Correlation between Mechanical and Morphological Properties of Polyphenol-Laden Xanthan Gum/Poly(vinyl alcohol) Composite Cryogels
Abstract
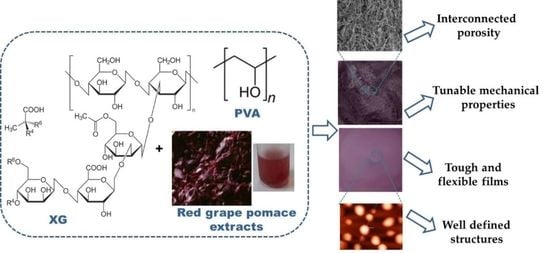
:1. Introduction
2. Results and Discussion
2.1. Compressive Behavior
2.2. Morphological Features
2.3. Viscoelastic Properties
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Water Swelling Measurements
4.2.2. Water Contact Angle Evaluation
4.2.3. Rheological Measurements
4.2.4. Uniaxial Compression Tests
4.2.5. Scanning Electron Microscopy (SEM)
4.2.6. Atomic Force Microscopy (AFM)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiss, K.; Ruszkai, C.; Takács-György, K. Examination of short supply chains based on circular economy and sustainability aspects. Resources 2019, 8, 161. [Google Scholar] [CrossRef] [Green Version]
- Raschip, I.E.; Fifere, N.; Dinu, M.V. Polysaccharide-based materials as promising alternatives to synthetic-based plastics for food packaging applications. In Bioplastics for Sustainable Development, 1st ed.; Roohi, M.K., Ed.; Springer Nature Publisher: Singapore, 2021; pp. 515–554. [Google Scholar]
- Huang, J.; Deng, Y.; Ren, J.; Chen, G.; Wang, G.; Wang, F.; Wu, X. Novel in situ forming hydrogel based on xanthan and chitosan re-gelifying in liquids for local drug delivery. Carbohydr. Polym. 2018, 186, 54. [Google Scholar] [CrossRef]
- Anwar, M.; Pervaiz, F.; Shoukat, H.; Noreen, S.; Shabbir, K.; Majeed, A.; Ijaz, S. Formulation and evaluation of interpenetrating network of xanthan gum and polyvinylpyrrolidone as a hydrophilic matrix for controlled drug delivery system. Polym. Bull. 2021, 78, 59. [Google Scholar] [CrossRef]
- Bhattacharya, S.S.; Shukla, S.; Banerjee, S.; Chowdhury, P.; Chakraborty, P.; Ghosh, A. Tailored IPN hydrogel bead of sodium carboxymethyl cellulose and sodium carboxymethyl xanthan gum for controlled delivery of diclofenac sodium. Polym. Plast. Technol. Eng. 2013, 52, 795–805. [Google Scholar] [CrossRef]
- Biswas, A.; Mondal, S.; Das, S.K.; Bose, A.; Thomas, S.; Ghosal, K.; Roy, S.; Provaznik, I. Development and characterization of natural product derived macromolecules based interpenetrating polymer network for therapeutic drug targeting. ACS Omega 2021, 6, 28699–28709. [Google Scholar] [CrossRef]
- Hanna, D.H.; Saad, G.R. Encapsulation of ciprofloxacin within modified xanthan gum-chitosan based hydrogel for drug. Bioorg. Chem. 2019, 84, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Platon, I.-V.; Ghiorghita, C.-A.; Lazar, M.M.; Raschip, I.E.; Dinu, M.V. Chitosan sponges with instantaneous shape recovery and multistrain antibacterial activity for controlled release of plant-derived polyphenols. Int. J. Mol. Sci. 2023, 24, 4452. [Google Scholar] [CrossRef] [PubMed]
- Martín-Illana, A.; Chinarro, E.; Cazorla-Luna, R.; Notario-Perez, F.; Veiga-Ochoa, M.D.; Rubio, J.; Tamayo, A. Optimized hydration dynamics in mucoadhesive xanthan-based trilayer vaginal films for the controlled release of tenofovir. Carbohydr. Polym. 2022, 278, 118958. [Google Scholar] [CrossRef]
