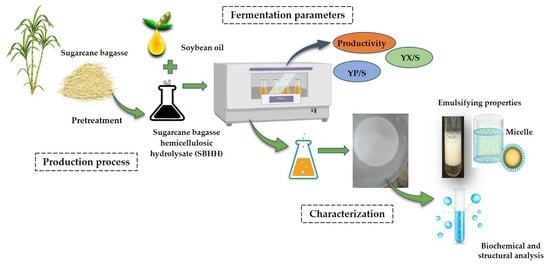
Production, Physicochemical and Structural Characterization of a Bioemulsifier Produced in a Culture Medium Composed of Sugarcane Bagasse Hemicellulosic Hydrolysate and Soybean Oil in the Context of Biorefineries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Sugarcane Bagasse Hemicellulosic Hydrolysate
2.3. Maintenance of Microorganisms
2.4. Inoculum Preparation with the S. shehatae 16-BR6-2AI
2.5. Bioemulsifier Production by S. shehatae 16-BR6-2AI
2.6. Bioemulsifier Isolation Produced by S. shehatae 16-BR6-2AI
2.7. Biochemical Characterization of the Bioemulsifier Produced by S. shehatae 16-BR6-2AI
2.8. Physicochemical Characterization of the Purified and Lyophilized Bioemulsifier Produced by S. shehatae 16-BR6-2AI
2.8.1. Molecular Weight
2.8.2. Elemental Analysis of Carbon (C), Hydrogen (H), Oxygen (O), and Nitrogen (N)
2.8.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.8.4. Nuclear Magnetic Resonance (1H NMR and 13C NMR)
2.8.5. Gas Chromatography and Mass Spectroscopy
2.8.6. X-ray Diffraction
2.8.7. Thermal Analysis of the Bioemulsifier
2.8.8. Zeta Potential
2.9. Effect of Bioemulsifier Concentration on the Stability of the Emulsion
2.10. Turbidity Measurement
2.11. Evaluation of Emulsifying Properties in Different Hydrophobic Substrates, Temperatures, pH Values, and Salinity
2.12. Emulsifying and Tensioactive Properties of Bioemulsifier Produced by S. shehatae 16-BR6-2AI
3. Results and Discussion
3.1. Characterization of Sugarcane Bagasse Hemicellulosic Hydrolysate
3.2. Bioemulsifier Production by S. shehatae 16-BR6-2AI in SBHH Combined with Soybean Oil
3.3. Isolation of Bioemulsifier Produced by S. shehatae 16-BR6-2AI in SBHH Combined with Soybean Oil
3.4. Biochemical Characterization of Bioemulsifier Produced by S. shehatae 16-BR6-2AI in SBHH Combined with Soybean Oil
3.5. Physicochemical Characterization of Bioemulsifier Produced by S. shehatae 16-BR6-2AI
3.6. Studies of the Emulsifying Properties of the Bioemulsifier Produced by S. shehatae
3.6.1. Evaluation of BE Concentration in the Emulsifying Property
3.6.2. Evaluation of Emulsifying Properties with Varied Hydrophobic Substrates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCBs | polychlorinated biphenyls |
| PAHs | polynuclear aromatic hydrocarbons |
| GRAS | generally recognized as safe |
| BE | bioemulsifiers |
| C-5 | 5 carbon carbs |
| C-6 | 6 carbon carbs |
| SBHH | sugarcane bagasse hemicellulosic hydrolysate |
| HPLC | high-performance liquid chromatography |
| YMA | yeast malt agar |
| EI24 | emulsifying index |
| ST | surface tension |
| PEO | polyethylene oxide |
| HMF | hydroxymethylfufural |
| Mw | average molecular mass |
| Tm | melting temperature |
| CMC | critical micelle concentration |
| FTIR | Fourier transform infrared spectroscopy |
| CG-MS | gas chromatography–mass spectrometry |
| TGA | thermos gravimetric analysis |
| DSC | differential scanning calorimetric |
| 1H NMR | 1H nuclear magnetic resonance |
| 13C NMR | 13C nuclear magnetic resonance |
| YX/S | yield coefficient of cell growth per substrate |
| YP/S | product yield coefficient per substrate |
| BSA | bovine serum albumin |
| GPC | gel permeation chromatography |
References
- Torres, E.M.M. A evolução da indústria petroquímica brasileira. Quím. Nova 1997, 20, 49–54. [Google Scholar] [CrossRef]
- Lara-Flores, A.A.; Araújo, R.G.; Rodríguez-Jasso, R.M.; Aguedo, M.; Aguilar, C.N.; Trajano, H.L.; Ruiz, H.A. Bioeconomy and biorefinery: Valorization of hemicellulose from lignocellulosic biomass and potential use of avocado residues as a promising resource of bioproducts. In Waste to Wealth, 2018th ed.; Singhania, R.R., Agarwal, R.A., Kumar, R.P., Sukumaran, R.K., Eds.; Springer: Singapore, 2018; Volume 1, pp. 141–170. [Google Scholar]
