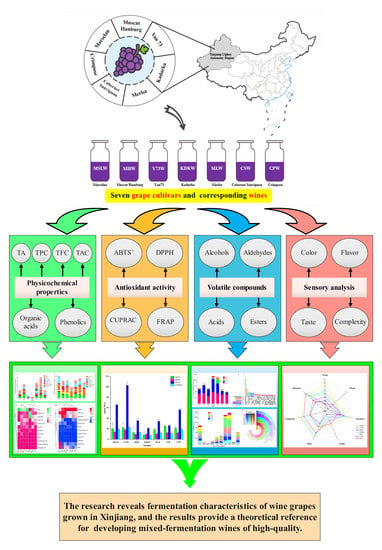
Chemical and Sensory Characteristics of Different Red Grapes Grown in Xinjiang, China: Insights into Wines Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wine Samples
2.2. Analytical Determinations
2.2.1. Determination of Classic Physicochemical Indexes
2.2.2. Determination of Total Phenolic, Total Flavonoid and Total Anthocyanin Content
2.2.3. Determination of Phenolic Compounds
2.2.4. Determination of Antioxidant Activity
2.2.5. Determination of Chromatic Characteristics
2.2.6. Determination of Volatile Compounds
2.2.7. Sensory Analysis
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Basic Physicochemical Properties
3.2. Cluster Analysis of Organic Acids
3.3. Content of Total Phenol, Total Flavonoid and Total Anthocyanin
3.4. Composition of Phenolic Compounds
3.5. Determination of Antioxidant Activity
3.6. Correlation Analysis of Phenolic Compounds and Antioxidant Activity
3.7. Color Analysis
3.8. Volatile Compounds Composition and Content
3.8.1. Alcohols
3.8.2. Esters
3.8.3. Acids
3.9. Principal Component Analysis
3.10. Identification of Aroma Fingerprints of Wines
3.11. Sensory Analysis of Different Wines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, Y.; Chen, K.; Yang, X.Y.; Li, J.M.; Li, X.W. Comparative study of the key aromatic compounds of Cabernet Sauvignon wine from the Xinjiang region of China. J. Food. Sci. Technol. 2021, 58, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- Titarenko, O.; Khalafyan, A.; Temerdashev, A.; Kaunova, A.; Abakumov, G. Identification of the Varietal and Regional Origin of Red Wines by Classification Analysis. J. Anal. Chem. 2018, 73, 195–206. [Google Scholar] [CrossRef]
- Miller, V.; Block, E. A review of wine fermentation process modeling. J. Food. Eng. 2020, 273, 225–226. [Google Scholar] [CrossRef]
- Gambuti, A.; Picariello, L.; Rinaldi, A.; Moio, L. Evolution of Sangiovese Wines with varied tannin and anthocyanin ratios during oxidative aging. Front. Chem. 2018, 6, 32–35. [Google Scholar] [CrossRef] [Green Version]
- Kassara, S.; Kennedy, J.A. Relationship between red wine grade and phenolics. 2. tannin composition and size. J. Agr. Food Chem. 2011, 59, 8409–8412. [Google Scholar] [CrossRef]
- Stamatina, K.; Yorgos, K.; Maria, K.; Niki, P.; Argirios, T.; Garifalia, K. Analytical phenolic composition and sensory assessment of selected rare Greek cultivars after extended bottle ageing. J. Sci. Food Agr. 2015, 95, 1638–1647. [Google Scholar]
- Jagatic Korenika, M.; Tomaz, I.; Preiner, D.; Plichta, V.; Jeromel, A. Impact of commercial yeasts on phenolic profile of Plavac Mali wines from Croatia. Fermentation 2021, 7, 92. [Google Scholar] [CrossRef]
- Lombardi, G.; Cossignani, L.; Giua, L.; Simonetti, S.; Maurizi, A.; Burini, G.; Coli, R.; Blasi, F. Phenol composition and antioxidant capacity of red wines produced in Central Italy changes after one-year storage. J. Appl. Bot. Food Qual. 2017, 90, 197–204. [Google Scholar]
- Jiao, J.; Xia, Y.; Yang, M.; Zheng, J.; Liu, Y.; Cao, Z. Differences in grape-surface yeast populations significantly influence the melatonin level of wine in spontaneous fermentation. LWT Food Sci. Technol. 2022, 163, 45–47. [Google Scholar] [CrossRef]
