Influence of the Deposition Rate and Substrate Temperature on the Morphology of Thermally Evaporated Ionic Liquids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Substrates
2.3. Thin-Film Deposition
2.4. Morphological Characterization
3. Results
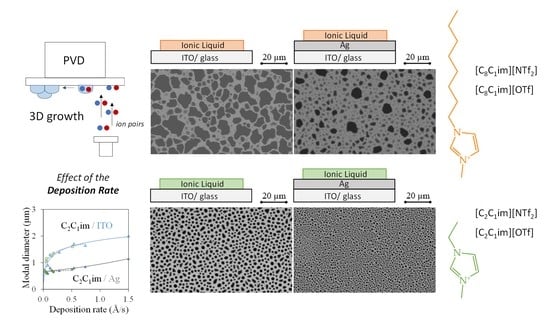
3.1. The Effect of the Mass Flow Rate on the Morphology of ILs Deposited on ITO and Ag Surfaces
3.2. Effect of the Substrate Temperature on the Morphology of ILs Deposited on ITO or Ag Surfaces

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [PubMed] [Green Version]
- Canongia Lopes, J.N.A.; Pádua, A.A.H. Nanostructural Organization in Ionic Liquids. J. Phys. Chem. B 2006, 110, 3330–3335. [Google Scholar] [CrossRef]
- Del Pópolo, M.G.; Voth, G.A. On the Structure and Dynamics of Ionic Liquids. J. Phys. Chem. B 2004, 108, 1744–1752. [Google Scholar] [CrossRef]
- Ghandi, K. A Review of Ionic Liquids, Their Limits and Applications. Green Sustain. Chem. 2014, 04, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [Green Version]
- Ren, F.; Wang, J.; Xie, F.; Zan, K.; Wang, S.; Wang, S. Applications of Ionic Liquids in Starch Chemistry: A Review. Green Chem. 2020, 22, 2162–2183. [Google Scholar] [CrossRef]
- Esperança, J.M.S.S.; Canongia Lopes, J.N.; Tariq, M.; Santos, L.M.N.B.F.; Magee, J.W.; Rebelo, L.P.N. Volatility of Aprotic Ionic Liquids—A Review. J. Chem. Eng. Data 2009, 55, 3–12. [Google Scholar] [CrossRef]
- Rocha, M.A.A.; Coutinho, J.A.P.; Santos, L.M.N.B.F. Vapor Pressures of 1,3-Dialkylimidazolium Bis(Trifluoromethylsulfonyl)Imide Ionic Liquids with Long Alkyl Chains. J. Chem. Phys. 2014, 141, 134502. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.C.S.; Alves, A.; Bastos, M.; Santos, L.M.N.B.F. The Impact of the Cation Alkyl Chain Length on the Wettability of Alkylimidazolium-Based Ionic Liquids at the Nanoscale. Phys. Chem. Chem. Phys. 2022, 24, 13343–13355. [Google Scholar] [CrossRef]
- Shahiduzzaman; Muslih, E.Y.; Hasan, A.K.M.; Wang, L.; Fukaya, S.; Nakano, M.; Karakawa, M.; Takahashi, K.; Akhtaruzzaman; Nunzi, J.-M.; et al. The Benefits of Ionic Liquids for the Fabrication of Efficient and Stable Perovskite Photovoltaics. Chem. Eng. J. 2021, 411, 128461. [Google Scholar] [CrossRef]
- Pei, Y.; Zhang, Y.; Ma, J.; Fan, M.; Zhang, S.; Wang, J. Ionic Liquids for Advanced Materials. Mater. Today Nano 2022, 17, 100159. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse Applications of Ionic Liquids: A Comprehensive Review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Sun, P.; Armstrong, D.W. Ionic Liquids in Analytical Chemistry. Anal. Chim. Acta 2010, 661, 1–16. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical Properties of Ionic Liquids: Database and Evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of Ionic Liquids in the Chemical Industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Borghi, F.; Podestà, A. Ionic Liquids under Nanoscale Confinement. Adv. Phys. X 2020, 5, 1736949. [Google Scholar] [CrossRef] [Green Version]
- Calandra, P.; Szerb, E.I.; Lombardo, D.; Algieri, V.; De Nino, A.; Maiuolo, L. A Presentation of Ionic Liquids as Lubricants: Some Critical Comments. Appl. Sci. 2021, 11, 5677. [Google Scholar] [CrossRef]
- Yoo, E.; Kim, J.; Hosono, E.; Zhou, H.; Kudo, T.; Honma, I. Large Reversible Li Storage of Graphene Nanosheet Families for Use in Rechargeable Lithium Ion Batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef]