- Conteratto, C.; Artuzo, F.D.; Santos, O.I.B.; Talamini, E. Biorefinery: A comprehensive concept for the sociotechnical transition toward bioeconomy. Renew. Sustain. Energy Rev. 2021, 151, 111527. [Google Scholar] [CrossRef]
- Djedri, S.; Issaadi, R.; Cerf, D.L.; Picton, L.; Moulai-Mostefa, N. Surfactants synthesis using petroleum fractions and crude oil: Application in microemulsion formulation. J. Dispers. Sci. Technol. 2010, 31, 877–882. [Google Scholar] [CrossRef]
- Jiménez-Peñalver, P.; Rodríguez, A.; Daverey, A.; Font, X.; Gea, T. Use of wastes for sophorolipids production as a transition to circular economy: State of the art and perspectives. Rev. Environ. Sci. Biotechnol. 2019, 18, 413–435. [Google Scholar] [CrossRef]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Malik, S.; Ghosh, A.; Saha, R.; Saha, B. A review on natural surfactants. RSC Adv. 2015, 5, 65757–65767. [Google Scholar] [CrossRef]
- Barbosa, F.G.; Ribeaux, D.R.; Rocha, T.; Costa, R.A.; Guzmán, R.R.; Marcelino, P.R.; Lacerda, T.M.; Silva, S.S. Biosurfactants: Sustainable and Versatile Molecules. J. Braz. Chem. Soc. 2022, 33, 870–893. [Google Scholar] [CrossRef]
- Jimoh, A.A.; Lin, J. Enhancement of Paenibacillus sp. D9 lipopeptide biosurfactant production through the optimization of medium composition and its application for biodegradation of hydrophobic pollutants. Appl. Biochem. Biotechnol. 2019, 187, 724–743. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Koul, Y.; Varjani, S.; Pandey, A.; Ngo, H.H.; Chang, J.S.; Wong, J.W.C.; Bui, X.T. A critical review on various feedstocks as sustainable substrates for biosurfactants production: A way towards cleaner production. Microb. Cell Factories 2021, 20, 120. [Google Scholar] [CrossRef]
- Campos-Takaki, G.M.; Sarubbo, L.A.; Albuquerque, C.D.C. Environmentally friendly biosurfactants produced by yeasts. In Biosurfactants, 1st ed.; Sen, R., Ed.; Springer: New York, NY, USA, 2010; Volume 672, pp. 250–260. [Google Scholar]
- Liepins, J.; Balina, K.; Soloha, R.; Berzina, I.; Lukasa, L.K.; Dace, E. Glycolipid biosurfactant production from waste cooking oils by yeast: Review of substrates, producers and products. Fermentation 2021, 7, 136. [Google Scholar] [CrossRef]
- Fenibo, E.O.; Douglas, S.I.; Stanley, H.O. A review on microbial surfactants: Production, classifications, properties and characterization. J. Adv. Microbiol. 2019, 18, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef] [PubMed]
- Amaral, P.F.; Coelho, M.A.Z.; Marrucho, I.M.; Coutinho, J.A. Biosurfactants, 1st ed.; Sen, R., Ed.; Springer: New York, NY, USA, 2010; Volume 672, pp. 236–249. [Google Scholar]
- Jain, R.M.; Mody, K.; Joshi, N.; Mishra, A.; Jha, B. Production and structural characterization of biosurfactant produced by an alkaliphilic bacterium, Klebsiella sp.: Evaluation of different carbon sources. Colloids Surf. B 2013, 108, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Franco Marcelino, P.R.; da Silva, V.L.; Rodrigues Philippini, R.; Von Zuben, C.J.; Contiero, J.; Dos Santos, J.C.; da Silva, S.S. Biosurfactants produced by Scheffersomyces stipitis cultured in sugarcane bagasse hydrolysate as new green larvicides for the control of Aedes aegypti, a vector of neglected tropical diseases. PLoS ONE 2017, 12, e0187125. [Google Scholar] [CrossRef] [Green Version]
- Marcelino, P.R.F.; Gonçalves, F.; Jimenez, I.M.; Carneiro, B.C.; Santos, B.B.; da Silva, S.S. Sustainable production of biosurfactants and their applications. In Lignocellulosic Biorefining Technologies, 1st ed.; Ingle, A.P., Chandel, A.K., da Silva, S.S., Eds.; John Wiley & Sons Ltd.: Noida, India, 2020; pp. 159–183. [Google Scholar] [CrossRef]