- Popa, N.; Novac, O.; Profire, L.; Lupusoru, C.E.; Popa, M.I. Hydrogels based on chitosan–xanthan for controlled release of theophylline. J. Mater. Sci. Mater. Med. 2010, 21, 1241–1248. [Google Scholar] [CrossRef]
- Raschip, I.E.; Hitruc, E.G.; Oprea, A.M.; Popescu, M.C.; Vasile, C. In vitro evaluation of the mixed xanthan/lignin hydrogels as vanillin carriers. J. Mol. Struct. 2011, 1003, 67–74. [Google Scholar] [CrossRef]
- Raschip, I.E.; Paduraru-Mocanu, O.M.; Nita, L.E.; Dinu, M.V. Antibacterial porous xanthan-based films containing flavoring agents evaluated by near infrared chemical imaging technique. J. Appl. Polym. Sci. 2020, 137, e49111. [Google Scholar] [CrossRef]
- Raschip, I.E.; Fifere, N.; Varganici, C.-D.; Dinu, M.V. Development of antioxidant and antimicrobial xanthan-based cryogels with tuned porous morphology and controlled swelling features. Int. J. Biol. Macromol. 2020, 156, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Raschip, I.E.; Dinu, M.V.; Fifere, N.; Darie-Nita, R.; Pamfil, D.; Popirda, A.; Logigan, C. Mechanical, thermal and surface properties of novel xanthan-based cryogels. Cell. Chem. Technol. 2020, 54, 915–924. [Google Scholar] [CrossRef]
- Raschip, I.E.; Fifere, N.; Dinu, M.V. A Comparative analysis on the effect of variety of grape pomace extracts on the ice-templated 3D cryogel features. Gels 2021, 7, 76. [Google Scholar] [CrossRef]
- Thakur, A.; Monga, S.; Wanchoo, R.K. Sorption and drug release studies from semi-interpenetrating polymer networks of chitosan and xanthan gum. Chem. Biochem. Eng. Q. 2014, 28, 105–115. [Google Scholar]
- Enache, A.A.; Serbezeanu, D.; Vlad-Bubulac, T.; Ipate, A.-M.; Suflet, D.M.; Drobota, M.; Barbalata-Mândru, M.; Udrea, R.M.; Rîmbu, C.M. Tunable properties via composition modulations of poly(vinyl alcohol)/xanthan gum/oxalic acid hydrogels. Materials 2022, 15, 2657. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, M.; Tan, K.B.; Lin, J.; Chen, M.; Zhu, Y. Polyvinyl alcohol/xanthan gum composite film with excellent food packaging, storage and biodegradation capability as potential environmentally-friendly alternative to commercial plastic bag. Int. J. Biol. Macromol. 2022, 212, 402–411. [Google Scholar] [CrossRef]
- Bernal-Chavez, S.A.; Alcala-Alcala, S.; Tapia-Guerrero, Y.S.; Magana, J.J.; Del Prado-Audelo, M.L.; Leyva-Gomez, G. Cross-linked polyvinyl alcohol-xanthan gum hydrogel fabricated by freeze/thaw technique for potential application in soft tissue engineering. RSC Adv. 2022, 12, 21713. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, X.M.; Wu, M.Y.; Wang, M.M.; Zhao, Y.Y.; Li, T.T. Synthesis and performance characterization of poly(vinyl alcohol)-xanthan gum composite hydrogel. React. Funct. Polym. 2019, 136, 34–43. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M. Construction of self-assembled polyelectrolyte complex hydrogel based on oppositely charged polysaccharides for sustained delivery of green tea polyphenols. Food Chem. 2020, 306, 125632. [Google Scholar] [CrossRef]
