; Zhang, F.; Pakrasi, H.B. Enhanced Limonene Production in a Fast-Growing Cyanobacterium through Combinatorial Metabolic Engineering. Metab. Eng. Commun. 2021, 12, e00164. [Google Scholar] [CrossRef]
- Klähn, S.; Baumgartner, D.; Pfreundt, U.; Voigt, K.; Schön, V.; Steglich, C.; Hess, W.R. Alkane Biosynthesis Genes in Cyanobacteria and Their Transcriptional Organization. Front. Bioeng. Biotechnol. 2014, 2, 24. [Google Scholar] [CrossRef] [Green Version]
- Chaves, J.E.; Melis, A. Engineering Isoprene Synthesis in Cyanobacteria. FEBS Lett. 2018, 592, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Kudoh, K.; Hotta, S.; Sekine, M.; Fujii, R.; Uchida, A.; Kubota, G.; Kawano, Y.; Ihara, M. Overexpression of Endogenous 1-Deoxy-d-Xylulose 5-Phosphate Synthase (DXS) in Cyanobacterium Synechocystis sp. PCC6803 Accelerates Protein Aggregation. J. Biosci Bioeng. 2017, 123, 590–596. [Google Scholar] [CrossRef]
- Gao, X.; Gao, F.; Liu, D.; Zhang, H.; Nie, X.; Yang, C. Engineering the Methylerythritol Phosphate Pathway in Cyanobacteria for Photosynthetic Isoprene Production from CO2. Energy Environ. Sci. 2016, 9, 1400–1411. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Zurbriggen, A.S.; Melis, A. Isoprene Hydrocarbons Production upon Heterologous Transformation of Saccharomyces cerevisiae: Isoprene Production in Yeast. J. Appl. Microbiol. 2012, 113, 52–65. [Google Scholar] [CrossRef]
- Xue, J.; Ahring, B.K. Enhancing Isoprene Production by Genetic Modification of the 1-Deoxy- d -Xylulose-5-Phosphate Pathway in Bacillus subtilis. Appl. Environ. Microbiol. 2011, 77, 2399–2405. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Wang, C.; Jang, H.-J.; Cha, M.-S.; Park, J.-E.; Jo, S.-Y.; Choi, E.-S.; Kim, S.-W. Isoprene Production by Escherichia coli through the Exogenous Mevalonate Pathway with Reduced Formation of Fermentation Byproducts. Microb. Cell. Fact. 2016, 15, 214. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Zhou, P.; Su, B.; Su, S.; Ye, L.; Yu, H. Enhanced Isoprene Production by Reconstruction of Metabolic Balance between Strengthened Precursor Supply and Improved Isoprene Synthase in Saccharomyces cerevisiae. ACS Synth. Biol. 2018, 7, 2308–2316. [Google Scholar] [CrossRef]
- Chaves, J.E.; Melis, A. Biotechnology of Cyanobacterial Isoprene Production. Appl. Microbiol. Biotechnol. 2018, 102, 6451–6458. [Google Scholar] [CrossRef] [PubMed]
- Englund, E.; Shabestary, K.; Hudson, E.P.; Lindberg, P. Systematic Overexpression Study to Find Target Enzymes Enhancing Production of Terpenes in Synechocystis PCC 6803, Using Isoprene as a Model Compound. Metab. Eng. 2018, 49, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Cid Gomes, L.; Rodrigues, J.S.; Yacout, D.M.M.; Arrou-Vignod, H.; Sjölander, J.; Vedin, N.P.; El Bakouri, O.; Stensjö, K.; Lindblad, P.; et al. A Combined Photobiological–Photochemical Route to C10 Cycloalkane Jet Fuels from Carbon Dioxide via Isoprene. Green. Chem. 2022, 24, 9602–9619. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Wang, Y.; Nie, Q.; Yi, X.; Ge, W.; Yang, J.; Xian, M. Production of Isoprene, One of the High-Density Fuel Precursors, from Peanut Hull Using the High-Efficient Lignin-Removal Pretreatment Method. Biotechnol. Biofuels 2017, 10, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodroffe, J.-D.; Harvey, B.G. Thermal Cyclodimerization of Isoprene for the Production of High-Performance Sustainable Aviation Fuel. Energy. Adv. 2022, 1, 338–343. [Google Scholar] [CrossRef]
- Rautela, A.; Kumar, S. Engineering Plant Family TPS into Cyanobacterial Host for Terpenoids Production. Plant Cell Rep. 2022, 41, 1791–1803. [Google Scholar] [CrossRef]
