Recent Development of Functional Chitosan-Based Hydrogels for Pharmaceutical and Biomedical Applications
Abstract
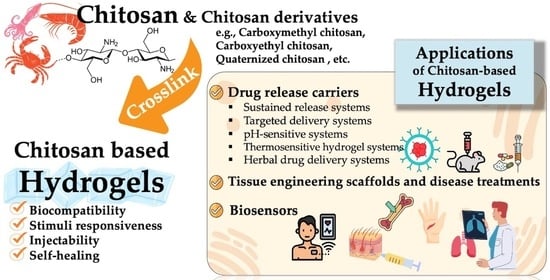
:1. Introduction
2. Gelation Techniques of Chitosan-Based Hydrogels
2.1. Synthesis of Water-Soluble Chitosan Derivatives for Self-Forming Gel

2.2. Physical Crosslinking
2.3. Chemical Crosslinking
2.4. Photo-Crosslinking
2.5. Hybrid Crosslinking
3. Pharmaceutical and Biomedical Applications of Chitosan-Based Hydrogels
3.1. Drug Delivery
3.1.1. Sustained Release Systems
3.1.2. Targeted Delivery Systems
| Drug Delivery Systems | Drug | Mode of Release | Composition of Hydrogel | Ref. |
|---|---|---|---|---|
| Sustained release systems | Clemastine fumarate | Topical route | Genipin/chitosan | [127] |
| Dexamethasone | Injection | CMC/oxidation dextran | [42] | |
| Diclofenac | Oral/topical route | Citral/chitosan | [109] | |
| Doxorubicin | Oral route | Chitosan/CMC/formadehyde/succinic anhydride | [163] | |
| Gabapentin | Injection | Chitosan-g-poly(acrylic acid-co-acrylamide) | [162] | |
| Insulin | Oral route | Layered double hydroxides beads coated with chitosan/alginate | [160] | |
| Insulin | Injection | Acrylamide-modified chitosan/tripolyphosphate | [159] | |
| Metformin | Oral route | Chitosan/PVA | [161] | |
| Valproic acid | Injection | Chitosan/alginate | [65] | |
| Targeted delivery systems | Betamethasone | Ocular routes | Chitosan/dialdehyde starch | [164] |
| Curcumin | Buccal route | Nanocapsules coated with chitosan | [165] | |
| Famotidine | Oral route | Montmorillonite/chitosan | [8] | |
| Doxorubicin | Injection | Chitosan/folate | [18] | |
| Lidocaine | Topical route | CMC/alginate | [41] | |
| pH-sensitive systems | Amoxicillin | Oral/topical route | Gamma-irradiated chitosan/PVA | [166] |
| Cefotaxime | Oral route | Chitosan/alginate | [167] | |
| Cytarabine | Oral route | Chitosan/tamarind/poly (methacrylic acid) | [168] | |
| Diclofenac | Oral route | Chitosan/Iron oxide | [169] | |
| Doxorubicin | Injection | Chitosan/Iron oxide | [87] | |
| Doxorubicin | Injection | CEC/dibenzaldehyde-terminated poly(ethylene glycol) | [29] | |
| Naproxen | Oral route | Bacterial cellulose/chitosan | [170] | |
| Phenylalanine | Injection | Oxidized hydroxypropyl cellulose/CMC | [171] | |
| Thermosensitive hydrogel systems | 2,6-diaminopurine | Oral route/Injection | Orotic acid-modified chitosan | [44] |
| Bupivacaine | Injection | Graphene oxide/chitosan | [172] | |
| Caffeic acid phenethyl ester | Depot | Acetylated CMC | [173] | |
| Diethyldithiocarbamate and copper ions | Topical route | Chitosan/βGP | [174] | |
| Doxorubicin/curcumin | Injection | Thiolated chitosan | [175] | |
| Etanercept | Injection | Chitosan/βGP/Pluronic F-127 | [176] | |
| Paracetamol | Topical route | Chitosan/βGP/genipin | [177] | |
| Herbal drug delivery systems | Berberine | Oral route | Amino acid-g-chitosan/βGP | [178] |
| Cannabidiol | Injection | Chitosan/sodium carboxymethylcellulose | [179] | |
| Carvacrol | Oral route | CMC/alginate | [151] | |
| Cortex moutan | Injection | N,N,N-trimethyl chitosan | [69] | |
| Curcumin | Injection | Chitosan-oligoconjugated linoleic acid | [88] | |
| Moringa oleifera leaf extract | Oral route | Chitosan/alginate | [180] | |
| Pueraria lobatae | Oral route | Chitosan/xanthan gum | [181] | |
| Quercetin | Injection | Chitosan/halloysite/graphitic-carbon nitride | [182] | |
| Resveratrol | Oral route | Chitosan/PVA | [183] |
3.1.3. pH-Sensitive Systems
3.1.4. Temperature-Sensitive Systems
3.1.5. Herbal Drug Delivery Systems
3.2. Tissue Engineering
3.3. Disease Treatment
3.4. Biosensor
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raina, N.; Pahwa, R.; Bhattacharya, J.; Paul, A.K.; Nissapatorn, V.; de Lourdes Pereira, M.; Oliveira, S.M.R.; Dolma, K.G.; Rahmatullah, M.; Wilairatana, P.; et al. Drug Delivery Strategies and Biomedical Significance of Hydrogels: Translational Considerations. Pharmaceutics 2022, 14, 574. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, A.; Haag, R.; Schedler, U. Hydrogels and Their Role in Biosensing Applications. Adv. Healthc. Mater. 2021, 10, 2100062. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, R.; Hu, J.; Wei, Z.; McClements, D.J.; Liu, S.; Li, B.; Li, Y. One-Step Dynamic Imine Chemistry for Preparation of Chitosan-Stabilized Emulsions Using a Natural Aldehyde: Acid Trigger Mechanism and Regulation and Gastric Delivery. J. Agric. Food Chem. 2020, 68, 5412–5425. [Google Scholar] [CrossRef]