- Anghel, N.; Dinu, M.V.; Zaltariov, M.; Pamfil, D.; Spiridon, I. New cellulose-collagen-alginate materials incorporated with quercetin, anthocyanins and lipoic acid. Int. J. Biol. Macromol. 2021, 181, 30–40. [Google Scholar] [CrossRef]
- Dragan, E.S.; Ghiorghita, C.A.; Dinu, M.V.; Duceac, I.A.; Coseri, S. Fabrication of self-antibacterial chitosan/oxidized starch polyelectrolyte complex sponges for controlled delivery of curcumin. Food Hydrocoll. 2023, 135, 108147. [Google Scholar] [CrossRef]
- Kamdem, D.P.; Shen, Z.; Nabinejad, O.; Zuju, S. Development of biodegradable composite chitosan based films incorporated with xylan and carvacrol for food packaging application. Food Packag. Shelf Life 2019, 21, 100344. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Kordowska-Wiater, M.; Nowakc, J.; Baraniak, B. Characterization of films based on chitosan lactate and its blends with oxidized starch and gelatin. Int. J. Biol. Macromol. 2015, 77, 350–359. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Active chitosan-polyvinyl alcohol films with natural extracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- Torres-Huerta, A.M.; Palma-Ramírez, D.; Domínguez-Crespo, M.A.; Del Angel-López, D.; de la Fuente, D. Comparative assessment of miscibility and degradability on PET/PLA and PET/chitosan blends. Eur. Polym. J. 2014, 61, 285–299. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Kim, M.H.; Cho, Y.-J.; Park, J.-S.; Nam, S.-Y.; Chun, B.-S. Gelatin-sodium alginate based films with Pseuderanthemum palatiferum (Nees) Radlk. Freeze-dried powder obtained by subcritical water extraction. Food Packag. Shelf Life 2020, 24, 100469. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, J.; Garcia-Cela, E.; Magan, N.; Rahatekar, S.S. Electrospinning alginate/polyethylene oxide and curcumin composite nanofibers. Mater. Lett. 2020, 270, 127662. [Google Scholar] [CrossRef]
- Esposito, T.; Silva, N.H.C.S.; Almeida, A.; Silvestre, A.J.D.; Piccinelli, A.; Aquino, R.P.; Sansone, F.; Mencherini, T.; Vilela, C.; Freire, C.S.R. Valorisation of chestnut spiny burs and roasted hazelnut skins extracts as bioactive additives for packaging films. Ind. Crops Prod. 2020, 151, 112491. [Google Scholar] [CrossRef]
- Narasagoudr, S.S.; Hegde, V.G.; Chougale, R.B.; Masti, S.P.; Vootla, S.; Malabadi, R.B. Physico-chemical and functional properties of rutin induced chitosan/poly (vinyl alcohol) bioactive films for food packaging applications. Food Hydrocoll. 2020, 109, 106096. [Google Scholar] [CrossRef]
- Dinu, M.V.; Gradinaru, A.C.; Lazar, M.M.; Dinu, I.A.; Raschip, I.E.; Ciocarlan, N.; Aprotosoaie, A.C. Physically cross-linked chitosan/dextrin cryogels entrapping Thymus vulgaris essential oil with enhanced mechanical, antioxidant and antifungal properties. Int. J. Biol. Macromol. 2021, 184, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Baek, S.-K.; Song, K.B. Physical and antioxidant properties of alginate films prepared from Sargassum fulvellum with black chokeberry extract. Food Packag. Shelf Life 2018, 18, 157–163. [Google Scholar] [CrossRef]