- Bhattacharyya, N.; Seth, S.; Tudu, B.; Tamuly, P.; Jana, A.; Ghosh, D.; Bandyopadhyay, R.; Bhuyan, M.; Sabhapandit, S. Detection of optimum fermentation time for black tea manufacturing using electronic nose. Sensor. Actuat. B Chem. 2007, 122, 627–634. [Google Scholar] [CrossRef]
- Peng, B.; Lei, Y.; Zhao, H.; Cui, L. Response surface methodology for optimization of fermentation process parameters for improving apple wine quality. J. Food Sci. Technol. 2015, 52, 7513–7518. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, T.; Tan, J.; Li, R. Geographical origin traceability of red wines based on chemometric classification via organic acid profiles. J. Food Qual. 2017, 54, 45–47. [Google Scholar] [CrossRef]
- Pons, A.; Allamy, L.; Schuttler, A.; Rauhut, D.; Thibon, C.; Darriet, P. What is the expected impact of climate change on wine aroma compounds and their precursors in grape? Oeno One 2017, 51, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Yang, J.; Deng, X.; Lei, Y.; Xie, S.; Guo, S.; Ren, R.; Li, J.; Zhang, Z.; Xu, T. Foliar-sprayed manganese sulfate improves flavonoid content in grape berry skin of Cabernet Sauvignon (Vitis vinifera L.) growing on alkaline soil and wine chromatic characteristics. Food Chem. 2020, 314, 45–49. [Google Scholar] [CrossRef]
- Bellvert, J.; Marsal, J.; Mata, M.; Girona, J. Yield, Must composition, and wine quality responses to preveraison water deficits in sparkling base wines of chardonnay. Am. J. Eno. Viticult. 2016, 67, 1–12. [Google Scholar] [CrossRef]
- Collombel, I.; Campos, M.; Hogg, T. Changes in the composition of the lactic acid bacteria behavior and the diversity of Oenococcus oeni isolated from red wines supplemented with selected grape phenolic compounds. Fermentation 2018, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo-Llopart, A.; Saurina, J. Multi-sensor characterization of sparkling wines based on data fusion. Chemosensors 2021, 9, 200. [Google Scholar] [CrossRef]
- Tao, Y.-S.; Li, H.; Wang, H. Data visualization of wine aroma compounds of Cabernet Sauvignon dry red wines from different origins in China. Chin. J. Anal. Chem. 2008, 36, 653–657. [Google Scholar]
- Zhang, K.; Yu, L.; Li, Q.; Wang, R.; Zhang, Z.Z. Comparison of the anthocyanins composition of five wine-making grape cultivars cultivated in the Wujiaqu area of Xinjiang, China. Oeno One 2019, 53, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Duan, W.-P.; Zhu, B.-Q.; Song, R.-R.; Zhang, B.; Lan, Y.-B.; Zhu, X.; Duan, C.-Q.; Han, S.-Y. Volatile composition and aromatic attributes of wine made with Vitisvinifera L.cv Cabernet Sauvignon grapes in the Xinjiang region of China: Effect of different commercial yeasts. Int. J. Food Prop. 2018, 21, 1423–1441. [Google Scholar] [CrossRef] [Green Version]
- Petronilho, S.; Rudnitskaya, A.; Coimbra, M.A.; Rocha, S.M. Comprehensive study of variety oenological potential using statistic tools for the efficient use of non-renewable resources. Appl. Sci. 2021, 11, 4003. [Google Scholar] [CrossRef]