- Marin, L.; Ailincai, D.; Mares, M.; Paslaru, E.; Cristea, M.; Nica, V.; Simionescu, B.C. Imino-chitosan biopolymeric films. Obtaining, self-assembling, surface and antimicrobial properties. Carbohydr. Polym. 2015, 117, 762–770. [Google Scholar] [CrossRef]
- Gyles, D.A.; Castro, L.D.; Silva, J.O.C.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Taokaew, S.; Kriangkrai, W. Chitinase-Assisted Bioconversion of Chitinous Waste for Development of Value-Added Chito-Oligosaccharides Products. Biology 2023, 12, 87. [Google Scholar] [CrossRef]
- Farhadnejad, H.; Mortazavi, S.A.; Jamshidfar, S.; Rakhshani, A.; Motasadizadeh, H.; Fatahi, Y.; Mahdieh, A.; Darbasizadeh, B. Montmorillonite-Famotidine/Chitosan Bio-nanocomposite Hydrogels as a Mucoadhesive/Gastroretentive Drug Delivery System. Iran. J. Pharm. Res. 2022, 21, e127035. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Preparation, characterization and antioxidant property of water-soluble ferulic acid grafted chitosan. Carbohydr. Polym. 2013, 96, 495–502. [Google Scholar] [CrossRef]
- Carroll, E.C.; Jin, L.; Mori, A.; Muñoz-Wolf, N.; Oleszycka, E.; Moran, H.B.T.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.M.; et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhou, Z.; Zheng, C.; Liu, Y.; Hao, R.; Ji, X.; Xi, Q.; Shen, J.; Li, Z. Chitosan oligosaccharide regulates AMPK and STAT1 pathways synergistically to mediate PD-L1 expression for cancer chemoimmunotherapy. Carbohydr. Polym. 2022, 277, 118869. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Szymczyk, P.; Rodziewicz, J.; Mielcarek, A. Effect of ionic and covalent crosslinking agents on properties of chitosan beads and sorption effectiveness of Reactive Black 5 dye. React. Funct. Polym. 2017, 114, 58–74. [Google Scholar] [CrossRef]
- Wu, M.-T.; Tsai, Y.-L.; Chiu, C.-W.; Cheng, C.-C. Synthesis, characterization, and highly acid-resistant properties of crosslinking β-chitosan with polyamines for heavy metal ion adsorption. RSC Adv. 2016, 6, 104754–104762. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, R.; Tu, M.; Zhao, J. Synthesis, characterization of biomimetic phosphorylcholine-bound chitosan derivative and in vitro drug release of their nanoparticles. J. Appl. Polym. Sci. 2013, 128, 153–160. [Google Scholar] [CrossRef]
- Shi, B.; Shen, Z.; Zhang, H.; Bi, J.; Dai, S. Exploring N-Imidazolyl-O-Carboxymethyl Chitosan for High Performance Gene Delivery. Biomacromolecules 2012, 13, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-J.; Sun, S.-L.; Chiu, C.-C.; Wang, L.-F. Retinol-encapsulated water-soluble succinated chitosan nanoparticles for antioxidant applications. J. Biomater. Sci. Polym. Ed. 2013, 24, 315–329. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Sarkar, K.; Bhattacharya, S.; Bhattacharyya, A.; Mishra, R.; Kundu, P.P. pH sensitive N-succinyl chitosan grafted polyacrylamide hydrogel for oral insulin delivery. Carbohydr. Polym. 2014, 112, 627–637. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Abdolahinia, E.D.; Nakhlband, A.; Aslzad, S.; Fathi, M.; Barar, J.; Omidi, Y. Smart chitosan–folate hybrid magnetic nanoparticles for targeted delivery of doxorubicin to osteosarcoma cells. Colloids Surf. B Biointerfaces 2022, 220, 112911. [Google Scholar] [CrossRef]
- Torğut, G.; Yazdıç, F.C.; Gürler, N. Synthesis, characterization, pH-sensitive swelling and antimicrobial activities of chitosan–graft-poly(hydroxyethyl methacrylate) hydrogel composites for biomedical applications. Polym. Eng. Sci. 2022, 62, 2552–2559. [Google Scholar] [CrossRef]
- Bari, S.S.; Mishra, S. Effect of calcium sulphate nanorods on mechanical properties of chitosan-hydroxyethyl methacrylate (HEMA) copolymer nanocomposites. Carbohydr. Polym. 2017, 157, 409–418. [Google Scholar] [CrossRef]