- Gutierrez-Pacheco, M.M.; Ortega-Ramirez, L.A.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Combinational approaches for antimicrobial packaging: Pectin and cinnamon leaf oil. In Antimicrobial food packaging; Academic Press: San Diego, CA, USA, 2016; pp. 609–617. [Google Scholar]
- Dridi, W.; Bordenave, N. Influence of polysaccharide concentration on polyphenol-polysaccharide interactions. Carbohydr. Polym. 2021, 274, 118670. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Bourvellec, C.L.; Renard, C.M.G.C.; Wessel, D.F.; Cardoso, S.M.; Coimbra, M.A. Interactions of arabinan-rich pectic polysaccharides with polyphenols. Carbohydr. Polym. 2020, 230, 115644. [Google Scholar] [CrossRef]
- Li, R.; Zeng, Z.; Fu, G.; Wan, Y.; Liu, C.; McClements, D.J. Formation and characterization of tannic acid/beta-glucan complexes: Influence of pH, ionic strength, and temperature. Food Res. Int. 2019, 120, 748–755. [Google Scholar] [CrossRef]
- Liu, X.; Bourvellec, C.L.; Renard, C.M.G.C. Interactions between cell wall polysaccharides and polyphenols: Effect of molecular internal structure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3574–3617. [Google Scholar] [CrossRef]
- Tudorache, M.; Bordenave, N. Phenolic compounds mediate aggregation of water-soluble polysaccharides and change their rheological properties: Effect of different phenolic compounds. Food Hydrocoll. 2019, 97, 105193. [Google Scholar] [CrossRef]
- Tudorache, M.; McDonald, J.-L.; Bordenave, N. Gallic acid reduces the viscosity and water binding capacity of soluble dietary fibers. Food Funct. 2020, 11, 5866. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.V.; Schwarz, S.; Dinu, I.A.; Drăgan, E.S. Comparative rheological study of ionic semi-IPN composite hydrogels based on polyacrylamide and dextran sulphate and of polyacrylamide hydrogels. Colloid Polym. Sci. 2012, 290, 1647–1657. [Google Scholar] [CrossRef]
- Amaral, C.N.R.; Oliveira, P.F.; Pedroni, L.G.; Mansur, C.R.E. Viscoelastic behavior of hydrogel-based xanthan gum/aluminum lactate with potential applicability for conformance control. J. Appl. Polym. Sci. 2021, 138, e50640. [Google Scholar] [CrossRef]
- Irfan, M.; Khan, M.; Rehman, T.; Ali, I.; Shah, L.A.; Khattak, N.S.; Khan, M.S. Synthesis and rheological survey of xanthan gum based terpolymeric hydrogels. Z. Phys. Chem. 2020, 235, 609–628. [Google Scholar] [CrossRef]
- Mikušová, V.; Ferková, J.; Žigrayová, D.; Krchňák, D.; Mikuš, P. Comparative study of polysaccharide-based hydrogels: Rheological and texture properties and ibuprofen release. Gels 2022, 8, 168. [Google Scholar] [CrossRef]
- Domínguez, E.; España, L.; López-Casado, G.; Cuartero, J.; Heredia, A. Biomechanics of isolated tomato (Solanum lycopersicum) fruit cuticles during ripening: The role of flavonoids. Funct. Plant Biol. 2009, 36, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Hwang, G.; Liu, Y.; Wang, Y.; Singh, A.P.; Vorsa, N.; Koo, H. Cranberry flavonoids modulate cariogenic properties of mixed-species biofilm through exopolysaccharides-matrix disruption. PLoS ONE 2015, 10, e0145844. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Reactivity of flavanols: Their fate in physical food processing and recent advances in their analysis by depolymerization. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4841–4880. [Google Scholar] [CrossRef]