- Asgher, M.; Afzal, M.; Qamar, S.A.; Khalid, N. Optimization of biosurfactant production from chemically mutated strain of Bacillus subtilis using waste automobile oil as low-cost substrate. Environ. Sustain. 2020, 3, 405–413. [Google Scholar] [CrossRef]
- Zambry, N.S.; Rusly, N.S.; Awang, M.S.; Md Noh, N.A.; Yahya, A.R.M. Production of lipopeptide biosurfactant in batch and fed-batch Streptomyces sp. PBD-410L cultures growing on palm oil. Bioprocess Biosyst. Eng. 2021, 44, 1577–1592. [Google Scholar] [CrossRef]
- Chandel, A.K.; Antunes, F.A.; Anjos, V.; Bell, M.J.; Rodrigues, L.N.; Polikarpov, I.; de Azevedo, E.R.; Bernardinelli, O.D.; Rosa, C.A.; Pagnocca, F.C.; et al. Multi-scale structural and chemical analysis of sugarcane bagasse in the process of sequential acid–base pretreatment and ethanol production by Scheffersomyces shehatae and Saccharomyces cerevisiae. Biotechnol. Biofuels 2014, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Canilha, L.; Chandel, A.K.; Milessi, T.S.S.; Antunes, F.A.F.; Freitas, W.L.C.; Felipe, M.G.A.; da Silva, S.S. Bioconversion of sugarcane biomass into ethanol: An overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J. Biotechnol. Biomed. 2012, 2012, 989572. [Google Scholar] [CrossRef] [Green Version]
- Marcelino, P.R.F.; Peres, G.F.D.; Terán-Hilares, R.; Pagnocca, F.C.; Rosa, C.A.; Lacerda, T.M.; dos Santos, J.C.; Da Silva, S.S. Biosurfactants production by yeasts using sugarcane bagasse hemicellulosic hydrolysate as new sustainable alternative for lignocellulosic biorefineries. Ind. Crops Prod. 2019, 129, 212–223. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Kitamoto, D.; Akiba, S.; Hioki, C.; Tabuchi, T. Extracellular accumulation of mannosylerythritol lipids by a strain of Candida antarctica. Agric. Biol. Chem. 1990, 54, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Kuiper, I.; Lagendijk, E.L.; Pickford, R.; Derrick, J.P.; Lamers, G.E.M.; Thomas-Oates, J.E.; Lugtenberg, B.J.J.; Bloemberg, G.V. Characterization of two Pseudomonas putida lipopeptide biosurfactants, putisolvin I and II, which inhibit biofilm formation and break down existing biofilms. Mol. Microbiol. 2004, 51, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.J.P.; Perfumo, A.; McClean, S.; Marchant, R.; Banat, I.M. Isolation and analysis of lipopeptides and high molecular weight biosurfactants. In Handbook of Hydrocarbon and Lipid Microbiology, 1st ed.; Timmis, K.N., Ed.; Springer: Berlin, Germany, 2010; Volume 1, pp. 3688–3704. [Google Scholar]
- Amaral, P.F.F.; da Silva, J.M.; Lehocky, M.; Barros-Timmons, A.M.V.; Coelho, M.A.Z.; Marrucho, I.M.; Coutinho, J.A.P. Production and characterization of a bioemulsifier from Yarrowia lipolytica. Process. Biochem. 2006, 41, 1894–1898. [Google Scholar] [CrossRef]
- Rufino, R.D.; de Luna, J.M.; Campos-Takaki, G.M.; Sarubbo, L.A. Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron. J. Biotechnol. 2014, 17, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, A.S.; Coutinho, J.O.P.A.; Júnior, A.C.; Rosa, C.A.; Siqueira, E.P.; Santos, V.L. Characterization of new biosurfactant produced by Trichosporon montevideense CLOA 72 isolated from dairy industry effluents. J. Basic. Microbiol. 2009, 49, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Izard, J.; Limberger, R.J. Rapid screening method for quantitation of bacterial cell lipids from whole cells. J. Microbiol. Methods 2003, 55, 411–418. [Google Scholar] [CrossRef]
- Dikit, P.; Maneerat, S.; Saimmai, A. The effective emulsifying property of biosurfactant–producing Marinobacter hydrocarbonoclasticus ST1 obtained from palm oil contaminated sites. Appl. Biochem. Microbiol. 2019, 55, 615–625. [Google Scholar] [CrossRef]
