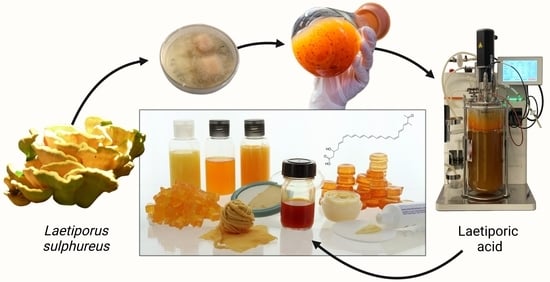
Pilot-Scale Production of the Natural Colorant Laetiporic Acid, Its Stability and Potential Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species and Media Preparation
2.2. Cultivation Parameters
2.3. In Situ Product Recovery
2.4. Analysis of Pigment Production
2.5. Stability Trials
2.6. Application of Laetiporic Acids
3. Results
3.1. Production Strain
3.2. Fermentation Experiments
3.3. In Situ Product Recovery
3.4. Pigment Stability
3.5. Color Fastness of Dyed Silk
3.6. Application in Various Matrices
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fried, R.; Oprea, I.; Fleck, K.; Rudroff, F. Biogenic colourants in the textile industry—A promising and sustainable alternative to synthetic dyes. Green Chem. 2022, 24, 13–35. [Google Scholar] [CrossRef]
- Sundström, C.; Sundström, E. Mit Pilzen Färben: Eine Fundgrube für Kunstgewerbler, Pilzsammler und Naturfreunde; Orell Füssli: Zürich, Switzerland, 1984; ISBN 3280014735. [Google Scholar]
- Tegeler, K.; Deutsche Gesellschaft für Mykologie e., V. Leitfaden zum Färben mit Pilzen, 2nd ed.; Josef Maria Christan: München, Germany, 2016. [Google Scholar]
- Hassan, K.; Kemkuignou, B.M.; Stadler, M. Two new triterpenes from basidiomata of the medicinal and edible mushroom, Laetiporus sulphureus. Molecules 2021, 26, 7090. [Google Scholar] [CrossRef]
- Klaus, A.; Kozarski, M.; Niksic, M.; Jakovljevic, D.; Todorovic, N.; Stefanoska, I.; van Griensven, L.J.L.D. The edible mushroom Laetiporus sulphureus as potential source of natural antioxidants. Int. J. Food Sci. Nutr. 2013, 64, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J. Will the sulphur polypore (Laetiporus sulphureus) become a new functional food? Glob. J. Med. Clin. Case Rep. 2019, 6, 6–9. [Google Scholar] [CrossRef] [Green Version]
- Turkoglu, A.; Duru, M.E.; Mercan, N.; Kivrak, I.; Gezer, K. Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem. 2007, 101, 267–273. [Google Scholar] [CrossRef]
- Petrović, J.; Glamočlija, J.; Stojković, D.S.; Ćirić, A.; Nikolić, M.; Bukvički, D.; Guerzoni, M.E.; Soković, M.D. Laetiporus sulphureus, edible mushroom from Serbia: Investigation on volatile compounds, in vitro antimicrobial activity and in situ control of Aspergillus flavus in tomato paste. Food Chem. Toxicol. 2013, 59, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Petrović, J.; Stojković, D.; Reis, F.S.; Barros, L.; Glamočlija, J.; Ćirić, A.; Ferreira, I.C.F.R.; Soković, M. Study on chemical, bioactive and food preserving properties of Laetiporus sulphureus (Bull.: Fr.) Murr. Food Funct. 2014, 5, 1441–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, R.W.; Mucci, A.; Davoli, P. Laetiporic acid, a new polyene pigment from the wood-rotting basidiomycete Laetiporus sulphureus (Polyporales, Fungi). Tetrahedron Lett. 2004, 45, 1075–1078. [Google Scholar] [CrossRef]
- Davoli, P.; Mucci, A.; Schenetti, L.; Weber, R.W.S. Laetiporic acids, a family of non-carotenoid polyene pigments from fruit-bodies and liquid cultures of Laetiporus sulphureus (Polyporales, Fungi). Phytochemistry 2005, 66, 817–823. [Google Scholar] [CrossRef]
