Development of Natural Active Agent-Containing Porous Hydrogel Sheets with High Water Content for Wound Dressings
Abstract
:1. Introduction
2. Results and Discussion
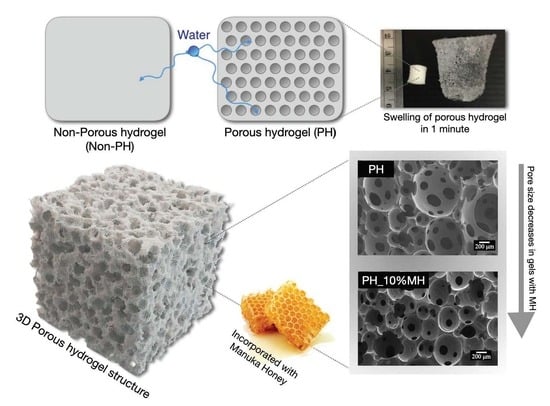
2.1. Comparisons of Non-Porous Hydrogels (NPH) and Porous Hydrogels (PH)
2.2. Comparisons of Porous Hydrogels (PH) and Porous Hydrogels with Manuka Honey (PH_MH)
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Non-Porous Hydrogels (NPH), Porous Hydrogels (PH), and Porous Hydrogels with Manuka Honey (PH_MH)
Synthesis of Hydrogels
4.3. Swelling Test
4.4. Surface Absorption
4.5. Morphological Observations
4.6. Rheological Measurement
4.7. Cytotoxicity Test
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosiak, J.M.; Yoshii, F. Hydrogels and Their Medical Applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1999, 151, 56–64. [Google Scholar] [CrossRef]
- Hicyilmaz, A.S.; Seckin, A.K.; Cerkez, I. Synthesis, Characterization and Chlorination of 2-Acrylamido-2-Methylpropane Sulfonic Acid Sodium Salt-Based Antibacterial Hydrogels. React. Funct. Polym. 2017, 115, 109–116. [Google Scholar] [CrossRef]
- Makino, K.; Hiyoshi, J.; Ohshima, H. Effects of Thermosensitivity of Poly (N-Isopropylacrylamide) Hydrogel upon the Duration of a Lag Phase at the Beginning of Drug Release from the Hydrogel. Colloids Surf. B Biointerfaces 2001, 20, 341–346. [Google Scholar] [CrossRef]
- Yooyod, M.; Ross, S.; Phewchan, P.; Daengmankhong, J.; Pinthong, T.; Tuancharoensri, N.; Mahasaranon, S.; Viyoch, J.; Ross, G.M. Homo- and Copolymer Hydrogels Based on N-Vinylformamide: An Investigation of the Impact of Water Structure on Controlled Release. Gels 2023, 9, 333. [Google Scholar] [CrossRef] [PubMed]
- Kongprayoon, A.; Ross, G.; Limpeanchob, N.; Mahasaranon, S.; Punyodom, W.; Topham, P.D.; Ross, S. Bio-Derived and Biocompatible Poly(Lactic Acid)/Silk Sericin Nanogels and Their Incorporation within Poly(Lactide-Co-Glycolide) Electrospun Nanofibers. Polym. Chem. 2022, 13, 3343–3357. [Google Scholar] [CrossRef]
- Ross, S.; Yooyod, M.; Limpeanchob, N.; Mahasaranon, S.; Suphrom, N.; Ross, G.M. Novel 3D Porous Semi-IPN Hydrogel Scaffolds of Silk Sericin and Poly(N-Hydroxyethyl Acrylamide) for Dermal Reconstruction. Express Polym. Lett. 2017, 11, 719–730. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A Review on Polymeric Hydrogel Membranes for Wound Dressing Applications: PVA-Based Hydrogel Dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Khurana, B.; Gierlich, P.; Meindl, A.; Gomes-Da-Silva, L.C.; Senge, M.O. Hydrogels: Soft Matters in Photomedicine. Photochem. Photobiol. Sci. 2019, 18, 2613–2656. [Google Scholar] [CrossRef]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Johnson, S. Superabsorbent Hydrogels for Removal of Divalent Toxic Ions. Part I: Synthesis and Swelling Characterization. React. Funct. Polym. 2005, 62, 271–283. [Google Scholar] [CrossRef]