- Singhal, A.; Schneible, J.D.; Lilova, R.L.; Hall, C.K.; Menegatti, S.; Grafmüller, A. A multiscale coarse-grained model to predict the molecular architecture and drug transport properties of modified chitosan hydrogels. Soft Matter 2020, 16, 10591–10610. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Jin, Z.; Zhao, K. Protective Effect of Composite Hydrogel Based on Hydroxypropyl Trimethylammonium Chloride Chitosan on Skin Photodamage. ACS Appl. Polym. Mater. 2022, 4, 7587–7598. [Google Scholar] [CrossRef]
- You, J.; Xie, S.; Cao, J.; Ge, H.; Xu, M.; Zhang, L.; Zhou, J. Quaternized Chitosan/Poly(acrylic acid) Polyelectrolyte Complex Hydrogels with Tough, Self-Recovery, and Tunable Mechanical Properties. Macromolecules 2016, 49, 1049–1059. [Google Scholar] [CrossRef]
- Han, W.; Chen, C.; Yang, K.; Wang, H.; Xia, H.; Zhao, Y.; Teng, Y.; Feng, G.; Chen, Y.M. Hyaluronic acid and chitosan-based injectable and self-healing hydrogel with inherent antibacterial and antioxidant bioactivities. Int. J. Biol. Macromol. 2023, 227, 373–383. [Google Scholar] [CrossRef]
- Wan, A.; Xu, Q.; Sun, Y.; Li, H. Antioxidant Activity of High Molecular Weight Chitosan and N,O-Quaternized Chitosans. J. Agric. Food Chem. 2013, 61, 6921–6928. [Google Scholar] [CrossRef]
- Wang, L.; Dong, J.; Zhao, Z.; Li, D.; Dong, W.; Lu, Y.; Jin, B.; Li, H.; Liu, Q.; Deng, B. Quarternized chitosan/quercetin/polyacrylamide semi-interpenetrating network hydrogel with recoverability, toughness and antibacterial properties for wound healing. Int. J. Biol. Macromol. 2023, 228, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, P.; Yao, Z.; Fang, Q.; Feng, L.; Guo, R.; Cheng, B. Preparation of Antimicrobial Hyaluronic Acid/Quaternized Chitosan Hydrogels for the Promotion of Seawater-Immersion Wound Healing. Front. Bioeng. Biotechnol. 2019, 7, 360. [Google Scholar] [CrossRef]
- Huang, Y.; Mu, L.; Zhao, X.; Han, Y.; Guo, B. Bacterial Growth-Induced Tobramycin Smart Release Self-Healing Hydrogel for Pseudomonas aeruginosa-Infected Burn Wound Healing. ACS Nano 2022, 16, 13022–13036. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180. [Google Scholar] [CrossRef]
- Guo, B.; Qu, J.; Zhao, X.; Zhang, M. Degradable conductive self-healing hydrogels based on dextran-graft-tetraaniline and N-carboxyethyl chitosan as injectable carriers for myoblast cell therapy and muscle regeneration. Acta Biomater. 2019, 84, 180–193. [Google Scholar] [CrossRef]
- Lin, P.; Liu, L.; He, G.; Zhang, T.; Yang, M.; Cai, J.; Fan, L.; Tao, S. Preparation and properties of carboxymethyl chitosan/oxidized hydroxyethyl cellulose hydrogel. Int. J. Biol. Macromol. 2020, 162, 1692–1698. [Google Scholar] [CrossRef]
- Bai, X.; Kong, M.; Xia, G.; Bi, S.; Zhou, Z.; Feng, C.; Cheng, X.; Chen, X. Systematic investigation of fabrication conditions of nanocarrier based on carboxymethyl chitosan for sustained release of insulin. Int. J. Biol. Macromol. 2017, 102, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, S.; Feng, X.; Chen, Y.; Chen, S.; Ma, J.; Zhou, F. Tannic acid-crosslinked O-carboxymethyl chitosan hydrogels for enhanced antibacterial activity and rapid hemostasis. J. Biomater. Sci. Polym. Ed. 2023, 34, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Kalliola, S.; Repo, E.; Srivastava, V.; Heiskanen, J.P.; Sirviö, J.A.; Liimatainen, H.; Sillanpää, M. The pH sensitive properties of carboxymethyl chitosan nanoparticles cross-linked with calcium ions. Colloids Surf. B Biointerfaces 2017, 153, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, J.; Yuan, Q.; Zhan, C.; Shang, Z.; Gu, Q.; Zhang, J.; Fu, G.; Hu, W. Characterization of ginsenoside compound K loaded ionically cross-linked carboxymethyl chitosan–calcium nanoparticles and its cytotoxic potential against prostate cancer cells. J. Ginseng Res. 2021, 45, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Wan, Y.; Xu, F.; Lu, J.; Yang, C.; Li, X.; Lu, Z.; Wang, X.; Wang, D. Ionic crosslinking of alginate/carboxymethyl chitosan fluorescent hydrogel for bacterial detection and sterilization. Carbohydr. Polym. 2023, 302, 120427. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Zhang, K.; Bai, X.; Liu, P.; Lyu, Z.; Li, A. Study on the Preparation and Properties of Carboxymethyl Chitosan as Fast Hemostatic Material. Polym. Sci. Ser. B 2021, 63, 843–852. [Google Scholar] [CrossRef]