- Erto, A.; Silvestre-Albero, A.; Silvestre-Albero, J.; Rodríguez-Reinoso, F.; Balsamo, M.; Lancia, A.; Montagnaro, F. Carbon-Supported Ionic Liquids as Innovative Adsorbents for CO2 Separation from Synthetic Flue-Gas. J. Colloid Interface Sci. 2015, 448, 41–50. [Google Scholar] [CrossRef]
- Fredlake, C.P.; Crosthwaite, J.M.; Hert, D.G.; Aki, S.N.V.K.; Brennecke, J.F. Thermophysical Properties of Imidazolium-Based Ionic Liquids. J. Chem. Eng. Data 2004, 49, 954–964. [Google Scholar] [CrossRef]
- Wishart, J.F. Energy Applications of Ionic Liquids. Energ. Environ. Sci. 2009, 2, 956. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.A.A.; Lima, C.F.R.A.C.; Gomes, L.R.; Schröder, B.; Coutinho, J.A.P.; Marrucho, I.M.; Esperança, J.M.S.S.; Rebelo, L.P.N.; Shimizu, K.; Lopes, J.N.C.; et al. High-Accuracy Vapor Pressure Data of the Extended [CnC1Im][Ntf2] Ionic Liquid Series: Trend Changes and Structural Shifts. J. Phys. Chem. B 2011, 115, 10919–10926. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.F.D.; Freire, M.G.; Fernandes, A.M.; Lopes-da-Silva, J.A.; Morgado, P.; Shimizu, K.; Filipe, E.J.M.; Canongia Lopes, J.N.; Santos, L.M.N.B.F.; Coutinho, J.A.P. Cation Alkyl Side Chain Length and Symmetry Effects on the Surface Tension of Ionic Liquids. Langmuir 2014, 30, 6408–6418. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Moosavi, F. The Effects of Temperature, Alkyl Chain Length, and Anion Type on Thermophysical Properties of the Imidazolium Based Amino Acid Ionic Liquids. J. Mol. Liq. 2018, 250, 121–130. [Google Scholar] [CrossRef]
- Katsyuba, S.A.; Vener, M.V.; Zvereva, E.E.; Fei, Z.; Scopelliti, R.; Laurenczy, G.; Yan, N.; Paunescu, E.; Dyson, P.J. How Strong Is Hydrogen Bonding in Ionic Liquids? Combined X-ray Crystallographic, Infrared/Raman Spectroscopic, and Density Functional Theory Study. J. Phys. Chem. B 2013, 117, 9094–9105. [Google Scholar] [CrossRef]
- Kurnia, K.A.; Lima, F.; Cláudio, A.F.M.; Coutinho, J.A.P.; Freire, M.G. Hydrogen-Bond Acidity of Ionic Liquids: An Extended Scale. Phys. Chem. Chem. Phys. 2015, 17, 18980–18990. [Google Scholar] [CrossRef] [Green Version]
- Bica, K.; Deetlefs, M.; Schröder, C.; Seddon, K.R. Polarisabilities of Alkylimidazolium Ionic Liquids. Phys. Chem. Chem. Phys. 2013, 15, 2703. [Google Scholar] [CrossRef]
- Fadeeva, Y.A.; Gruzdev, M.S.; Kudryakova, N.O.; Shmukler, L.E.; Safonova, L.P. Physico-Chemical Characterization of Alkyl-Imidazolium Protic Ionic Liquids. J. Mol. Liq. 2020, 297, 111305. [Google Scholar] [CrossRef]
- Sanchora, P.; Pandey, D.K.; Kagdada, H.L.; Materny, A.; Singh, D.K. Impact of Alkyl Chain Length and Water on the Structure and Properties of 1-Alkyl-3-Methylimidazolium Chloride Ionic Liquids. Phys. Chem. Chem. Phys. 2020, 22, 17687–17704. [Google Scholar] [CrossRef]
- Rocha, M.A.A.; Neves, C.M.S.S.; Freire, M.G.; Russina, O.; Triolo, A.; Coutinho, J.A.P.; Santos, L.M.N.B.F. Alkylimidazolium Based Ionic Liquids: Impact of Cation Symmetry on Their Nanoscale Structural Organization. J. Phys. Chem. B 2013, 117, 10889–10897. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Gurung, E.; Tamas, G.; Koh, Y.P.; Shadeck, M.; Simon, S.L.; Maroncelli, M.; Quitevis, E.L. Effect of Alkyl Chain Branching on Physicochemical Properties of Imidazolium-Based Ionic Liquids. J. Chem. Eng. Data 2016, 61, 1078–1091. [Google Scholar] [CrossRef]