- Borazan, A.; Bozan, B. The influence of pectolytic enzyme addition and prefermentative mash heating during the winemaking process on the phenolic composition of Okuzgozu red wine. Food Chem. 2013, 138, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Samaniego-Sanchez, C.; Marin-Garcia, G.; Quesada-Granados, J. A new fermented beverage from sugarcane (Saccharum officinarum L.) molasses: Analysis of physicochemical properties and antioxidant capacity, and comparison with other industrial alcohol products. LWT Food Sci. Technol. 2020, 128, 324–345. [Google Scholar] [CrossRef]
- Murtaza, A.; Huma, N.; Shabbir, A.; Murtaza, S.; Anees-Ur-Rehman, M. Survival of micro-organisms and organic acid profile of probiotic Cheddar cheese from buffalo milk during accelerated ripening. Int. J. Dairy Technol. 2017, 70, 562–571. [Google Scholar] [CrossRef]
- Andlauer, W.; Stumpf, C.; Furst, P. Influence of the acetification process on phenolic compounds. J. Agr. Food Chem. 2000, 48, 3533–3536. [Google Scholar] [CrossRef]
- Abu Bakar, F.; Mohamed, M.; Rahmat, A.; Fry, J. Phytochemicals and antioxidant activity of different parts of bambangan (Mangifera pajang) and tarap (Artocarpus odoratissimus). Food Chem. 2009, 113, 479–483. [Google Scholar] [CrossRef]
- Anon, A.; Lopez, F.; Hernando, D.; Orriols, I.; Revilla, E.; Losada, M. Effect of five enological practices and of the general phenolic composition on fermentation-related aroma compounds in Mencia young red wines. Food Chem. 2014, 148, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Briz-Cid, N.; Figueiredo-Gonzalez, M.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gandara, J. Effect of two anti-fungal treatments (Metrafenone and Boscalid Plus Kresoxim-methyl) applied to vines on the color and phenol profile of different red wines. Molecules 2014, 19, 8093–8111. [Google Scholar] [CrossRef] [Green Version]
- Stopka, P.; Krizova, J.; Vrchotova, N.; Babikova, P.; Triska, J.; Balik, J.; Kyselakova, M. Antioxidant activity of wines and related matters studied by EPR spectroscopy. Czech J. Food Sci. 2008, 26, S49–S54. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wu, J.; Wang, X.; Li, S.; Zheng, C.; Du, H.; Xu, J.; Wang, S. Composition of phenolic compounds and antioxidant activity in the leaves of blueberry cultivars. J. Funct. Foods 2015, 16, 295–304. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agr. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Di Mattia, D.; Piva, A.; Martuscelli, M.; Mastrocola, D.; Sachetti, G. Effect of sulfites on the in vitro antioxidant activity of wines. Ital. J. Food Sci. 2015, 27, 505–512. [Google Scholar]
- Mutanen, J.; Raty, J.; Gornov, E.; Lehtonen, P.; Peiponen, K.-E.; Jaaskelainen, T. Measurement of color, refractive index, and turbidity of red wines. Am. J. Enolo. Viticult. 2007, 58, 387–392. [Google Scholar] [CrossRef]
- Poggio, C.; Vialba, L.; Berardengo, A.; Federico, R.; Colombo, M.; Beltrami, R.; Scribante, A. Color Stability of New Esthetic Restorative Materials: A Spectrophotometric Analysis. J. Funct Biomater. 2017, 8, 26. [Google Scholar] [CrossRef]
- Cellamare, L.; D’Auria, M.; Emanuele, L.; Racioppi, R. The effect of light on the composition of some volatile compounds in wine: An HS-SPME-GC-MS study. Int. J. Food Sci. Technol. 2009, 44, 2377–2384. [Google Scholar] [CrossRef]