- Karami, F.; Saber-Samandari, S. Synthesis and characterization of a novel hydrogel based on carboxymethyl chitosan/sodium alginate with the ability to release simvastatin for chronic wound healing. Biomed. Mater. 2023, 18, 025001. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, Z.; Lyu, Y.; Yang, J.; Lin, L.; Bai, H.; Li, Y.; Feng, Y.; Chen, Y. Electroactive injectable hydrogel based on oxidized sodium alginate and carboxymethyl chitosan for wound healing. Int. J. Biol. Macromol. 2023, 230, 123231. [Google Scholar] [CrossRef]
- Hu, K.; Jia, E.; Zhang, Q.; Zheng, W.; Sun, R.; Qian, M.; Tan, Y.; Hu, W. Injectable carboxymethyl chitosan-genipin hydrogels encapsulating tea tree oil for wound healing. Carbohydr. Polym. 2023, 301, 120348. [Google Scholar] [CrossRef]
- Wu, M.; Lin, M.; Li, P.; Huang, X.; Tian, K.; Li, C. Local anesthetic effects of lidocaine-loaded carboxymethyl chitosan cross-linked with sodium alginate hydrogels for drug delivery system, cell adhesion, and pain management. J. Drug Deliv. Sci. Technol. 2023, 79, 104007. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, X.; Kong, Y.; Li, R.; Li, T.; Wang, R.; Ma, Z.; Liang, Y.-m.; Ma, S.; Zhou, F. Injectable carboxymethyl chitosan/nanosphere-based hydrogel with dynamic crosslinking network for efficient lubrication and sustained drug release. Int. J. Biol. Macromol. 2023, 229, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Liu, Y.; Wang, X.; Yan, H.; Wang, L.; Qu, L.; Kong, D.; Qiao, M.; Wang, L. Injectable hydrogel composed of hydrophobically modified chitosan/oxidized-dextran for wound healing. Mater. Sci. Eng. C 2019, 104, 109930. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Feng, G.; Wang, J.; Zhang, R.; Zhong, S.; Wang, J.; Cui, X. Injectable chitosan-based self-healing supramolecular hydrogels with temperature and pH dual-responsivenesses. Int. J. Biol. Macromol. 2023, 227, 1038–1047. [Google Scholar] [CrossRef]
- Salama, H.E.; Abdel Aziz, M.S. Novel biocompatible and antimicrobial supramolecular O-carboxymethyl chitosan biguanidine/zinc physical hydrogels. Int. J. Biol. Macromol. 2020, 163, 649–656. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, C.; Liu, Y.; Luo, W.; Deng, H.; Shen, J. Chitosan biguanide induced mitochondrial inhibition to amplify the efficacy of oxygen-sensitive tumor therapies. Carbohydr. Polym. 2022, 295, 119878. [Google Scholar] [CrossRef]
- Cho, I.S.; Cho, M.O.; Li, Z.; Nurunnabi, M.; Park, S.Y.; Kang, S.-W.; Huh, K.M. Synthesis and characterization of a new photo-crosslinkable glycol chitosan thermogel for biomedical applications. Carbohydr. Polym. 2016, 144, 59–67. [Google Scholar] [CrossRef]
- Kim, D.-E.; Lee, Y.B.; Shim, H.-E.; Song, J.J.; Han, J.-S.; Moon, K.-S.; Huh, K.M.; Kang, S.-W. Application of Hexanoyl Glycol Chitosan as a Non-cell Adhesive Polymer in Three-Dimensional Cell Culture. ACS Omega 2022, 7, 18471–18480. [Google Scholar] [CrossRef]
- Jang, B.S.; Park, K.H.; Suh, E.Y.; Lee, B.-S.; Kang, S.-W.; Huh, K.M. Non-cell adhesive hexanoyl glycol chitosan hydrogels for stable and efficient formation of 3D cell spheroids with tunable size and density. Int. J. Biol. Macromol. 2021, 187, 955–963. [Google Scholar] [CrossRef]
- Cao, Z.; Su, C.; Sun, X.; Shao, K.; Wang, X.; Mu, Y.; Chen, X.; Feng, C. Enhanced mechanical properties of hydroxybutyl chitosan hydrogel through anchoring interface effects of diatom biosilica. Carbohydr. Polym. 2022, 296, 119975. [Google Scholar] [CrossRef]
- Wan, Z.; Dong, Q.; Guo, X.; Bai, X.; Zhang, X.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. A dual-responsive polydopamine-modified hydroxybutyl chitosan hydrogel for sequential regulation of bone regeneration. Carbohydr. Polym. 2022, 297, 120027. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, L.; Gao, C.; Huang, J.; Li, Q.; Zhang, Z. Highly resilient, biocompatible, and antibacterial carbon nanotube/hydroxybutyl chitosan sponge dressing for rapid and effective hemostasis. J. Mater. Chem. B 2021, 9, 9754–9763. [Google Scholar] [CrossRef]
- Inanan, T. Chitosan Co-polymeric nanostructures for catalase immobilization. React. Funct. Polym. 2019, 135, 94–102. [Google Scholar] [CrossRef]
- Apryatina, K.V.; Tkachuk, E.K.; Smirnova, L.A. Influence of macromolecules conformation of chitosan on its graft polymerization with vinyl monomers and the copolymer properties. Carbohydr. Polym. 2020, 235, 115954. [Google Scholar] [CrossRef] [PubMed]