- Perkin, S.; Crowhurst, L.; Niedermeyer, H.; Welton, T.; Smith, A.M.; Gosvami, N.N. Self-Assembly in the Electrical Double Layer of Ionic Liquids. Chem. Commun. 2011, 47, 6572. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.C.S.; Mendes, A.; Santos, L.M.N.B.F. Morphology of Imidazolium-Based Ionic Liquids as Deposited by Vapor Deposition: Micro-/Nanodroplets and Thin Films. ChemPhysChem 2016, 17, 2123–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, J.C.S.; Coelho, A.F.S.M.G.; Mendes, A.; Santos, L.M.N.B.F. Nucleation and Growth of Microdroplets of Ionic Liquids Deposited by Physical Vapor Method onto Different Surfaces. Appl. Surf. Sci. 2018, 428, 242–249. [Google Scholar] [CrossRef]
- Campos, R.M.; Alves, A.C.P.M.; Lima, M.A.L.; Farinha, A.F.M.; Cardoso, J.P.S.; Mendes, A.; Costa, J.C.S.; Santos, L.M.N.B.F. Morphology, Structure, and Dynamics of Pentacene Thin Films and Their Nanocomposites with [C2C1Im][NTf2] and [C2C1Im][OTF] Ionic Liquids. ChemPhysChem 2020, 21, 1814–1825. [Google Scholar] [CrossRef] [PubMed]
- Cremer, T.; Killian, M.; Gottfried, J.M.; Paape, N.; Wasserscheid, P.; Maier, F.; Steinrück, H.-P. Physical Vapor Deposition of [EMIM][Tf2N]: A New Approach to the Modification of Surface Properties with Ultrathin Ionic Liquid Films. ChemPhysChem 2008, 9, 2185–2190. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Rocha, R.M.; Vaz, I.C.M.; Torres, M.C.; Mendes, A.; Santos, L.M.N.B.F. Description and Test of a New Multilayer Thin Film Vapor Deposition Apparatus for Organic Semiconductor Materials. J. Chem. Eng. Data 2015, 60, 3776–3791. [Google Scholar] [CrossRef] [Green Version]
- Rietzler, F.; May, B.; Steinrück, H.-P.; Maier, F. Switching Adsorption and Growth Behavior of Ultrathin [C2C1Im][OTf] Films on Au(111) by Pd Deposition. Phys. Chem. Chem. Phys. 2016, 18, 25143–25150. [Google Scholar] [CrossRef] [Green Version]
- Richey, N.E.; de Paula, C.; Bent, S.F. Understanding Chemical and Physical Mechanisms in Atomic Layer Deposition. J. Chem. Phys. 2020, 152, 040902. [Google Scholar] [CrossRef]
- Venables, J.A.; Spiller, G.D.T.; Hanbucken, M. Nucleation and Growth of Thin Films. Rep. Prog. Phys. 1984, 47, 399–459. [Google Scholar] [CrossRef]
- Ratsch, C.; Venables, J.A. Nucleation Theory and the Early Stages of Thin Film Growth. J. Vac. Sci. Technol. A 2003, 21, S96–S109. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Campos, R.M.; Castro, A.C.M.; Farinha, A.F.M.; Oliveira, G.N.P.; Araújo, J.P.; Santos, L.M.N.B.F. The Effect of Ionic Liquids on the Nucleation and Growth of Perylene Films Obtained by Vapor Deposition. CrystEngComm 2023, 25, 913–924. [Google Scholar] [CrossRef]
- Young, T., III. An Essay on the Cohesion of Fluids. Philos. Trans. Roy. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- ADAM, N.K. Use of the Term “Young’s Equation” for Contact Angles. Nature 1957, 180, 809–810. [Google Scholar] [CrossRef]
- Teixeira, M.S.M.; Santos, L.M.N.B.F.; Costa, J.C.S. Nucleation, Coalescence, and Thin-Film Growth of Triflate-Based Ionic Liquids on ITO, Ag, and Au Surfaces. Colloids Interfaces 2022, 6, 46. [Google Scholar] [CrossRef]
- Tariq, M.; Freire, M.G.; Saramago, B.; Coutinho, J.A.P.; Lopes, J.N.C.; Rebelo, L.P.N. Surface Tension of Ionic Liquids and Ionic Liquid Solutions. Chem. Soc. Rev. 2012, 41, 829–868. [Google Scholar] [CrossRef]