- Nogueira, I.B.; Rodríguez, D.M.; da Silva Andradade, R.F.; Lins, A.B.; Bione, A.P.; da Silva, I.G.S.; Franco, L.O.; de Campos-Takaki, G.M. Bioconversion of agroindustrial waste in the production of bioemulsifier by Stenotrophomonas maltophilia UCP 1601 and application in bioremediation process. Int. J. Chem. Eng. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Chandankere, R.; Yao, J.; Cai, M.; Masakorala, K.; Jain, A.K.; Choi, M.M. Properties and characterization of biosurfactant in crude oil biodegradation by bacterium Bacillus methylotrophicus USTBa. Fuel 2014, 122, 140–148. [Google Scholar] [CrossRef]
- Morita, T.; Fukuoka, T.; Konishi, M.; Imura, T.; Yamamoto, S.; Kitagawa, M.; Sogabe, A.; Kitamoto, D. Production of a novel glycolipid biosurfactant, mannosylmannitol lipid, by Pseudozyma parantarctica and its interfacial properties. Appl. Microbiol. Biotechnol. 2009, 83, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Ohadi, M.; Dehghannoudeh, G.; Forootanfar, H.; Shakibaie, M.; Rajaee, M. Investigation of the structural, physicochemical properties, and aggregation behavior of lipopeptide biosurfactant produced by Acinetobacter junii B6. Int. J. Biol. Macromol. 2018, 112, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Bharucha, C.; Jha, S.; Yadav, S.; Nerurkar, A.; Desai, A.J. Biosurfactant production using molasses and whey under thermophilic conditions. Bioresour. Technol. 2008, 99, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.G.; Goldenberg, B.G. Surface-active agents from two Bacillus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef] [Green Version]
- Gírio, F.M.; Fonseca, C.; Carvalheiro, F.; Duarte, L.C.; Marques, S.; Lukasik, R.M. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef]
- Yu, X.; Li, X.; Jin, T.; Su, Y.; Li, J.; Gao, M.; Zheng, L.; Tan, S.; Chen, G. Integrated biorefinery process for production of sophorolipids from corn straw. Biochem. Eng. J. 2021, 167, 107908. [Google Scholar] [CrossRef]
- Martiniano, S.E.; Chandel, A.K.; Soares, L.C.; Pagnocca, F.C.; da Silva, S.S. Evaluation of novel xylose-fermenting yeast strains from Brazilian forests for hemicellulosic ethanol production from sugarcane bagasse. 3 Biotech. 2013, 3, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Bonan, C.I.; Biazi, L.E.; Santos, S.C.; Soares, L.B.; Dionísio, S.R.; Hoffmam, Z.B.; Costa, A.C.; Ienczak, J.L. Online monitoring of the redox potential in microaerobic and anaerobic Scheffersomyces stipitis fermentations. Biotechnol. Lett. 2019, 41, 753–761. [Google Scholar] [CrossRef]
- Thraeib, J.Z.; Altemimi, A.B.; Jabbar Abd Al-Manhel, A.; Abedelmaksoud, T.G.; El-Maksoud, A.A.A.; Madankar, C.S.; Cacciola, F. Production and Characterization of a Bioemulsifier Derived from Microorganisms with Potential Application in the Food Industry. Life 2022, 12, 924. [Google Scholar] [CrossRef]
- da Silva, J.F.; da Silva, L.A.R.; Barbosa, M.R.; Houllou, L.M.; Malafaia, C.B. Bioemulsifier produced by Yarrowia lipolytica using residual glycerol as a carbon source. J. Env. Anal. Progr. 2020, 5, 031–037. [Google Scholar] [CrossRef]
- Satpute, S.K.; Banpurkar, A.G.; Dhakephalkar, P.K.; Banat, I.M.; Chopade, B.A. Methods for investigating biosurfactants and bioemulsifiers: A review. Crit. Rev. Biotechnol. 2010, 30, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Pasa, K.C. Recovery of biosurfactant produced by Kluyveromyces marxianus. Trabalho de Conclusão de Curso, Universidade Tecnológica Federal do Paraná, Paraná, Brazil, 2019. Available online: https://repositorio.utfpr.edu.br/jspui/handle/1/11466 (accessed on 29 September 2022).