- Zhao, L.; Chang, W.; Xiao, Y.; Liu, H.; Liu, P. Methylerythritol Phosphate Pathway of Isoprenoid Biosynthesis. Annu. Rev. Biochem. 2013, 82, 497–530. [Google Scholar] [CrossRef] [Green Version]
- Bentley, F.K.; Zurbriggen, A.; Melis, A. Heterologous Expression of the Mevalonic Acid Pathway in Cyanobacteria Enhances Endogenous Carbon Partitioning to Isoprene. Mol. Plant 2014, 7, 71–86. [Google Scholar] [CrossRef] [Green Version]
- Pade, N.; Erdmann, S.; Enke, H.; Dethloff, F.; Dühring, U.; Georg, J.; Wambutt, J.; Kopka, J.; Hess, W.R.; Zimmermann, R.; et al. Insights into Isoprene Production Using the Cyanobacterium Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2016, 9, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suva, L.J.; Cooper, A.; Watts, A.E.; Ebetino, F.H.; Price, J.; Gaddy, D. Bisphosphonates in Veterinary Medicine: The New Horizon for Use. Bone 2021, 142, 115711. [Google Scholar] [CrossRef]
- Park, J.; Pandya, V.R.; Ezekiel, S.J.; Berghuis, A.M. Phosphonate and Bisphosphonate Inhibitors of Farnesyl Pyrophosphate Synthases: A Structure-Guided Perspective. Front. Chem. 2021, 8, 612728. [Google Scholar] [CrossRef] [PubMed]
- Rasulov, B.; Talts, E.; Kännaste, A.; Niinemets, Ü. Bisphosphonate Inhibitors Reveal a Large Elasticity of Plastidic Isoprenoid Synthesis Pathway in Isoprene-Emitting Hybrid Aspen. Plant Physiol. 2015, 168, 532–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satta, A.; Esquirol, L.; Ebert, B.E.; Newman, J.; Peat, T.S.; Plan, M.; Schenk, G.; Vickers, C.E. Molecular Characterization of Cyanobacterial Short-chain Prenyltransferases and Discovery of a Novel GGPP Phosphatase. FEBS J. 2022, 289, 6672–6693. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Morgan, R.M.L.; Fraser, P.D.; Hellgardt, K.; Nixon, P.J. Crystal Structure of Geranylgeranyl Pyrophosphate Synthase (CrtE) Involved in Cyanobacterial Terpenoid Biosynthesis. Front. Plant Sci. 2020, 11, 589. [Google Scholar] [CrossRef]
- Russell, D.W.; Sambrook, J. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2001; Volume 1, p. 112. [Google Scholar]
- Yu, J.; Liberton, M.; Cliften, P.F.; Head, R.D.; Jacobs, J.M.; Smith, R.D.; Koppenaal, D.W.; Brand, J.J.; Pakrasi, H.B. Synechococcus elongatus UTEX 2973, a Fast-Growing Cyanobacterial Chassis for Biosynthesis Using Light and CO2. Sci. Rep. 2015, 5, 8132. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.S.; Krupa, R.A.; Zhang, F.; Hajimorad, M.; Holtz, W.J.; Prasad, N.; Lee, S.K.; Keasling, J.D. BglBrick Vectors and Datasheets: A Synthetic Biology Platform for Gene Expression. J. Biol. Eng. 2011, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Elhai, J.; Vepritskiy, A.; Muro-Pastor, A.M.; Flores, E.; Wolk, C.P. Reduction of Conjugal Transfer Efficiency by Three Restriction Activities of Anabaena sp. Strain PCC 7120. J. Bacteriol. 1997, 179, 1998–2005. [Google Scholar] [CrossRef] [Green Version]
- Gale, G.A.R.; Schiavon Osorio, A.A.; Puzorjov, A.; Wang, B.; McCormick, A.J. Genetic Modification of Cyanobacteria by Conjugation Using the CyanoGate Modular Cloning Toolkit. J. Vis. Exp. 2019, 152, e60451. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.P.; Rastogi, R.P.; Häder, D.-P.; Sinha, R.P. An Improved Method for Genomic DNA Extraction from Cyanobacteria. World J. Microbiol. Biotechnol. 2011, 27, 1225–1230. [Google Scholar] [CrossRef]