- Dragoni, D.; Manini, N.; Ballone, P. Interfacial Layering of a Room-Temperature Ionic Liquid Thin Film on Mica: A Computational Investigation. ChemPhysChem 2012, 13, 1772–1780. [Google Scholar] [CrossRef]
- Weijs, J.H.; Marchand, A.; Andreotti, B.; Lohse, D.; Snoeijer, J.H. Origin of Line Tension for a Lennard-Jones Nanodroplet. Phys. Fluid. 2011, 23, 022001. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, P.; Borg, M.K.; Reese, J.M.; Wen, D. A Critical Assessment of the Line Tension Determined by the Modified Young’s Equation. Phys. Fluid. 2018, 30, 082003. [Google Scholar] [CrossRef] [Green Version]
- Lexow, M.; Talwar, T.; Heller, B.S.J.; May, B.; Bhuin, R.G.; Maier, F.; Steinrück, H.-P. Time-Dependent Changes in the Growth of Ultrathin Ionic Liquid Films on Ag(111). Phys. Chem. Chem. Phys. 2018, 20, 12929–12938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lexow, M.; Heller, B.S.J.; Maier, F.; Steinrück, H.-P. Anion Exchange at the Liquid/Solid Interface of Ultrathin Ionic Liquid Films on Ag(111). ChemPhysChem 2018, 19, 2978–2984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lexow, M.; Heller, B.S.J.; Partl, G.; Bhuin, R.G.; Maier, F.; Steinrück, H.-P. Cation Exchange at the Interfaces of Ultrathin Films of Fluorous Ionic Liquids on Ag(111). Langmuir 2018, 35, 398–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, Y.; Wu, G.; Hu, J. Coexistence of Liquid and Solid Phases of Bmim-PF6 Ionic Liquid on Mica Surfaces at Room Temperature. J. Am. Chem. Soc. 2006, 128, 7456–7457. [Google Scholar] [CrossRef]
- Lexow, M.; Maier, F.; Steinrück, H.-P. Ultrathin Ionic Liquid Films on Metal Surfaces: Adsorption, Growth, Stability and Exchange Phenomena. Adv. Phys. X 2020, 5, 1761266. [Google Scholar] [CrossRef]
- Delcheva, I.; Ralston, J.; Beattie, D.A.; Krasowska, M. Static and Dynamic Wetting Behaviour of Ionic Liquids. Adv. Colloid Interface Sci. 2015, 222, 162–171. [Google Scholar] [CrossRef]
- Deyko, A.; Cremer, T.; Rietzler, F.; Perkin, S.; Crowhurst, L.; Welton, T.; Steinrück, H.-P.; Maier, F. Interfacial Behavior of Thin Ionic Liquid Films on Mica. J. Phys. Chem. C 2013, 117, 5101–5111. [Google Scholar] [CrossRef]
- Gong, X.; Li, L. Understanding the Wettability of Nanometer-Thick Room Temperature Ionic Liquids (RTILs) on Solid Surfaces. Chin. Chem. Lett. 2017, 28, 2045–2052. [Google Scholar] [CrossRef]
- Beattie, D.A.; Espinosa-Marzal, R.M.; Ho, T.T.M.; Popescu, M.N.; Ralston, J.; Richard, C.J.E.; Sellapperumage, P.M.F.; Krasowska, M. Molecularly-Thin Precursor Films of Imidazolium-Based Ionic Liquids on Mica. J. Phys. Chem. C 2013, 117, 23676–23684. [Google Scholar] [CrossRef]
- Borghi, F.; Mirigliano, M.; Lenardi, C.; Milani, P.; Podestà, A. Nanostructure Determines the Wettability of Gold Surfaces by Ionic Liquid Ultrathin Films. Front. Chem. 2021, 9, 619432. [Google Scholar] [CrossRef]
- Li, S.; Han, K.S.; Feng, G.; Hagaman, E.W.; Vlcek, L.; Cummings, P.T. Dynamic and Structural Properties of Room-Temperature Ionic Liquids near Silica and Carbon Surfaces. Langmuir 2013, 29, 9744–9749. [Google Scholar] [CrossRef] [PubMed]
- Pensado, A.S.; Malberg, F.; Gomes, M.F.C.; Pádua, A.A.H.; Fernández, J.; Kirchner, B. Interactions and Structure of Ionic Liquids on Graphene and Carbon Nanotubes Surfaces. RSC Adv. 2014, 4, 18017–18024. [Google Scholar] [CrossRef]