- Patel, P.K.; Mistry, S.N.; Patel, G.J.; Bharadia, P.D.; Pandya, V.M.; Modi, D.A. Recent in Controlled Drug Delivery System: Superporous Hydrogels. IJPI’s J. Pharm. Cosmetol. 2011, 1, 53–65. [Google Scholar]
- Zhang, T.; Sanguramath, R.A.; Israel, S.; Silverstein, M.S. Emulsion Templating: Porous Polymers and Beyond. Macromolecules 2019, 52, 5445–5479. [Google Scholar] [CrossRef] [Green Version]
- Whang, K.; Goldstick, T.K.; Healy, K.E. Control of Protein Release from Emulsion Freeze-Dried Scaffolds with Unique Microarchitecture. Scopus 1996, 2, 774. [Google Scholar]
- Baker, S.C.; Rohman, G.; Southgate, J.; Cameron, N.R. The Relationship between the Mechanical Properties and Cell Behaviour on PLGA and PCL Scaffolds for Bladder Tissue Engineering. Biomaterials 2009, 30, 1321–1328. [Google Scholar] [CrossRef]
- Pramanik, R.; Narayanan, A.; Rajan, A.; Konar, S.; Arockiarajan, A. Transversely Isotropic Freeze-Dried PVA Hydrogels: Theoretical Modelling and Experimental Characterization. Int. J. Eng. Sci. 2019, 144, 103144. [Google Scholar] [CrossRef]
- Gong, X.; Rohm, K.; Su, Z.; Zhao, B.; Renner, J.; Manas-Zloczower, I.; Feke, D.L. Porous Hydrogels Templated from Soy-Protein-Stabilized High Internal Phase Emulsions. J. Mater. Sci. 2020, 55, 17284–17301. [Google Scholar] [CrossRef]
- Hori, K.; Sano, M.; Suzuki, M.; Hanabusa, K. Preparation of Porous Polymer Materials Using Water-in-Oil Gel Emulsions as Templates. Polym. Int. 2018, 67, 909–916. [Google Scholar] [CrossRef]
- Omidian, H.; Rocca, J.G.; Park, K. Advances in Superporous Hydrogels. J. Control. Release 2005, 102, 3–12. [Google Scholar] [CrossRef]
- Rungrod, A.; Kapanya, A.; Punyodom, W.; Molloy, R.; Meerak, J.; Somsunan, R. Synthesis of Poly(ε-Caprolactone) Diacrylate for Micelle-Cross-Linked Sodium AMPS Hydrogel for Use as Controlled Drug Delivery Wound Dressing. Biomacromolecules 2021, 22, 3839–3859. [Google Scholar] [CrossRef] [PubMed]
- Sivan, S.S.; Roberts, S.; Urban, J.P.G.; Menage, J.; Bramhill, J.; Campbell, D.; Franklin, V.J.; Lydon, F.; Merkher, Y.; Maroudas, A.; et al. Injectable Hydrogels with High Fixed Charge Density and Swelling Pressure for Nucleus Pulposus Repair: Biomimetic Glycosaminoglycan Analogues. Acta Biomater. 2014, 10, 1124–1133. [Google Scholar] [CrossRef]
- Ghatak, S.; Maytin, E.V.; MacK, J.A.; Hascall, V.C.; Atanelishvili, I.; Moreno Rodriguez, R.; Markwald, R.R.; Misra, S. Roles of Proteoglycans and Glycosaminoglycans in Wound Healing and Fibrosis. Int. J. Cell Biol. 2015, 2015, 834893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bialik-Was, K.; Pluta, K.; Malina, D.; Barczewski, M.; Malarz, K.; Mrozek-Wilczkiewicz, A. The Effect of Glycerin Content in Sodium Alginate/Poly(Vinyl Alcohol)-Based Hydrogels for Wound Dressing Application. Int. J. Mol. Sci. 2021, 22, 12022. [Google Scholar] [CrossRef] [PubMed]
- Kalaithong, W.; Molloy, R.; Nalampang, K.; Somsunan, R. Design and Optimization of Polymerization Parameters of Carboxymethyl Chitosan and Sodium 2-Acrylamido-2-Methylpropane Sulfonate Hydrogels as Wound Dressing Materials. Eur. Polym. J. 2021, 143, 110186. [Google Scholar] [CrossRef]