- Valadon, L.R.G.; Mummery, R.S. A new carotenoid from Laetiporus sulphureus. Ann. Bot. 1969, 33, 879–882. [Google Scholar] [CrossRef]
- Seibold, P.S.; Lenz, C.; Gressler, M.; Hoffmeister, D. The Laetiporus polyketide synthase LpaA produces a series of antifungal polyenes. J. Antibiot. 2020, 73, 711–720. [Google Scholar] [CrossRef]
- Pleszczyńska, M.; Wiater, A.; Siwulski, M.; Szczodrak, J. Successful large-scale production of fruiting bodies of Laetiporus sulphureus (Bull.: Fr.) Murrill on an artificial substrate. World J. Microbiol. Biotechnol. 2013, 29, 753–758. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Zorn, H.; Krings, U.; Berger, R.G. Characteristic volatiles from young and aged fruiting bodies of wild Polyporus sulfureus (Bull.:Fr.) Fr. J. Agric. Food Chem. 2005, 53, 4524–4528. [Google Scholar] [CrossRef]
- Methodensammlung der Bund/Länder-AG Gentechnik (LAG): Molekularbiologische Identifizierung von Pilzen mittels ITS-PCR und nachfolgender Sequenzierung. J. Verbr. Lebensm. 2012, 7, 71–76. [CrossRef]
- Sprecher, E. Über die guttation bei pilzen. Planta 1959, 53, 565–574. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Zschätzsch, M.; Steudler, S.; Reinhardt, O.; Bergmann, P.; Ersoy, F.; Stange, S.; Wagenführ, A.; Walther, T.; Berger, R.G.; Werner, A. Production of natural colorants by liquid fermentation with Chlorociboria aeruginascens and Laetiporus sulphureus and prospective applications. Eng. Life Sci. 2021, 21, 270–282. [Google Scholar] [CrossRef]
- Bergmann, P.; Takenberg, M.; Frank, C.; Zschätzsch, M.; Werner, A.; Berger, R.G.; Ersoy, F. Cultivation of Inonotus hispidus in stirred tank and wave bag bioreactors to produce the natural colorant hispidin. Fermentation 2022, 8, 541. [Google Scholar] [CrossRef]
- Deutsches Institut für Normung, e.V. Textilien—Farbechtheitsprüfungen—Teil C06: Farbechtheit bei der Haushaltswäsche und der Gewerblichen Wäsche; Beuth Verlag GmbH: Berlin, Germany, 2010. [Google Scholar]
- Deutsches Institut für Normung, e.V. Farbmetrik—Teil 4: CIE 1976 L*a*b* Farbraum; Beuth Verlag GmbH: Berlin, Germany, 2020. [Google Scholar]
- Dresch, P.; Aguanno, M.N.D.; Rosam, K.; Grienke, U.; Rollinger, J.M.; Peintner, U. Fungal strain matters: Colony growth and bioactivity of the European medicinal polypores Fomes fomentarius, Fomitopsis pinicola and Piptoporus betulinus. AMB Express 2015, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suay, I.; Arenal, F.; Asensio, F.J.; Basilio, A.; Cabello, M.A.; Díez, M.T.; García, J.B.; del Val, A.G.; Gorrochategui, J.; Hernández, P.; et al. Screening of basidiomycetes for antimicrobial activities. Antonie Van Leeuwenhoek 2000, 78, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Czuryło, A.; Frąc, M.; Rola, B.; Sulej, J.; Pawlik, A.; Siwulski, M.; Rogalski, J. Laccase production and metabolic diversity among Flammulina velutipes strains. World J. Microbiol. Biotechnol. 2015, 31, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Krahe, N.-K.; Berger, R.G.; Witt, M.; Zorn, H.; Omarini, A.B.; Ersoy, F. Monokaryotic pleurotus sapidus strains with intraspecific variability of an alkene cleaving DyP-type peroxidase activity as a result of gene mutation and differential gene expression. Int. J. Mol. Sci. 2021, 22, 1363. [Google Scholar] [CrossRef] [PubMed]