- Meusel, M.; Gezmis, A.; Jaekel, S.; Lexow, M.; Bayer, A.; Maier, F.; Steinrück, H.-P. Time- and Temperature-Dependent Growth Behavior of Ionic Liquids on Au(111) Studied by Atomic Force Microscopy in Ultrahigh Vacuum. J. Phys. Chem. C 2021, 125, 20439–20449. [Google Scholar] [CrossRef]
- Cremer, T.; Stark, M.; Deyko, A.; Steinrück, H.-P. Liquid/Solid Interface of Ultrathin Ionic Liquid Films: [C1C1Im][Tf2N] and [C8C1Im][Tf2N] on Au(111). Langmuir 2011, 27, 3662–3671. [Google Scholar] [CrossRef] [PubMed]
- Rola, K.P.; Zajac, A.; Szpecht, A.; Kowal, D.; Cybinska, J.; Smiglak, M.; Komorowska, K. Interaction of electron beam with ionic liquids and its application for micropatterning. Eur. Polym. J. 2021, 156, 110615. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Topp, A.; Siegesmund, A.; Papcke, A.; Kockerling, M.; Verevkin, S.P. In the Footsteps of August Michaelis: Syntheses and Thermodynamics of Extremely Low-Volatile Ionic Liquids. ChemistryOpen 2021, 10, 243–247. [Google Scholar] [CrossRef]
- Maruyama, S.; Takeyama, Y.; Taniguchi, H.; Fukumoto, H.; Itoh, M.; Kumigashira, H.; Oshima, M.; Yamamoto, T.; Matsumoto, Y. Molecular Beam Deposition of Nanoscale Ionic Liquids in Ultrahigh Vacuum. ACS Nano 2010, 4, 5946–5952. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Maruyama, S. Laser Deposition of Nano-Ionic Liquids and their Process Applications in a Vacuum. Ionic Liquid Devices 2017, 136–167. [Google Scholar] [CrossRef]
- Takeyama, Y.; Mantoku, S.; Maruyama, S.; Matsumoto, Y. Growth Behaviours of Pentacene Films Confined in Engineered Shapes of Ionic-Liquid in Vacuum. CrystEngComm 2014, 16, 684–689. [Google Scholar] [CrossRef]
- Delcheva, I.; Beattie, D.A.; Ralston, J.; Krasowska, M. Dynamic Wetting of Imidazolium-Based Ionic Liquids on Gold and Glass. Phys. Chem. Chem. Phys. 2018, 20, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Hessey, S.G.; Jones, R.G. On the Evaporation, Bonding, and Adsorbate Capture of an Ionic Liquid on Au(111). Chem. Sci. 2013, 4, 2519–2529. [Google Scholar] [CrossRef]
- Rietzler, F.; Piermaier, M.; Deyko, A.; Steinruck, H.-P.; Maier, F. Electrospray Ionization Deposition of Ultrathin Ionic Liquid Films: [C8C1Im]Cl and [C8C1Im][Tf2N] on Au(111). Langmuir 2014, 30, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Chopra, K.L.; Randlett, M.R. Influence of Deposition Parameters on the Coalescence Stage of Growth of Metal Films. J. Appl. Phys. 1968, 39, 1874–1881. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, R.M.; Neto, C.; Santos, L.M.N.B.F.; Bastos, M.; Costa, J.C.S. Influence of the Deposition Rate and Substrate Temperature on the Morphology of Thermally Evaporated Ionic Liquids. Fluids 2023, 8, 105. https://doi.org/10.3390/fluids8030105
Carvalho RM, Neto C, Santos LMNBF, Bastos M, Costa JCS. Influence of the Deposition Rate and Substrate Temperature on the Morphology of Thermally Evaporated Ionic Liquids. Fluids. 2023; 8(3):105. https://doi.org/10.3390/fluids8030105
Chicago/Turabian StyleCarvalho, Rita M., Cândida Neto, Luís M. N. B. F. Santos, Margarida Bastos, and José C. S. Costa. 2023. "Influence of the Deposition Rate and Substrate Temperature on the Morphology of Thermally Evaporated Ionic Liquids" Fluids 8, no. 3: 105. https://doi.org/10.3390/fluids8030105