- Zheng, M.; Su, H.; Xiao, R.; Chen, J.; Chen, H.; Tan, K.B.; Zhu, Y. Effects of Polygonatum cyrtonema extracts on the antioxidant ability, physical and structure properties of carboxymethyl cellulose-xanthan gum-flaxseed gum active packaging films. Food Chem. 2023, 403, 134320. [Google Scholar] [CrossRef]
- Giannouli, P.; Morris, E.R. Cryogelation of xanthan. Food Hydrocoll. 2003, 17, 495–501. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Kurochkin, I.N.; Kurochkin, I.I. Study of cryostructuring of polymer systems. 33. Effect of rate of chilling aqueous poly(vinyl alcohol) solutions during their freezing on physicochemical properties and porous structure of resulting cryogels. Colloid J. 2012, 74, 319–327. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V.; Ghiorghita, A.C. Chitosan-based polyelectrolyte complex cryogels with elasticity, toughness and delivery of curcumin engineered by polyions pair and cryostructuration steps. Gels 2022, 8, 240. [Google Scholar] [CrossRef]
- Dimofte, A.; Dinu, M.V.; Anghel, N.; Doroftei, F.; Spiridon, I. Xanthan and alginate-matrix used as transdermal delivery carrier for piroxicam and ketoconazole. Int. J. Biol. Macromol. 2022, 209, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Peng, X.; Xi, L.; Wan, C.; Shi, S.; Wang, Y.; Yu, X. An agar–polyvinyl alcohol hydrogel loaded with tannic acid with efficient hemostatic and antibacterial capacity for wound dressing. Food Funct. 2022, 13, 9622. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Hu, X.; Qi, X.; Yu, H.; Liu, Y.; Li, J.; Zhang, J.; Dong, W. A novel thermo-responsive hydrogel based on salecan and poly(N-isopropylacrylamide): Synthesis and characterization. Colloids Surf. B Biointerfaces 2015, 125, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Feng, L.; Xie, A.; Wei, W.; Wang, S.; Zhang, J.; Dong, W. Synthesis and characterization of a novel hydrogel: Salecan/polyacrylamide semi-IPN hydrogel with a desirable pore structure. J. Mater. Chem. B 2014, 2, 3646–3658. [Google Scholar] [CrossRef]
- Qi, X.; Hu, X.; Wei, W.; Yu, H.; Li, J.; Zhang, J.; Dong, W. Investigation of salecan/poly(vinyl alcohol) hydrogels prepared by freeze/thaw method. Carbohydr. Polym. 2015, 118, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, F.; Cofelice, M.; Lopez, F. Rheological Characterization of Hydrogels from Alginate-Based Nanodispersion. Polymers 2019, 11, 259. [Google Scholar] [CrossRef] [Green Version]
- Preda, P.; Enciu, A.-M.; Tanase, C.; Dudau, M.; Albulescu, L.; Maxim, M.-E.; Darie-Ni, R.N.; Brincoveanu, O.; Avram, M. Assessing Polysaccharides/Aloe Vera–Based Hydrogels for Tumor Spheroid Formation. Gels 2023, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Chae, B.S.; Lee, Y.S.; Jhon, M.S. The scaling behavior of a highly aggregated colloidal suspension microstructure and its change in shear flow. Colloid Polym. Sci. 2004, 282, 236–242. [Google Scholar] [CrossRef]