- Luangharn, T.; Karunarathna, S.C.; Hyde, K.D.; Chukeatirote, E. Optimal conditions of mycelia growth of Laetiporus sulphureus sensu lato. Mycology 2014, 5, 221–227. [Google Scholar] [CrossRef]
- Hwang, H.S.; Lee, S.H.; Baek, Y.M.; Kim, S.W.; Jeong, Y.K.; Yun, J.W. Production of extracellular polysaccharides by submerged mycelial culture of Laetiporus sulphureus var. miniatus and their insulinotropic properties. Appl. Microbiol. Biotechnol. 2008, 78, 419–429. [Google Scholar] [CrossRef]
- Sun, L.; Kwak, S.; Jin, Y.-S. Vitamin a production by engineered saccharomyces cerevisiae from xylose via two-phase in situ extraction. ACS Synth. Biol. 2019, 8, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Grosse, M.; Strauss, E.; Krings, U.; Berger, R.G. Response of the sesquiterpene synthesis in submerged cultures of the basidiomycete tyromyces floriformis to the medium composition. Mycologia 2019, 111, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Oudshoorn, A.; Steinbusch, K.J.J.; Kerste, R.W.H.; Woolner, D. Integrated System for Biocatalytically Producing and Recovering an Organic Substance. U.S. Patent 2022/0275323 A1, 1 September 2022. [Google Scholar]
- Räisänen, R.; Primetta, A.; Nikunen, S.; Honkalampi, U.; Nygren, H.; Pihlava, J.-M.; Berghe, I.V.; von Wright, A. Examining safety of biocolourants from fungal and plant sources-examples from cortinarius and tapinella, salix and Tanacetum spp. and dyed woollen fabrics. Antibiotics 2020, 9, 266. [Google Scholar] [CrossRef]






| Label | Dye | WOF 1 | Ethanol | Curing |
|---|---|---|---|---|
| a | L. sulphureus, freeze-dried biomass | 20% | 1% | - |
| b | L. sulphureus, freeze-dried biomass | 20% | 1% | vinegar |
| c | L. sulphureus, freeze-dried biomass | 20% | 30% | - |
| d | L. sulphureus, wet biomass | 20% | 1% | - |
| e | Dyer’s madder | 50% | - | - |
| f | Simplicol textile dye | 40% | - | - |
| g | Industrially dyed silk | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergmann, P.; Frank, C.; Reinhardt, O.; Takenberg, M.; Werner, A.; Berger, R.G.; Ersoy, F.; Zschätzsch, M. Pilot-Scale Production of the Natural Colorant Laetiporic Acid, Its Stability and Potential Applications. Fermentation 2022, 8, 684. https://doi.org/10.3390/fermentation8120684
Bergmann P, Frank C, Reinhardt O, Takenberg M, Werner A, Berger RG, Ersoy F, Zschätzsch M. Pilot-Scale Production of the Natural Colorant Laetiporic Acid, Its Stability and Potential Applications. Fermentation. 2022; 8(12):684. https://doi.org/10.3390/fermentation8120684
Chicago/Turabian StyleBergmann, Pia, Christina Frank, Olena Reinhardt, Meike Takenberg, Anett Werner, Ralf G. Berger, Franziska Ersoy, and Marlen Zschätzsch. 2022. "Pilot-Scale Production of the Natural Colorant Laetiporic Acid, Its Stability and Potential Applications" Fermentation 8, no. 12: 684. https://doi.org/10.3390/fermentation8120684