- Bentley, F.K.; Melis, A. Diffusion-Based Process for Carbon Dioxide Uptake and Isoprene Emission in Gaseous/Aqueous Two-Phase Photobioreactors by Photosynthetic Microorganisms. Biotechnol. Bioeng. 2012, 109, 100–109. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Angarica, V.E.; Sancho, J.; Fillat, M.F. The FurA Regulon in Anabaena sp. PCC 7120: In Silico Prediction and Experimental Validation of Novel Target Genes. Nucleic Acids Res. 2014, 42, 4833–4846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formighieri, C.; Melis, A. A phycocyanin· phellandrene synthase fusion enhances recombinant protein expression and β-phellandrene (monoterpene) hydrocarbons production in Synechocystis (cyanobacteria). Metab. Eng. 2015, 32, 116–124. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford Method for Protein Quantitation. Methods Mol. Biol. 1994, 32, 9–15. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Gan, J.; Xiao, Z.-X.; Cao, Y. FitDock: Protein-Ligand Docking by Template Fitting. Brief. Bioinform. 2022, 23, bbac087. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.-X.; Cao, Y. CB-Dock2: Improved Protein–Ligand Blind Docking by Integrating Cavity Detection, Docking and Homologous Template Fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef] [PubMed]
- Ivleva, N.B.; Bramlett, M.R.; Lindahl, P.A.; Golden, S.S. LdpA: A Component of the Circadian Clock Senses Redox State of the Cell. EMBO J. 2005, 24, 1202–1210. [Google Scholar] [CrossRef] [Green Version]
- Camsund, D.; Heidorn, T.; Lindblad, P. Design and Analysis of LacI-Repressed Promoters and DNA-Looping in a Cyanobacterium. J. Biol. Eng. 2014, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, F.; Pacheco, C.C.; Ferreira, D.; Moradas-Ferreira, P.; Tamagnini, P. Selection of Suitable Reference Genes for RT-QPCR Analyses in Cyanobacteria. PLoS ONE 2012, 7, e34983. [Google Scholar] [CrossRef]
- Lin, P.-C.; Zhang, F.; Pakrasi, H.B. Enhanced Production of Sucrose in the Fast-Growing Cyanobacterium Synechococcus elongatus UTEX 2973. Sci. Rep. 2020, 10, 390. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Chu, X.; Qiu, Y.; Wu, L.; Qiao, Y.; Wu, J.; Li, D. Discovery of Potent Inhibitor for Farnesyl Pyrophosphate Synthase in the Mevalonate Pathway. Chem. Commun. 2010, 46, 5340. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Lindert, S.; Zhu, W.; Wang, K.; McCammon, J.A.; Oldfield, E. Taxodione and Arenarone Inhibit Farnesyl Diphosphate Synthase by Binding to the Isopentenyl Diphosphate Site. Proc. Natl. Acad. Sci. USA 2014, 111, E2530–E2539. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-L.; Cao, R.; Wang, Y.; Oldfield, E. Farnesyl Diphosphate Synthase Inhibitors with Unique Ligand-Binding Geometries. ACS Med. Chem. Lett. 2015, 6, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Gadelha, A.P.R.; Brigagao, C.M.; da Silva, M.B.; Rodrigues, A.B.M.; Guimarães, A.C.R.; Paiva, F.; de Souza, W.; Henriques, C. Insights about the Structure of Farnesyl Diphosphate Synthase (FPPS) and the Activity of Bisphosphonates on the Proliferation and Ultrastructure of Leishmania and Giardia. Parasites Vectors 2020, 13, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves, J.E.; Kirst, H.; Melis, A. Isoprene Production in Synechocystis under Alkaline and Saline Growth Conditions. J. Appl. Phycol. 2015, 27, 1089–1097. [Google Scholar] [CrossRef]
- Saccardo, A.; Bezzo, F.; Sforza, E. Microalgae growth in ultra-thin steadystate continuous photobioreactors: Assessing self-shading effects. Front. Bioeng. Biotechnol. 2022, 10, 977429. [Google Scholar] [CrossRef]





 represents light illumination.
represents light illumination.
 represents light illumination.
represents light illumination.