- Granato, D.; Katayama, U.; de Castro, A. Phenolic composition of South American red wines classified according to their antioxidant activity, retail price and sensory quality. Food Chem. 2011, 129, 366–373. [Google Scholar] [CrossRef]
- Rolle, L.; Torchio, F.; Giacosa, S.; Segade, R.; Cagnasso, E.; Gerbi, V. Assessment of physicochemical differences in Nebbiolo grape berries from different production areas and sorted by flotation. Am. J. Enolo. Viticult. 2012, 63, 195–204. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, T.; Chen, Y.; Wang, H.; Gong, G.; Li, Y. Microwave irradiation: Impacts on physicochemical properties of red wine. Cyta J. Food 2020, 18, 281–290. [Google Scholar] [CrossRef]
- Ricciutelli, M.; Moretti, S.; Galarini, R.; Sagratini, G.; Mari, M.; Lucarini, S.; Vittori, S.; Caprioli, G. Identification and quantification of new isomers of isopropyl-malic acid in wine by LC-IT and LC-Q-Orbitrap. Food Chem. 2019, 294, 390–396. [Google Scholar] [CrossRef]
- Ma, Y.; Li, T.; Xu, X.; Ji, Y.; Jiang, X.; Shi, X.; Wang, B. Investigation of volatile compounds, microbial succession, and their relation during spontaneous fermentation of Petit Manseng. Front. Microbiol. 2021, 12, 34–36. [Google Scholar] [CrossRef]
- Valentao, P.; Seabra, M.; Lopes, G.; Silva, R.; Martins, V.; Trujillo, E.; Velazquez, E.; Andrade, B. Influence of Dekkera bruxellensis on the contents of anthocyanins, organic acids and volatile phenols of Dao red wine. Food Chem. 2007, 100, 64–70. [Google Scholar] [CrossRef]
- Wang, Q.; Su, N.; Zhang, H.; Yang, P. The effects of pulsed electric fields applied to red and white wines during bottle ageing on organic acid contents. J. Food Sci Technol. 2015, 52, 171–180. [Google Scholar] [CrossRef]
- Hosu, A.; Cristea, M.; Cimpoiu, C. Analysis of total phenolic, flavonoids, anthocyanins and tannins content in Romanian red wines: Prediction of antioxidant activities and classification of wines using artificial neural networks. Food Chem. 2014, 150, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Shavandi, A.; Harrison, R.; Bekhit, A. Characterization of Phenolic Compounds in Wine Lees. Antioxidants 2018, 7, 25–27. [Google Scholar]
- Zeng, L.; Teissedre, L.; Jourdes, M. Structures of polymeric pigments in red wine and their derived quantification markers revealed by high-resolution quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Sp. 2016, 30, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Hettiarachchi, G.; Morton, W.; Razic, S. Analysis of phenolics in wine by high performance thin-layer chromatography with gradient elution and high resolution plate imaging. J. Pharmaceut. Biomed. 2015, 102, 93–99. [Google Scholar] [CrossRef]
- Garaguso, I.; Nardini, M. Polyphenols content, phenolics profile and antioxidant activity of organic red wines produced without sulfur dioxide/sulfites addition in comparison to conventional red wines. Food Chem. 2015, 179, 336–342. [Google Scholar] [CrossRef]
- Guan, L.; Li, H.; Fan, G.; Li, H.; Fang, B.; Dai, W.; Delrot, S.; Wang, J.; Wu, H. Regulation of anthocyanin biosynthesis in tissues of a Teinturier grape cultivar under sunlight exclusion. Am. J. Enolo. Viticult. 2014, 65, 363–374. [Google Scholar] [CrossRef]