- Jones, O.G.; McClements, D.J. Functional biopolymer particles: Design, fabrication, and applications. Compr. Rev. Food Sci. Food Saf. 2010, 9, 374–397. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, L.A.; Marçal, M.C.; Neves, M.L.C.; Silva, M.P.C.; Porto, L.F.; Campos-Takaki, G.M. Bioemulsifier production in batch culture using glucose as carbon source by Candida lipolytica. Appl. Biochem. Biotechnol. 2001, 95, 59–67. [Google Scholar] [CrossRef]
- Bhaumik, M.; Dhanarajan, G.; Chopra, J.; Kumar, R.; Hazra, C.; Sen, R. Production, partial purification and characterization of a proteoglycan bioemulsifier from an oleaginous yeast. Bioprocess Biosyst. Eng. 2020, 43, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Sen, I.K.; Mandal, A.K.; Chakraborty, R.; Behera, B.; Yadav, K.K.; Maiti, T.K.; Islam, S.S. Structural and immunological studies of an exopolysaccharide from Acinetobacter junii BB1A. Carbohydr. Polym. 2014, 101, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Uzoigwe, C.; Burguess, J.G.; Ennis, C.J.; Rahman, P.K.S.M. Bioemulsifiers are not biosurfactants and require different screening approaches. Front. Microbiol. 2015, 6, 245. [Google Scholar] [CrossRef] [Green Version]
- Cirigliano, M.C.; Carman, G.M. Purification and Characterization of Liposan, a Bioemulsifier from Candida lipolytica. Appl Environ Microbiol. 1985, 50, 846–850. [Google Scholar] [CrossRef] [Green Version]
- Campos, J.M.; Stamford, T.L.M.; Sarubbo, L.A. Characterization and application of a biosurfactant isolated from Candida utilis in salad dressings. Biodegradation 2019, 30, 313–324. [Google Scholar] [CrossRef]
- Vera, E.C.S.; de Azevedo, P.O.D.S.; Domínguez, J.M.; de Souza Oliveira, R.P. Optimization of biosurfactant and bacteriocin-like inhibitory substance (BLIS) production by Lactococcus lactis CECT-4434 from agroindustrial waste. Biochem. Eng. J. 2018, 133, 168–178. [Google Scholar] [CrossRef]
- Vecino, X.; Barbosa-Pereira, L.; Devesa-Rey, R.; Cruz, J.M.; Moldes, A.B. Optimization of extraction conditions and fatty acid characterization of Lactobacillus pentosus cell-bound biosurfactant/bioemulsifier. J. Sci. Food Agric. 2015, 95, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Thavasi, R.; Jayalakshmi, S.; Balasubramanian, T.; Banat, I.M. Biosurfactant production by Corynebacterium kutscheri from waste motor lubricant oil and peanut oil cake. Lett. Appl. Microbiol. 2007, 45, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Gil, C.V.; Rebocho, A.T.; Esmail, A.; Sevrin, C.; Grandfils, C.; Torres, C.A.; Reis, M.A.M.; Freitas, F. Characterization of the Thermostable Biosurfactant Produced by Burkholderia thailandensis DSM 13276. Polymers 2022, 14, 2088. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Chen, L.Y.; Tian, Z.J.; Sun, Y.; Liu, J.B.; Huang, L. Characterization and application of a novel bioemulsifier in crude oil degradation by Acinetobacter beijerinckii ZRS. J. Basic Microbiol. 2016, 56, 184–195. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Pereira, J.F.B.; Costa, R.; Evtuguin, D.V.; Coutinho, J.A.P.; Teixeira, J.A.; Rodrigues, L.R. Novel bioemulsifier produced by a Paenibacillus strain isolated from crude oil. Microb. Cell Fact. 2015, 14, 14. [Google Scholar] [CrossRef] [Green Version]
- Beltrani, T.; Chiavarini, S.; Cicero, D.O.; Grimaldi, M.; Ruggeri, C.; Tamburini, E.; Cremisini, C. Chemical characterization and surface properties of a new bioemulsifier produced by Pedobacter sp. strain MCC-Z. Int. J. Biol. Macromol. 2015, 72, 1090–1096. [Google Scholar] [CrossRef]
- Kourmentza, C.; Araujo, D.; Sevrin, C.; Roma-Rodriques, C.; Ferreira, J.L.; Freitas, F.; Dionisio, M.; Baptista, P.V.; Fernandes, A.R.; Grandfils, C.; et al. Occurrence of non-toxic bioemulsifiers during polyhydroxyalkanoate production by Pseudomonas strains valorizing crude glycerol by-product. Bioresour. Technol. 2019, 281, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Ramani, K.; Jain, C.; Mandal, A.B.; Sekaran, G. Microbial induced lipoprotein biosurfactant from slaughterhouse lipid waste and its application to the removal of metal ions from aqueous solution. Colloids Surf. B Biointerfaces 2012, 97, 254–263. [Google Scholar] [CrossRef]