- Dimonie, D.; Dima, Ş.-O.; Petrache, M. Influence of centrifugation on the molecular parameters of chitosan solubilized in weakly acidic aqueous solutions. Dig. J. Nanomater. Biostruct. 2013, 8, 1799–1809. [Google Scholar]
- Mochalova, A.E.; Kruglova, E.N.; Yunin, P.A.; Apryatina, K.V.; Smirnova, O.N.; Smirnova, L.A. Graft and block copolymers of chitosan with vinyl monomers: Synthesis, structure, and properties. Polym. Sci. Ser. B 2015, 57, 93–105. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Maté, J.I.; Gardrat, C.; Coma, V. Effect of chitosan molecular weight on the antimicrobial activity and release rate of carvacrol-enriched films. Food Hydrocoll. 2015, 51, 60–68. [Google Scholar] [CrossRef]
- Vörös-Horváth, B.; Živković, P.; Bánfai, K.; Bóvári-Biri, J.; Pongrácz, J.; Bálint, G.; Pál, S.; Széchenyi, A. Preparation and Characterization of ACE2 Receptor Inhibitor-Loaded Chitosan Hydrogels for Nasal Formulation to Reduce the Risk of COVID-19 Viral Infection. ACS Omega 2022, 7, 3240–3253. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, D.R.; Chon, J.W.; Chung, D.J. Hemostatic Patches Based on Crosslinked Chitosan Films Applied in Interventional Procedures. Polymers 2021, 13, 2402. [Google Scholar] [CrossRef]
- Sánchez-Andica, R.A.; Páez-Melo, M.I.; Sánchez-Domínguez, M. Preparation and characterization of a controlled-release formulation based on carbofuran loaded in ionically cross-linked chitosan microparticles. J. Polym. Res. 2020, 27, 332. [Google Scholar] [CrossRef]
- Gwak, M.A.; Lee, S.J.; Lee, D.; Park, S.A.; Park, W.H. Highly gallol-substituted, rapidly self-crosslinkable, and robust chitosan hydrogel for 3D bioprinting. Int. J. Biol. Macromol. 2023, 227, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Gordienko, M.G.; Palchikova, V.V.; Kalenov, S.V.; Lebedev, E.A.; Belov, A.A.; Menshutina, N.V. The alginate–chitosan composite sponges with biogenic Ag nanoparticles produced by combining of cryostructuration, ionotropic gelation and ion replacement methods. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 34–44. [Google Scholar] [CrossRef]
- Sheir, M.M.; Nasra, M.M.A.; Abdallah, O.Y. Chitosan alginate nanoparticles as a platform for the treatment of diabetic and non-diabetic pressure ulcers: Formulation and in vitro/in vivo evaluation. Int. J. Pharm. 2021, 607, 120963. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Feng, Z.; Li, Z.; Lin, Z.; Chen, L.; Li, B.; Chen, Z.; Wu, Z.; Zeng, J.; Zhang, J.; et al. Carboxymethyl chitosan/sodium alginate hydrogels with polydopamine coatings as promising dressings for eliminating biofilm and multidrug-resistant bacteria induced wound healing. Int. J. Biol. Macromol. 2023, 225, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Jafarimanesh, M.A.; Ai, J.; Shojae, S.; Khonakdar, H.A.; Darbemamieh, G.; Shirian, S. Sustained release of valproic acid loaded on chitosan nanoparticles within hybrid of alginate/chitosan hydrogel with/without stem cells in regeneration of spinal cord injury. Prog. Biomater. 2023, in press. [Google Scholar] [CrossRef]
- Zarandona, I.; Bengoechea, C.; Álvarez-Castillo, E.; de la Caba, K.; Guerrero, A.; Guerrero, P. 3D Printed Chitosan-Pectin Hydrogels: From Rheological Characterization to Scaffold Development and Assessment. Gels 2021, 7, 175. [Google Scholar] [CrossRef]
- Song, K.; Hao, Y.; Liu, Y.; Cao, R.; Zhang, X.; He, S.; Wen, J.; Zheng, W.; Wang, L.; Zhang, Y. Preparation of pectin-chitosan hydrogels based on bioadhesive-design micelle to prompt bacterial infection wound healing. Carbohydr. Polym. 2023, 300, 120272. [Google Scholar] [CrossRef]
- Gubanova, G.N.; Petrova, V.A.; Kononova, S.V.; Popova, E.N.; Smirnova, V.E.; Bugrov, A.N.; Klechkovskaya, V.V.; Skorik, Y.A. Thermal Properties and Structural Features of Multilayer Films Based on Chitosan and Anionic Polysaccharides. Biomolecules 2021, 11, 762. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.-l.; Siu, W.S.; Kan, C.-W.; Leung, P.-C.; Wanxue, C.; Chiou, J.-C. Influence of pH-responsive compounds synthesized from chitosan and hyaluronic acid on dual-responsive (pH/temperature) hydrogel drug delivery systems of Cortex Moutan. Int. J. Biol. Macromol. 2021, 168, 163–174. [Google Scholar] [CrossRef]