- Liang, J.; Karakoçak, B.B.; Struckhoff, J.J.; Ravi, N. Synthesis and Characterization of Injectable Sulfonate-Containing Hydrogels. Biomacromolecules 2016, 17, 4064–4074. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Gupta, A.; Gupta, B. Scar Free Healing Mediated by the Release of Aloe Vera and Manuka Honey from Dextran Bionanocomposite Wound Dressings. Int. J. Biol. Macromol. 2018, 120, 1581–1590. [Google Scholar] [CrossRef]
- Sh Ahmed, A.; Taher, M.; Mandal, U.K.; Jaffri, J.M.; Susanti, D.; Mahmood, S.; Zakaria, Z.A. Pharmacological Properties of Centella Asiatica Hydrogel in Accelerating Wound Healing in Rabbits. BMC Complement. Altern. Med. 2019, 19, 213. [Google Scholar] [CrossRef] [Green Version]
- Yotsawimonwat, S.; Rattanadechsakul, J.; Rattanadechsakul, P.; Okonogi, S. Skin Improvement and Stability of Echinacea Purpurea Dermatological Formulations. Int. J. Cosmet. Sci. 2010, 32, 340–346. [Google Scholar] [CrossRef]
- Frydman, G.H.; Olaleye, D.; Annamalai, D.; Layne, K.; Yang, I.; Kaafarani, H.M.A.; Fox, J.G. Manuka Honey Microneedles for Enhanced Wound Healing and the Prevention and/or Treatment of Methicillin-Resistant Staphylococcus Aureus (MRSA) Surgical Site Infection. Sci. Rep. 2020, 10, 13229. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Biological Properties and Therapeutic Activities of Honey in Wound Healing: A Narrative Review and Meta-Analysis. J. Tissue Viability 2016, 25, 98–118. [Google Scholar] [CrossRef]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef] [Green Version]
- Hixon, K.R.; Bogner, S.J.; Ronning-Arnesen, G.; Janowiak, B.E.; Sell, S.A. Investigating Manuka Honey Antibacterial Properties When Incorporated into Cryogel, Hydrogel, and Electrospun Tissue Engineering Scaffolds. Gels 2019, 5, 7–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, K.; Carville, K.; Chadwick, P.; Moore, Z.; Nicodème, M.; Percival, S.L.; Romanelli, M.; Schultz, G.; Tariq, G. Wuwhs Consensus Document: Executive Summary Wound Exudate: Effective Assessment and Management Introduction. Wound Int. 2019. [Google Scholar]
- Hilliard, G.; DeClue, C.E.; Minden-Birkenmaier, B.A.; Dunn, A.J.; Sell, S.A.; Shornick, L.P. Preliminary Investigation of Honey-Doped Electrospun Scaffolds to Delay Wound Closure. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2019, 107, 2620–2628. [Google Scholar] [CrossRef] [PubMed]
- Pormohammad, A.; Monych, N.K.; Ghosh, S.; Turner, D.L.; Turner, R.J. Nanomaterials in Wound Healing and Infection Control. Antibiotics 2021, 10, 473. [Google Scholar] [CrossRef]
- Sung, H.W.; Huang, R.N.; Huang, L.L.; Tsai, C.C. In Vitro Evaluation of Cytotoxicity of a Naturally Occurring Cross-Linking Reagent for Biological Tissue Fixation. J. Biomater. Sci. Polym. Ed. 1999, 10, 63–78. [Google Scholar] [CrossRef]