- Saranya, P.; Swarnalatha, S.; Sekaran, G. Lipoprotein biosurfactant production from an extreme acidophile using fish oil and its immobilization in nanoporous activated carbon for the removal of Ca2+ and Cr3+ in aqueous solution. RSC Adv. 2014, 4, 34144–34155. [Google Scholar] [CrossRef]
- Tokle, T.; McClements, D.J. Physicochemical properties of lactoferrin stabilized oil-in-water emulsions: Effects of pH, salt and heating. Food Hydrocoll. 2011, 25, 976–982. [Google Scholar] [CrossRef]
- Guzey, D.; McClements, D.J. Formation, stability and properties of multilayer emulsions for application in the food industry. Adv. Colloid Interface Sci. 2006, 128–130, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Mnif, I.; Ghribi, D. High molecular weight bioemulsifiers, main properties and potential environmental and biomedical applications. World J. Microbiol. Biotechnol. 2015, 31, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Ravera, F.; Dziza, K.; Santini, E.; Cristofolini, L.; Liggieri, L. Emulsification and emulsion stability: The role of the interfacial properties. Adv. Colloid Interface Sci. 2021, 288, 102344. [Google Scholar] [CrossRef] [PubMed]
- Lukondeh, T.; Ashbolt, N.J.; Rogers, P.L. Evaluation of Kluyveromyces marxianus FII 510700 grown on a lactose-based medium as a source of a natural bioemulsifier. J. Ind. Microbiol. Biotechnol. 2003, 30, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Domingues, V.; Monteiro, A.S.; Freitas, G.; Santos, V.L. Solid flocculation and emulsifying activities of the lipopolysaccharide produced by Trichosporon mycotoxinivorans CLA2. Appl. Biochem. Biotechnol. 2017, 182, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.S.; Cameotra, S.S. An update on the use of unconventional substrates for biosurfactant production and their new applications. Appl. Microbiol. Biotechnol. 2002, 58, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. Lipopeptides: From self-assembly to bioactivity. Chem. Comm. 2015, 51, 8574–8583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, M.; Aranda, F.J.; Espuny, M.J.; Marqués, A.; Teruel, J.A.; Manresa, A.; Ortiz, A. Aggregation behaviour of a dirhamnolipid biosurfactant secreted by Pseudomonas aeruginosa in aqueous media. J. Colloid Interface Sci. 2007, 307, 246–253. [Google Scholar] [CrossRef]
- Pornsunthorntawee, O.; Chavadej, S.; Rujiravanit, R. Solution properties and vesicle formation of rhamnolipid biosurfactants produced by Pseudomonas aeruginosa SP4. Colloids Surf. B Biointerfaces 2009, 72, 6–15. [Google Scholar] [CrossRef]
- Rufino, R.D.; Sarubbo, L.A.; Campos-Takaki, G.M. Enhancement of stability of biosurfactant produced by Candida lipolytica using industrial residue as substrate. World J. Microbiol. Biotechnol. 2007, 23, 729–734. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Olaniran, A.O. Production and characterization of bioemulsifiers from Acinetobacter strains isolated from lipid-rich wastewater. 3 Biotech. 2019, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Durval, I.J.; Ribeiro, B.G.; Aguiar, J.S.; Rufino, R.D.; Converti, A.; Sarubbo, L.A. Application of a Biosurfactant Produced by Bacillus cereus UCP 1615 from Waste Frying Oil as an Emulsifier in a Cookie Formulation. Fermentation 2021, 7, 189. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Salgueiro, A.A.; Sarubbo, L.A. Synthesis and evaluation of biosurfactant produced by Candida lipolytica using animal fat and corn steep liquor. J. Pet. Sci. Eng. 2013, 105, 43–50. [Google Scholar] [CrossRef]
- Bergmann, D. Cosmetic Products Quality Control Guide. 2008. Available online: https://www.gov.br/anvisa/pt-br/centraisdeconteudo/publicacoes/cosmeticos/manuais-e-guias/guia-de-controle-de-qualidade-de-produtos-cosmeticos.GuiadeControledeQualidadedeProdutosCosméticos.pdf/view (accessed on 8 October 2022).