- Miranda, D.G.; Malmonge, S.M.; Campos, D.M.; Attik, N.G.; Grosgogeat, B.; Gritsch, K. A chitosan-hyaluronic acid hydrogel scaffold for periodontal tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1691–1702. [Google Scholar] [CrossRef]
- Sun, J.; Schiffman, J.D.; Perry, S.L. Linear Viscoelasticity and Time–Alcohol Superposition of Chitosan/Hyaluronic Acid Complex Coacervates. ACS Appl. Polym. Mater. 2022, 4, 1617–1625. [Google Scholar] [CrossRef]
- Yuan, F.-Z.; Wang, H.-F.; Guan, J.; Fu, J.-N.; Yang, M.; Zhang, J.-Y.; Chen, Y.-R.; Wang, X.; Yu, J.-K. Fabrication of Injectable Chitosan-Chondroitin Sulfate Hydrogel Embedding Kartogenin-Loaded Microspheres as an Ultrasound-Triggered Drug Delivery System for Cartilage Tissue Engineering. Pharmaceutics 2021, 13, 1487. [Google Scholar] [CrossRef] [PubMed]
- Ismillayli, N.; Hadi, S.; Andayani, I.G.A.S.; Honiar, R.; Mariana, B.; Sanjaya, R.K.; Hermanto, D. Synthesize of self-electrostatic interaction chitosan-carrageenan membrane and its properties. J. Phys. Conf. Ser. 2021, 1943, 012177. [Google Scholar] [CrossRef]
- Srivastava, N.; Richa; Choudhury, A.R. Enhanced encapsulation efficiency and controlled release of co-encapsulated Bacillus coagulans spores and vitamin B9 in gellan/κ-carrageenan/chitosan tri-composite hydrogel. Int. J. Biol. Macromol. 2023, 227, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Papagiannopoulos, A.; Nikolakis, S.-P.; Pamvouxoglou, A.; Koutsopoulou, E. Physicochemical properties of electrostatically crosslinked carrageenan/chitosan hydrogels and carrageenan/chitosan/Laponite nanocomposite hydrogels. Int. J. Biol. Macromol. 2023, 225, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Hasan, A.; Pandey, L.M. Antibacterial nano-biocomposite scaffolds of Chitosan, Carboxymethyl Cellulose and Zn & Fe integrated Hydroxyapatite (Chitosan-CMC-FZO@HAp) for bone tissue engineering. Cellulose 2021, 28, 9207–9226. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Karperien, M.; Johnbosco, C.; Mahmood, A.; Kousar, M. Chitosan and carboxymethyl cellulose-based 3D multifunctional bioactive hydrogels loaded with nano-curcumin for synergistic diabetic wound repair. Int. J. Biol. Macromol. 2023, 227, 1203–1220. [Google Scholar] [CrossRef]
- Ensandoost, R.; Izadi-Vasafi, H.; Adelnia, H. Anti-Bacterial Activity of Chitosan-Alginate-Poly (Vinyl Alcohol) Hydrogel Containing Entrapped Peppermint Essential Oil. J. Macromol. Sci. Pt. B 2022, 61, 225–237. [Google Scholar] [CrossRef]
- Ahmed, R.; Afreen, A.; Tariq, M.; Zahid, A.A.; Masoud, M.S.; Ahmed, M.; Ali, I.; Akram, Z.; Hasan, A. Bone marrow mesenchymal stem cells preconditioned with nitric-oxide-releasing chitosan/PVA hydrogel accelerate diabetic wound healing in rabbits. Biomed. Mater. 2021, 16, 035014. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, W.; Chen, G.; Li, X.; Wang, Y.; Xiong, J.; Wei, L. Preparation and Characterization of Polyvinyl Alcohol-Chitosan/Cerium Hydrogel with Significant Antibacterial Activity. Starch Stärke 2021, 73, 2000253. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Wang, Y.; Zhou, G.; Ge, K.; Jiang, L. Adhesive, antibacterial and double crosslinked carboxylated polyvinyl alcohol/chitosan hydrogel to enhance dynamic skin wound healing. Int. J. Biol. Macromol. 2023, 228, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Supper, S.; Anton, N.; Seidel, N.; Riemenschnitter, M.; Schoch, C.; Vandamme, T. Rheological Study of Chitosan/Polyol-phosphate Systems: Influence of the Polyol Part on the Thermo-Induced Gelation Mechanism. Langmuir 2013, 29, 10229–10237. [Google Scholar] [CrossRef] [PubMed]
- Hashad, R.A.; Ishak, R.A.H.; Fahmy, S.; Mansour, S.; Geneidi, A.S. Chitosan-tripolyphosphate nanoparticles: Optimization of formulation parameters for improving process yield at a novel pH using artificial neural networks. Int. J. Biol. Macromol. 2016, 86, 50–58. [Google Scholar] [CrossRef]