- Luan, Y.; Zhang, W.; Xi, M.; Huo, S.; Ma, N. Comparing the effects of exogenous abscisic acid on the phenolic composition of Yan 73 and Cabernet Sauvignon (Vitis vinifera L.) wines. Eur. Food Res. Technol. 2014, 239, 203–213. [Google Scholar] [CrossRef]
- Xu, F.; Luan, Y.; Zhang, W.; Huo, S.; Gao, X.; Fang, L.; Xi, M. Phenolic profiles and antioxidant properties of young wines made from Yan73 (Vitis vinifera L.) and Cabernet Sauvignon (Vitis vinifera L.) grapes treated by 24-Epibrassinolide. Molecules 2014, 19, 10189–10207. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Velez, M.; Martinez-Martinez, F.; Del Valle-Ribes, C. The study of phenolic compounds as natural antioxidants in wine. Crit. Rev. Food Sci. 2003, 43, 233–244. [Google Scholar] [CrossRef]
- Manolescu, N.; Oprea, E.; Bodolica, M.; Simion, C. Is wine industrialization lowering natural qualities of white wines? Rev. Roum. Chim. 2016, 61, 199–203. [Google Scholar]
- Ghatak, A.; Chaturvedi, A.; Desai, S. Indian grape wines: A potential source of phenols, polyphenols, and antioxidants. Int. J. Food Prop. 2014, 17, 818–828. [Google Scholar] [CrossRef]
- Jose Jara-Palacios, M.; Gordillo, B.; Lourdes Gonzalez-Miret, M.; Hernanz, D.; Luisa Escudero-Gilete, M.; Heredia, J. Comparative study of the enological potential of different winemaking byproducts: Implications in the antioxidant activity and color expression of red wine anthocyanins in a model solution. J. Agr. Food Chem. 2014, 62, 6975–6983. [Google Scholar] [CrossRef]
- Briz-Cid, N.; Figueiredo-Gonzalez, M.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gandara, J. The measure and control of effects of botryticides on phenolic profile and color quality of red wines. Food Control 2015, 50, 942–948. [Google Scholar] [CrossRef]
- Fragoso, S.; Guasch, J.; Acena, L.; Mestres, M.; Busto, O. Prediction of red wine colour and phenolic parameters from the analysis of its grape extract. Int. J. Food Sci. Technol. 2011, 46, 2569–2575. [Google Scholar] [CrossRef]
- He, F.; Liang, N.; Mu, L.; Pan, H.; Wang, J.; Reeves, J.; Duan, Q. Anthocyanins and their variation in red wines II. anthocyanin derived pigments and their color evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef] [Green Version]
- Gordillo, B.; Jesus Cejudo-Bastante, M.; Rodriguez-Pulido, J.; Lourdes Gonzalez-Miret, M.; Heredia, J. Application of the differential colorimetry and polyphenolic profile to the evaluation of the chromatic quality of Tempranillo red wines elaborated in warm climate. Influence of the presence of oak wood chips during fermentation. Food Chem. 2013, 141, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Du, G.; Gao, T.; Wang, W.; Meng, D.; Li, J.; Brennan, C.; Wang, Y.; Zhao, H.; Wang, Y.; et al. The effect of carbonic maceration during winemaking on the color, aroma and sensory properties of ‘Muscat Hamburg’ wine. Molecules 2019, 24, 3120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilc, T.; Werck-Reichhart, D.; Navrot, N. Meta-analysis of the core aroma components of grape and wine aroma. Front. Plant Sci. 2016, 7, 24–27. [Google Scholar] [CrossRef] [Green Version]