- Opt Veld, R.C.; Walboomers, X.F.; Jansen, J.A.; Wagener, F.A.D.T.G. Design Considerations for Hydrogel Wound Dressings: Strategic and Molecular Advances. Tissue Eng.-Part B Rev. 2020, 26, 230–248. [Google Scholar] [CrossRef]
- Sergeeva, A.; Vikulina, A.S.; Volodkin, D. Porous Alginate Scaffolds Assembled Using Vaterite CaCO3 Crystals. Micromachines 2019, 10, 357. [Google Scholar] [CrossRef] [Green Version]
- Foudazi, R.; Zowada, R.; Manas-Zloczower, I.; Feke, D.L. Porous Hydrogels: Present Challenges and Future Opportunities. Langmuir 2023, 39, 2092–2111. [Google Scholar] [CrossRef]
- El-Senduny, F.F.; Hegazi, N.M.; Abd Elghani, G.E.; Farag, M.A. Manuka Honey, a Unique Mono-Floral Honey. A Comprehensive Review of Its Bioactives, Metabolism, Action Mechanisms, and Therapeutic Merits. Food Biosci. 2021, 42, 101038. [Google Scholar] [CrossRef]
- Minden-Birkenmaier, B.A.; Cherukuri, K.; Smith, R.A.; Radic, M.Z.; Bowlin, G.L. Manuka Honey Modulates the Inflammatory Behavior of a DHL-60 Neutrophil Model under the Cytotoxic Limit. Int. J. Biomater. 2019, 11, 6132581. [Google Scholar] [CrossRef] [Green Version]










| Samples | Average Pore Size (µm) | % Porosity |
|---|---|---|
| Porous hydrogel (PH) | 108.5 ± 46.0 | 37.10 ± 34.5 |
| Porous hydrogel with 1% Manuka Honey (PH_1% MH) | 51.5 ± 24.2 | 21.73 ± 15.5 |
| Porous hydrogel with 10% Manuka Honey (PH_10% MH) | 50.5 ± 11.2 | 42.22 ± 11.1 |
| Sample | AMPs (g) | DI Water/F127 (g) | DI Water (g) | XL (g) | TEMED (g) | APS (g) | BA (g) | MAA (g) | MH (g) |
|---|---|---|---|---|---|---|---|---|---|
| Non-Porous hydrogel (NPH) | 5.00 | - | 4.00 | 0.20 | - | - | - | - | - |
| Porous hydrogel (PH) | 5.00 | 4.00 | - | 0.20 | 0.65 | 0.65 | 0.50 | 0.25 | - |
| Porous hydrogel with 1% Manuka honey (PH_1% MH) | 5.00 | 3.90 | - | 0.20 | 0.65 | 0.65 | 0.50 | 0.25 | 0.1040 |
| Porous hydrogel with 10% Manuka honey (PH_10% MH) | 5.00 | 1.96 | - | 0.20 | 0.65 | 0.65 | 0.50 | 0.25 | 1.0400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinthong, T.; Yooyod, M.; Daengmankhong, J.; Tuancharoensri, N.; Mahasaranon, S.; Viyoch, J.; Jongjitwimol, J.; Ross, S.; Ross, G.M. Development of Natural Active Agent-Containing Porous Hydrogel Sheets with High Water Content for Wound Dressings. Gels 2023, 9, 459. https://doi.org/10.3390/gels9060459
Pinthong T, Yooyod M, Daengmankhong J, Tuancharoensri N, Mahasaranon S, Viyoch J, Jongjitwimol J, Ross S, Ross GM. Development of Natural Active Agent-Containing Porous Hydrogel Sheets with High Water Content for Wound Dressings. Gels. 2023; 9(6):459. https://doi.org/10.3390/gels9060459
Chicago/Turabian StylePinthong, Thanyaporn, Maytinee Yooyod, Jinjutha Daengmankhong, Nantaprapa Tuancharoensri, Sararat Mahasaranon, Jarupa Viyoch, Jirapas Jongjitwimol, Sukunya Ross, and Gareth M. Ross. 2023. "Development of Natural Active Agent-Containing Porous Hydrogel Sheets with High Water Content for Wound Dressings" Gels 9, no. 6: 459. https://doi.org/10.3390/gels9060459