- Aoki, T.; Decker, E.A.; McClements, D.J. Influence of environmental stresses on stability of O/W emulsions containing droplets stabilized by multilayered membranes produced by a layer-by-layer electrostatic deposition technique. Food Hydrocoll. 2005, 19, 209–220. [Google Scholar] [CrossRef]
- Chandran, P.; Das, N. Biosurfactant production and diesel oil degradation by yeast species Trichosporon asahii isolated from petroleum hydrocarbon contaminated soil. Int. J. Eng. Sci. Technol. 2010, 2, 6942–6953. [Google Scholar]
- Luna, J.M.; Rufino, R.D.; Sarubbo, L.A.; Campos-Takaki, G.M. Characterisation, surface properties and biological activity of a biosurfactant produced from industrial waste by Candida sphaerica UCP0995 for application in the petroleum industry. Colloids Surf. B Biointerfaces 2013, 103, 202–209. [Google Scholar] [CrossRef]
- Sobrinho, H.B.S.; Rufino, R.D.; Luna, J.M.; Salgueiro, A.A.; Campos-Takaki, G.M.; Leite, L.F.C.; Sarubbo, L.A. Utilization of two agroindustrial by-products for the production of a surfactant by Candida sphaerica UCP0995. Process Biochem. 2008, 43, 912–917. [Google Scholar] [CrossRef]









| Solvents | EI24 (%) | T.S (mN/m) | BE (g/L) |
|---|---|---|---|
| Chloroform: methanol | 48.3 ± 0.33 a | 61.9 | 7.4 ± 0.07 a |
| Chloroform | 47.6 ± 0.26 a | 60.1 | 6.5 ± 0.06 b |
| Ethanol | 58.3 ± 0.33 b | 63.9 | 7.8 ± 0.07 c |
| Ethyl acetate | 48.3 ± 0.32 a | 63.5 | 7.7 ± 0.08 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, F.G.; Marcelino, P.R.F.; Lacerda, T.M.; Philippini, R.R.; Giancaterino, E.T.; Mancebo, M.C.; dos Santos, J.C.; da Silva, S.S. Production, Physicochemical and Structural Characterization of a Bioemulsifier Produced in a Culture Medium Composed of Sugarcane Bagasse Hemicellulosic Hydrolysate and Soybean Oil in the Context of Biorefineries. Fermentation 2022, 8, 618. https://doi.org/10.3390/fermentation8110618
Barbosa FG, Marcelino PRF, Lacerda TM, Philippini RR, Giancaterino ET, Mancebo MC, dos Santos JC, da Silva SS. Production, Physicochemical and Structural Characterization of a Bioemulsifier Produced in a Culture Medium Composed of Sugarcane Bagasse Hemicellulosic Hydrolysate and Soybean Oil in the Context of Biorefineries. Fermentation. 2022; 8(11):618. https://doi.org/10.3390/fermentation8110618
Chicago/Turabian StyleBarbosa, Fernanda Gonçalves, Paulo Ricardo Franco Marcelino, Talita Martins Lacerda, Rafael Rodrigues Philippini, Emma Teresa Giancaterino, Marcos Campos Mancebo, Júlio Cesar dos Santos, and Silvio Silvério da Silva. 2022. "Production, Physicochemical and Structural Characterization of a Bioemulsifier Produced in a Culture Medium Composed of Sugarcane Bagasse Hemicellulosic Hydrolysate and Soybean Oil in the Context of Biorefineries" Fermentation 8, no. 11: 618. https://doi.org/10.3390/fermentation8110618