| Plasmids and Strains | Description | Antibiotic Resistance | Reference |
|---|---|---|---|
| Plasmid | |||
| pBA2SkIKmA2 | Contains codon-optimized IspS gene for cyanobacteria | Kanamycin | [4] |
| pAM2991 | One-step cloning vector for overexpression between EcoRI and BamHI restriction sites | Spectinomycin | [43] |
| pRL443 | Conjugal plasmid suitable for the mobilization of cargo plasmids in cyanobacteria | Ampicillin | [34] |
| pRL623 | Helper plasmid for bacterial conjugal DNA transfer | Chloramphenicol | [34] |
| pBbE1k-RFP | Contains the RFP gene between NdeI and BamHI | Kanamycin | [33] |
| pAM2991-IspS | Derivative of pAM2991, IspS gene cloned between EcoRI and BamHI sites under the Ptrc promoter | Spectinomycin | This work |
| pBbE1k-IDI-NSIII | Made from pBbE1k-RFP, IDI gene cloned between NdeI and BamHI sites under the Ptrc promoter | Kanamycin | This work |
| Strain | |||
| UTEX 2973 | Wild-type | None | [32] |
| UTEX 2973 IspS | The IspS gene integrated at the NSI site of the UTEX 2973 genome | Spectinomycin | This work |
| UTEX 2973 IspS.IDI | IspS and IDI genes integrated at the NSI and NSIII sites of the UTEX 2973 genome, respectively | Spectinomycin and kanamycin | This work |
| Residue (SeCrtE) | Type of Interaction (IPP) | Residue (SeCrtE) | Types of Interaction (Alendronate) |
|---|---|---|---|
| Ser86 | Hydrogen bond | Ser86 | Hydrogen bond |
| Leu87 | Hydrophobic | Leu87 | Hydrophobic |
| Asp90 | Hydrophobic | Asp90 | Hydrophobic |
| Asp91 | Hydrogen bond | Asp91 | Hydrogen bond |
| Asp96 | Hydrogen bond | Asp96 | Hydrogen bond |
| Asp98 | Hydrogen bond | Asp98 | Hydrogen bond |
| Arg101 | Salt bridge | Arg101 | Salt bridge |
| Lys187 | Salt bridge | Leu159 | Hydrophobic |
| Gln163 | Hydrophobic | ||
| Lys187 | Salt bridge |
| Cyanobacterial Strain | Engineering Strategy | Production Conditions | Cumulative Isoprene Production (Production Studies in Days) | Volumetric Productivity (μg/L/h) | Reference |
|---|---|---|---|---|---|
| Synechocystis sp. PCC 6803 | IspS gene integration under native PpsbA2 promoter in host genome | Closed-vessel cultivation | 50 μg/g DCW (1 day) | -- | [4] |
| Synechocystis sp. PCC 6803 | IspS gene integration under native PpsbA2 promoter in host genome | Fed-batch cultivation in a closed system | 150 μg/L (8 days) | 0.78 | [37] |
| Synechocystis sp. PCC 6803 | IspS and MVA pathway enzymes were expressed | Fed-batch cultivation in a closed system | 250 μg/g DCW (8 days) | 1.53 | [24] |
| Synechocystis sp. PCC 6803 | IspS expressed under PpsbA2 promoter | Closed cultivation system, alkaline, and saline conditions | 120 μg/L (4 days) | 1.25 | [51] |
| Synechocystis sp. PCC 6803 | IspS gene inserted in pVZ325 replicative plasmid under various promoters | Closed and open cultivation systems, plasmid-based expression | 93 μg/g DCW (1 day; closed system) 336 μg/g DCW (1 day; open system) | 1.2 4.2 | [25] |
| Synechococcus elongatus PCC 7942 | IspS, DXS, IspG, and IDI genes were cloned under PpsbA2, Ptrc, and PcpcB promoters | Open cultivation system, aerated with 5% CO2 | 1260 mg/L (21 days) | 2500 | [12] |
| Synechocystis sp. PCC 6803 | IspS, IDI, and DXS gene integration | Open cultivation system | 1.60 mg/L (4 days) | 16.6 | [19] |
| Synechococcus elongatus UTEX 2973 | IspS and IDI genes integrated under Ptrc promoter in cyanobacterial genome | Closed cultivation system, supplemented with alendronate as an inhibitor | 1920 μg/g DCW or 740 μg/L (6 days) | 5.14 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, I.; Rautela, A.; Gangwar, A.; Kesari, V.; Padhi, A.K.; Kumar, S. Geranyl Diphosphate Synthase (CrtE) Inhibition Using Alendronate Enhances Isoprene Production in Recombinant Synechococcus elongatus UTEX 2973: A Step towards Isoprene Biorefinery. Fermentation 2023, 9, 217. https://doi.org/10.3390/fermentation9030217
Yadav I, Rautela A, Gangwar A, Kesari V, Padhi AK, Kumar S. Geranyl Diphosphate Synthase (CrtE) Inhibition Using Alendronate Enhances Isoprene Production in Recombinant Synechococcus elongatus UTEX 2973: A Step towards Isoprene Biorefinery. Fermentation. 2023; 9(3):217. https://doi.org/10.3390/fermentation9030217
Chicago/Turabian StyleYadav, Indrajeet, Akhil Rautela, Agendra Gangwar, Vigya Kesari, Aditya K. Padhi, and Sanjay Kumar. 2023. "Geranyl Diphosphate Synthase (CrtE) Inhibition Using Alendronate Enhances Isoprene Production in Recombinant Synechococcus elongatus UTEX 2973: A Step towards Isoprene Biorefinery" Fermentation 9, no. 3: 217. https://doi.org/10.3390/fermentation9030217