- Allam, A.F.; Hagras, N.A.-E.; Farag, H.F.; Osman, M.M.; Shalaby, T.I.; Kazem, A.H.; Shehab, A.Y.; Mogahed, N.M.F.H. Remarkable histopathological improvement of experimental toxoplasmosis after receiving spiramycin-chitosan nanoparticles formulation. J. Parasit. Dis. 2022, 46, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Khoerunnisa, F.; Nurhayati, M.; Dara, F.; Rizki, R.; Nasir, M.; Aziz, H.A.; Hendrawan, H.; Poh, N.E.; Kaewsaneha, C.; Opaprakasit, P. Physicochemical Properties of TPP-Crosslinked Chitosan Nanoparticles as Potential Antibacterial Agents. Fibers Polym. 2021, 22, 2954–2964. [Google Scholar] [CrossRef]
- Barrera-Martínez, C.L.; Padilla-Vaca, F.; Liakos, I.; Meléndez-Ortiz, H.I.; Cortez-Mazatan, G.Y.; Peralta-Rodríguez, R.D. Chitosan microparticles as entrapment system for trans- cinnamaldehyde: Synthesis, drug loading, and in vitro cytotoxicity evaluation. Int. J. Biol. Macromol. 2021, 166, 322–332. [Google Scholar] [CrossRef]
- Barkhordari, S.; Alizadeh, A.; Yadollahi, M.; Namazi, H. One-pot synthesis of magnetic chitosan/iron oxide bio-nanocomposite hydrogel beads as drug delivery systems. Soft Mater. 2021, 19, 373–381. [Google Scholar] [CrossRef]
- Liu, H.; Meng, X.; Li, L.; Xia, Y.; Hu, X.; Fang, Y. The incorporated hydrogel of chitosan-oligoconjugated linoleic acid vesicles and the protective sustained release for curcumin in the gel. Int. J. Biol. Macromol. 2023, 227, 17–26. [Google Scholar] [CrossRef]
- Cai, Y.; Lapitsky, Y. Formation and dissolution of chitosan/pyrophosphate nanoparticles: Is the ionic crosslinking of chitosan reversible? Colloids Surf. B Biointerfaces 2014, 115, 100–108. [Google Scholar] [CrossRef]
- Huang, Y.; Lapitsky, Y. Determining the Colloidal Behavior of Ionically Cross-Linked Polyelectrolytes with Isothermal Titration Calorimetry. J. Phys. Chem. B 2013, 117, 9548–9557. [Google Scholar] [CrossRef]
- Sacco, P.; Paoletti, S.; Cok, M.; Asaro, F.; Abrami, M.; Grassi, M.; Donati, I. Insight into the ionotropic gelation of chitosan using tripolyphosphate and pyrophosphate as cross-linkers. Int. J. Biol. Macromol. 2016, 92, 476–483. [Google Scholar] [CrossRef]
- Sacco, P.; Brun, F.; Donati, I.; Porrelli, D.; Paoletti, S.; Turco, G. On the Correlation between the Microscopic Structure and Properties of Phosphate-Cross-Linked Chitosan Gels. ACS Appl. Mater. Interfaces 2018, 10, 10761–10770. [Google Scholar] [CrossRef]
- Skwarczynska, A.; Kaminska, M.; Owczarz, P.; Bartoszek, N.; Walkowiak, B.; Modrzejewska, Z. The structural (FTIR, XRD, and XPS) and biological studies of thermosensitive chitosan chloride gels with β-glycerophosphate disodium. J. Appl. Polym. Sci. 2018, 135, 46459. [Google Scholar] [CrossRef]
- Pieklarz, K.; Galita, G.; Tylman, M.; Maniukiewicz, W.; Kucharska, E.; Majsterek, I.; Modrzejewska, Z. Physico-Chemical Properties and Biocompatibility of Thermosensitive Chitosan Lactate and Chitosan Chloride Hydrogels Developed for Tissue Engineering Application. J. Funct. Biomater. 2021, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Filion, D.; Buschmann, M.D. Chitosan–glycerol-phosphate (GP) gels release freely diffusible GP and possess titratable fixed charge. Carbohydr. Polym. 2013, 98, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, V.Y.; Burova, T.V.; Grinberg, N.V.; Tikhonov, V.E.; Dubovik, A.S.; Moskalets, A.P.; Khokhlov, A.R. Thermodynamic insight into the thermoresponsive behavior of chitosan in aqueous solutions: A differential scanning calorimetry study. Carbohydr. Polym. 2020, 229, 115558. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Starnes, T.; Ko, J.-W.K.; Laurencin, C.T. Development of Injectable Thermogelling Chitosan–Inorganic Phosphate Solutions for Biomedical Applications. Biomacromolecules 2007, 8, 3779–3785. [Google Scholar] [CrossRef]
- Ceccaldi, C.; Assaad, E.; Hui, E.; Buccionyte, M.; Adoungotchodo, A.; Lerouge, S. Optimization of Injectable Thermosensitive Scaffolds with Enhanced Mechanical Properties for Cell Therapy. Macromol. Biosci. 2017, 17, 1600435. [Google Scholar] [CrossRef]
- Deng, A.; Kang, X.; Zhang, J.; Yang, Y.; Yang, S. Enhanced gelation of chitosan/β-sodium glycerophosphate thermosensitive hydrogel with sodium bicarbonate and biocompatibility evaluated. Mater. Sci. Eng. C 2017, 78, 1147–1154. [Google Scholar] [CrossRef]
- Alinejad, Y.; Adoungotchodo, A.; Hui, E.; Zehtabi, F.; Lerouge, S. An injectable chitosan/chondroitin sulfate hydrogel with tunable mechanical properties for cell therapy/tissue engineering. Int. J. Biol. Macromol. 2018, 113, 132–141. [Google Scholar] [CrossRef]