- Saenz-Navajas, M.-P.; Arias, I.; Ferrero-del-Teso, S.; Fernandez-Zurbano, P.; Escudero, A.; Ferreira, V. Chemo-sensory approach for the identification of chemical compounds driving green character in red wines. Food Res. Int. 2018, 109, 138–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambropoulos, I.; Roussis, G. Inhibition of the decrease of volatile esters and terpenes during storage of wines and a model wine medium by wine phenolic extracts. Food Technol. Biotech. 2007, 45, 147–155. [Google Scholar]
- Petronilho, S.; Lopez, R.; Ferreira, V.; Coimbra, A.; Rocha, M. Revealing the usefulness of aroma networks to explain wine aroma properties: A case study of portuguese wines. Molecules 2020, 25, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tufariello, M.; Capone, S.; Siciliano, P. Volatile components of Negroamaro red wines produced in Apulian Salento area. Food Chem. 2012, 132, 2155–2164. [Google Scholar] [CrossRef]
- De-la-Fuente-Blanco, A.; Saenz-Navajas, P.; Ferreira, V. On the effects of higher alcohols on red wine aroma. Food Chem. 2016, 210, 107–114. [Google Scholar] [CrossRef]
- Song, Q.; Li, H.; Liang, Y.; Tao, S.; Mi, Q.; Qian, C.; Wang, H. Characterisation of volatile components of red and sparkling wines from a new wine grape cultivar ‘Meili’ (Vitis vinifera L.). Vitis 2013, 52, 41–48. [Google Scholar]
- Rutan, E.; Herbst-Johnstone, M.; Kilmartin, A. Effect of cluster thinning vitis vinifera cv. Pinot Noir on wine volatile and phenolic composition. J. Agr. Food Chem. 2018, 66, 10053–10066. [Google Scholar] [CrossRef]
- Han, B.; Gao, J.; Han, X.; Deng, H.; Wu, T.; Li, C.; Zhan, J.; Huang, W.; You, Y. Hanseniaspora uvarum FS35 degrade putrescine in wine through the direct oxidative deamination pathway of copper amine oxidase 1. Food Res. Int. 2022, 162, 25–28. [Google Scholar] [CrossRef]
- Fabani, P.; Ravera, J.A.; Wunderlin, A. Markers of typical red wine varieties from the Valley of Tulum (San Juan-Argentina) based on VOCs profile and chemometrics. Food Chem. 2013, 141, 1055–1062. [Google Scholar] [CrossRef]
- Ouyang, X.; Zhu, B.; Liu, R.; Gao, Q.; Lin, G.; Wu, J.; Hu, Z.; Zhang, B. Comparison of volatile composition and color attributes of mulberry wine fermented by different commercial yeasts. J. Food Process. Pres. 2018, 42, 450–453. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, C.; de Revel, G. Esters in wines: New insight through the establishment of a database of French wines. Am. J. Enolo. Viticult. 2014, 65, 293–304. [Google Scholar] [CrossRef]
- Lytra, G.; Tempere, S.; Marchand, S.; de Revel, G.; Barbe, C. How do esters and dimethyl sulphide concentrations affect fruity aroma perception of red wine? Demonstration by dynamic sensory profile evaluation. Food Chem. 2016, 194, 196–200. [Google Scholar] [CrossRef]
- Philipp, C.; Nauer, S.; Sari, S.; Eder, P.; Patzl-Fischerleitner, E.; Eder, R. Quantification of 38 volatile ester compounds by means of SIDA-HS-SPME-GC-MS in ‘Pinot blanc’ wines and comparison with other important Austrian varieties. Mitteilungen Klosterneuburg. 2019, 69, 93–114. [Google Scholar]