- Rial, R.; Soltero, J.F.A.; Verdes, P.V.; Liu, Z.; Ruso, J.M. Mechanical Properties of Composite Hydrogels for Tissue Engineering. Curr. Top. Med. Chem. 2018, 18, 1214–1223. [Google Scholar] [CrossRef]
- Ross-Murphy, S.B.; Shatwell, K.P. Polysaccharide strong and weak gels. Biorheology 1993, 30, 217–227. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, H.; Pan, X.; Zhang, C.; Zhang, K.; Chen, Z.; Dong, W.; Xie, A.; Qi, X. Dendritic hydrogels with robust inherent antibacterial properties for promoting bacteria-infected wound healing. ACS Appl. Mater. Interfaces 2022, 14, 11144–11155. [Google Scholar] [CrossRef]
- Martinez-Ruvalcaba, A.; Chornet, E.; Rodrigue, D. Viscoelastic properties of dispersed chitosan/xanthan hydrogels. Carbohydr. Polym. 2007, 67, 586–595. [Google Scholar] [CrossRef]
- Ferry, D.J. Viscoelastic Properties of Polymers, 3rd ed.; John Wiley and Sons Inc.: New York, NY, USA, 1980; pp. 68–80. [Google Scholar]
- Menard, K.P. Dynamic Mechanical Analysis: A Practical Introduction; CRC Press: New York, NY, USA, 1999. [Google Scholar]
- Tao, Y.; Zhang, R.; Xu, W.; Bai, Z.; Zhou, Y.; Zhao, S.; Xu, Y.; Yu, D. Rheological behavior and microstructure of release-controlled hydrogels based on xanthan gum crosslinked with sodium trimetaphosphate. Food Hydrocoll. 2016, 52, 923–933. [Google Scholar] [CrossRef]
- Popescu, I.; Turtoi, M.; Suflet, D.M.; Dinu, M.V.; Darie-Nita, R.; Anghelache, M.; Calin, M.; Constantin, M. Alginate/poloxamer hydrogel obtained by thiol-acrylate photopolymerization for the alleviation of the inflammatory response of human keratinocytes. Int. J. Biol. Macromol. 2021, 180, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Gorbacheva, S.N.; Yadykova, A.Y.; Ilyin, S.O. Rheological and tribological properties of low-temperature greases based on cellulose acetate butyrate gel. Carbohydr. Polym. 2021, 272, 118509. [Google Scholar] [CrossRef] [PubMed]
- Amri, N.; Ghemati, D.; Bouguettaya, N.; Aliouche, D. Swelling kinetics and rheological behavior of chitosan-PVA/ montmorillonite hybrid polymers. Period. Polytech. Chem. Eng. 2019, 63, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Zarandona, I.; Bengoechea, C.; Álvarez-Castillo, E.; de la Caba, K.; Guerrero, A.; Guerrero, P. 3D Printed chitosan-pectin hydrogels: From rheological characterization to scaffold development and assessment. Gels 2021, 7, 175. [Google Scholar] [CrossRef] [PubMed]
- Luchian, C.E.; Cotea, V.V.; Vlase, L.; Toiu, A.M.; Colibaba, L.C.; Raschip, I.E.; Nadas, G.; Gheldiu, A.M.; Tuchilus, C.; Rotaru, L. Antioxidant and antimicrobial effects of grape pomace extracts. In Proceedings of the 42ndWorld Congress of Vine and Wine, Les Ulis, France, 15–19 July 2019; Volume 15, p. 04006. [Google Scholar]
- Luchian, C.E.; Scutarasu, E.C.; Colibaba, L.C.; Cotea, V.V.; Vlase, L.; Toiu, A.M. Evaluation of byproducts from the wine-making industry by identification of bioactive compounds. In Proceedings of the 41st World Congress of Vine and Wine, Les Ulis, France, 15–19 July 2019; Volume 12, p. 04007. [Google Scholar]
- Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Designing smart triple-network cationic cryogels with outstanding efficiency and selectivity for deep cleaning of phosphate. Chem. Eng. J. 2021, 426, 131411. [Google Scholar] [CrossRef]
- Sáez, P.; Dinu, I.A.; Rodríguez, A.; Gómez, J.M.; Lazar, M.M.; Rossini, D.; Dinu, M.V. Composite cryo-beads of chitosan reinforced with natural zeolites with remarkable elasticity and switching on/off selectivity for heavy metal ions. Int. J. Biol. Macromol. 2020, 164, 2432–2449. [Google Scholar] [CrossRef] [PubMed]
- Raschip, I.E.; Hitruc, G.E.; Vasile, C.; Popescu, M.-C. Effect of the lignin type on the morphology and thermal properties of the xanthan/lignin hydrogels. Int. J. Biol. Macromol. 2013, 54, 230–237. [Google Scholar] [CrossRef]












| Sample Code | XG:PVA | Amount of Extract, v/v% | Swelling Ratio 1 g/g | Contact Angle 2 θ° |
|---|---|---|---|---|
| XG100.V | - | 0 | 63 ± 7 | 70 ± 3 |
| PVA100.V | - | 0 | - | 89 ± 4 |
| XG100.VII | - | 0 | 62 ± 10 | 71 ± 4 |
| PVA100.VII | - | 0 | - | 88 ± 4 |
| CG90.VII | 9:1 | 0 | 59 ± 9 | 79 ± 3 |
| CG90F.III | 9:1 | 10 | 22 ± 8 | 81 ± 5 |
| CG90F.V | 9:1 | 10 | 20 ± 5 | 82 ± 6 |
| CG90F.VII | 9:1 | 10 | 20 ± 3 | 83 ± 4 |
| CG75F.III | 3:1 | 10 | 21 ± 3 | 81 ± 4 |
| CG75F.V | 3:1 | 10 | 17 ± 5 | 85 ± 3 |
| CG75F.VII | 3:1 | 10 | 16 ± 6 | 85 ± 4 |
| CG50F.III | 1:1 | 10 | 13 ± 5 | 83 ± 3 |
| CG50F.V | 1:1 | 10 | 12 ± 5 | 87 ± 3 |
| CG50F.VII | 1:1 | 10 | 11 ± 3 | 92 ± 6 |
| Scanning Area 1 | 5 × 5 μm2 | 10 × 10 μm2 | 20 × 20 μm2 | 30 × 30 μm2 | 40 × 40 μm2 |
|---|---|---|---|---|---|
| XGVII | 17 ± 0.2 | 18 ± 1 | 22 ± 8 | 42 ± 2 | 63 ± 1 |
| XG90F.III | 23 ± 1 | 25 ± 1 | 30 ± 4 | 40 ± 3 | 46 ± 5 |
| XG50F.III | 76 ± 12 | 85 ± 5 | 92 ± 2 | 95 ± 3 | 98 ± 1 |
| Sample | G0′ (Pa) | n′ | n″ | −m | R2 (G′) | R2 (|η*|) |
|---|---|---|---|---|---|---|
| CG90F.III | 766 ± 15 | 0.026 | 0.020 | −0.974 | 0.652 | 0.999 |
| CG90F.V | 399 ± 8 | 0.093 | 0.066 | −0.907 | 0.944 | 0.999 |
| CG90F.VII | 1003 ± 21 | 0.033 | 0.056 | −0.965 | 0.574 | 0.999 |
| CG75F.III | 582 ± 10 | 0.087 | 0.115 | −0.912 | 0.912 | 0.999 |
| CG75F.V | 313 ± 5 | 0.121 | 0.085 | −0.881 | 0.968 | 0.999 |
| CG75F.VII | 305 ± 4 | 0.130 | 0.100 | −0.871 | 0.967 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raschip, I.E.; Darie-Nita, R.N.; Fifere, N.; Hitruc, G.-E.; Dinu, M.V. Correlation between Mechanical and Morphological Properties of Polyphenol-Laden Xanthan Gum/Poly(vinyl alcohol) Composite Cryogels. Gels 2023, 9, 281. https://doi.org/10.3390/gels9040281
Raschip IE, Darie-Nita RN, Fifere N, Hitruc G-E, Dinu MV. Correlation between Mechanical and Morphological Properties of Polyphenol-Laden Xanthan Gum/Poly(vinyl alcohol) Composite Cryogels. Gels. 2023; 9(4):281. https://doi.org/10.3390/gels9040281
Chicago/Turabian StyleRaschip, Irina Elena, Raluca Nicoleta Darie-Nita, Nicusor Fifere, Gabriela-Elena Hitruc, and Maria Valentina Dinu. 2023. "Correlation between Mechanical and Morphological Properties of Polyphenol-Laden Xanthan Gum/Poly(vinyl alcohol) Composite Cryogels" Gels 9, no. 4: 281. https://doi.org/10.3390/gels9040281