- Rossetti, F.; Jouin, A.; Jourdes, M.; Teissedre, L.; Foligni, R.; Longo, E.; Boselli, E. Impact of different stoppers on the composition of red and rose Lagrein, Schiava (Vernatsch) and Merlot wines stored in bottle. Molecules 2020, 25, 4276. [Google Scholar] [CrossRef] [PubMed]
- San Juan, F.; Cacho, J.; Ferreira, V.; Escudero, A. Aroma chemical composition of red wines from different price categories and its relationship to quality. J. Agr. Food Chem. 2012, 60, 5045–5056. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Grana, M.; Oliveira, M. Determination of odorants in varietal wines from international grape cultivars (Vitis vinifera) grown in NW Spain. S. Afr. J. Enol. Viticult. 2013, 34, 212–222. [Google Scholar] [CrossRef] [Green Version]
- Gomez Garcia-Carpintero, E.; Sanchez-Palomo, E.; Gomez Gallego, A.; Gonzalez-Vinas, A. Characterization of impact odorants and sensory profile of Bobal red wines from Spain’s La Mancha region. Flavour. Frag. J. 2012, 27, 60–68. [Google Scholar] [CrossRef]
- Garcia-Carpintero, G.; Sanchez-Palomo, E.; Gomez Gallego, A.; Gonzalez-Vinas, A. Effect of cofermentation of grape varieties on aroma profiles of La Mancha red wines. J. Food Sci. 2011, 76, C1169–C1180. [Google Scholar] [CrossRef]
- Capone, S.; Tufariello, M.; Siciliano, P. Analytical characterisation of Negroamaro red wines by “Aroma Wheels”. Food Chem. 2013, 141, 2906–2915. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, Y.S.; Wen, Y.; Wang, H. Aroma evaluation of young Chinese Merlot wines with denomination of origin. S. Afr. J. Enolo. Viticult. 2013, 34, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, K.; Elmaci, Y.; Ova, G.; Altug, T.; Yuecel, U. Descriptive analysis of red wines from different grape cultivars in turkey. Int. J. Food Prop. 2007, 10, 93–102. [Google Scholar] [CrossRef]
- Koussissi, E.; Paterson, A.; Piggott, R. Sensory flavour discrimination of Greek dry red wines. J. Sci. Food. Agr. 2003, 83, 797–808. [Google Scholar] [CrossRef]
- Saenz-Navajas, P.; Martin-Lopez, C.; Ferreira, V.; Fernandez-Zurbano, P. Sensory properties of premium Spanish red wines and their implication in wine quality perception. Aust. J. Grape Wine R 2011, 17, 9–19. [Google Scholar] [CrossRef]
- Llobodanin, G.; Barroso, P.; Castro, A. Sensory characterization of young South American red wines classified by varietal and origin. J. Food Sci. 2014, 79, S1595–S1603. [Google Scholar] [CrossRef] [PubMed]
- McKay, M.; Buica, A. Factors influencing olfactory perception of selected off-flavour-causing compounds in red wine–A Review. S. Afr. J. Enolo. Viticult. 2020, 41, 56–71. [Google Scholar]
- Cao, W.; Shu, N.; Wen, J.; Yang, Y.; Jin, Y.; Lu, W. Characterization of the key aroma volatile compounds in nine different grape varieties wine by headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS), odor activity values (OAV) and sensory analysis. Foods 2022, 11, 2767. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, A.; Jose Rodriguez-Bencomo, J.; Garrido, I.; Martin-Alvarez, J.; Victoria Moreno-Arribas, M.; Bartolome, B. Assessment of the impact of the addition of antimicrobial plant extracts to wine: Volatile and phenolic composition. J. Sci. Food Agr. 2013, 93, 2507–2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo, C.; Pardo, F.; Zalacain, A.; Alonso, L.; Salinas, R. Differentiation of co-winemaking wines by their aroma composition. Euro Food Res. Techol. 2008, 227, 777–787. [Google Scholar] [CrossRef]
- Pavez, C.; Steinhaus, M.; Casaubon, G.; Schieberle, P.; Agosin, E. Identification, quantitation and sensory evaluation of methyl 2-and methyl 3-methylbutanoate in varietal red wines. Aust. J. Grape Wine Res. 2015, 21, 189–193. [Google Scholar] [CrossRef]
- Radeka, S.; Lukic, I.; Persuric, D. Influence of different maceration treatments on the aroma profile of rose and red wines from croatian aromatic cv. Muskat ruza porecki (Vitis vinifera L.). Food Technol. Biotech. 2012, 50, 442–453. [Google Scholar]
- Sanchez-Palomo, E.; Gomez Garcia-Carpintero, E.; Alonso-Villegas, R.; Gonzalez-Vinas, A. Characterization of aroma compounds of Verdejo white wines from the La Mancha region by odour activity values. Flavour. Frag. J. 2010, 25, 456–462. [Google Scholar] [CrossRef]
- Sanchez-Palomo, E.; Trujillo, M.; Garcia Ruiz, A.; Gonzalez Vinas, A. Aroma profile of malbec red wines from La Mancha region: Chemical and sensory characterization. Food Res. Int. 2017, 100, 201–208. [Google Scholar] [CrossRef] [PubMed]











| Variety | Total Sugar (g/L) | pH | Total Acidity (g/L) | Alcohol % (v/v) |
|---|---|---|---|---|
| MSLG | 229.56 ± 0.16 b | 3.98 ± 0.34 cdef | 6.43 ± 0.02 f | - |
| Y73G | 178.31 ± 0.12 f | 3.63 ± 0.2 fg | 6.43 ± 0.09 f | - |
| MHG | 204.81 ± 0.21 a | 4.11 ± 0.15 bcd | 7.23 ± 0.26 de | - |
| KDKG | 207.23 ± 0.13 d | 4.17 ± 0.24 bc | 7.14 ± 0.43 e | - |
| MLG | 228.06 ± 0.23 c | 4.48 ± 0.11 b | 5.52 ± 0.22 g | - |
| CSG | 166.11 ± 0.34 e | 4.89 ± 0.34 a | 6.31 ± 0.25 f | - |
| CPG | 223.12 ± 0.24 g | 4.07 ± 0.12 cde | 6.52 ± 0.18 f | - |
| MSLW | 1.82 ± 0.07 j | 3.73 ± 0.12 defg | 7.42 ± 0.13 cde | 13.47 ± 0.47 a |
| Y73W | 1.83 ± 0.12 j | 3.47 ± 0.13 g | 6.13 ± 0.35 f | 9.79 ± 0.19 c |
| MHW | 1.81 ± 0.14 j | 3.97 ± 0.24 cdef | 7.56 ± 0.31 bcd | 11.35 ± 0.63 b |
| KDKW | 3.12 ± 0.34 h | 3.95 ± 0.13 cdef | 7.69 ± 0.03 bc | 12.18 ± 0.77 b |
| MLW | 2.31 ± 0.16 i | 3.48 ± 0.21 g | 7.92 ± 0.12 ab | 13.42 ± 0.62 a |
| CSW | 2.89 ± 0.06 h | 3.67 ± 0.25 efg | 8.11 ± 0.14 a | 8.52 ± 0.42 d |
| CPW | 1.64 ± 0.11 j | 3.81 ± 0.15 cdefg | 7.31 ± 0.09 cde | 13.29 ± 0.52 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, Y.; Wang, H.; Xu, X.; Ye, P.; Wu, H.; Zhao, R.; Shi, X.; Cai, F. Chemical and Sensory Characteristics of Different Red Grapes Grown in Xinjiang, China: Insights into Wines Composition. Fermentation 2022, 8, 689. https://doi.org/10.3390/fermentation8120689
Miao Y, Wang H, Xu X, Ye P, Wu H, Zhao R, Shi X, Cai F. Chemical and Sensory Characteristics of Different Red Grapes Grown in Xinjiang, China: Insights into Wines Composition. Fermentation. 2022; 8(12):689. https://doi.org/10.3390/fermentation8120689
Chicago/Turabian StyleMiao, Yuanyuan, Huan Wang, Xiaoyu Xu, Piping Ye, Huimin Wu, Ruirui Zhao, Xuewei Shi, and Fei Cai. 2022. "Chemical and Sensory Characteristics of Different Red Grapes Grown in Xinjiang, China: Insights into Wines Composition" Fermentation 8, no. 12: 689. https://doi.org/10.3390/fermentation8120689