- Stanzione, A.; Polini, A.; La Pesa, V.; Quattrini, A.; Romano, A.; Gigli, G.; Moroni, L.; Gervaso, F. Thermosensitive chitosan-based hydrogels supporting motor neuron-like NSC-34 cell differentiation. Biomater. Sci. 2021, 9, 7492–7503. [Google Scholar] [CrossRef] [PubMed]
- Dang, P.A.; Palomino-Durand, C.; Elsafi Mabrouk, M.; Marquaille, P.; Odier, C.; Norvez, S.; Pauthe, E.; Corté, L. Rational formulation design of injectable thermosensitive chitosan-based hydrogels for cell encapsulation and delivery. Carbohydr. Polym. 2022, 277, 118836. [Google Scholar] [CrossRef] [PubMed]
- Coburn, P.T.; Herbay, A.C.; Berrini, M.; Li-Jessen, N.Y.K. An in vitro assessment of the response of THP-1 macrophages to varying stiffness of a glycol-chitosan hydrogel for vocal fold tissue engineering applications. J. Biomed. Mater. Res. Pt. A 2021, 109, 1337–1352. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.; Morimitsu, Y.; Hisadome, T.; Inomoto, F.; Yoshida, M.; Takei, T. Optimized hydrophobically modified chitosan cryogels for strength and drug delivery systems. J. Biosci. Bioeng. 2021, 132, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Olaru, A.-M.; Marin, L.; Morariu, S.; Pricope, G.; Pinteala, M.; Tartau-Mititelu, L. Biocompatible chitosan based hydrogels for potential application in local tumour therapy. Carbohydr. Polym. 2018, 179, 59–70. [Google Scholar] [CrossRef]
- Iftime, M.-M.; Morariu, S.; Marin, L. Salicyl-imine-chitosan hydrogels: Supramolecular architecturing as a crosslinking method toward multifunctional hydrogels. Carbohydr. Polym. 2017, 165, 39–50. [Google Scholar] [CrossRef]
- Liu, C.; Dong, C.; Liu, S.; Yang, Y.; Zhang, Z. Multiple chiroptical switches and logic circuit based on salicyl-imine-chitosan hydrogel. Carbohydr. Polym. 2021, 257, 117534. [Google Scholar] [CrossRef]
- Marin, L.; Ailincai, D.; Morariu, S.; Tartau-Mititelu, L. Development of biocompatible glycodynameric hydrogels joining two natural motifs by dynamic constitutional chemistry. Carbohydr. Polym. 2017, 170, 60–71. [Google Scholar] [CrossRef]
- Ailincai, D.; Porzio, W.; Marin, L. Hydrogels Based on Imino-Chitosan Amphiphiles as a Matrix for Drug Delivery Systems. Polymers 2020, 12, 2687. [Google Scholar] [CrossRef]
- Damiri, F.; Bachra, Y.; Bounacir, C.; Laaraibi, A.; Berrada, M. Synthesis and Characterization of Lyophilized Chitosan-Based Hydrogels Cross-Linked with Benzaldehyde for Controlled Drug Release. J. Chem. 2020, 2020, 8747639. [Google Scholar] [CrossRef]
- Bratskaya, S.; Privar, Y.; Skatova, A.; Slobodyuk, A.; Kantemirova, E.; Pestov, A. Carboxyalkylchitosan-based hydrogels with “imine clip”: Enhanced stability and amino acids-induced disassembly under physiological conditions. Carbohydr. Polym. 2021, 274, 118618. [Google Scholar] [CrossRef]
- Bratskaya, S.; Skatova, A.; Privar, Y.; Boroda, A.; Kantemirova, E.; Maiorova, M.; Pestov, A. Stimuli-Responsive Dual Cross-Linked N-Carboxyethylchitosan Hydrogels with Tunable Dissolution Rate. Gels 2021, 7, 188. [Google Scholar] [CrossRef]
- Kłosiński, K.K.; Wach, R.A.; Girek-Bąk, M.K.; Rokita, B.; Kołat, D.; Kałuzińska-Kołat, Ż.; Kłosińska, B.; Duda, Ł.; Pasieka, Z.W. Biocompatibility and Mechanical Properties of Carboxymethyl Chitosan Hydrogels. Polymers 2023, 15, 144. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, J.; Tai, J.; Han, Q.; Wang, L.; Wang, K.; Wang, S.; Fan, Y. Comparison of chitosan microsphere versus O-carboxymethyl chitosan microsphere for drug delivery systems. J. Bioact. Compat. Polym. 2017, 32, 469–486. [Google Scholar] [CrossRef]
- Vo, N.T.N.; Huang, L.; Lemos, H.; Mellor, A.L.; Novakovic, K. Genipin-crosslinked chitosan hydrogels: Preliminary evaluation of the in vitro biocompatibility and biodegradation. J. Appl. Polym. Sci. 2021, 138, 50848. [Google Scholar] [CrossRef]
- Kaufmann, G.; Klein, M.P.; Goettert, M.I.; Aguirre, T.A.S. Development and cytotoxicity evaluation of a cylindrical pH-responsive chitosan-genipin hydrogel for the oral delivery of diclofenac sodium. Eur. Polym. J. 2022, 181, 111649. [Google Scholar] [CrossRef]
- Dimida, S.; Demitri, C.; De Benedictis, V.M.; Scalera, F.; Gervaso, F.; Sannino, A. Genipin-cross-linked chitosan-based hydrogels: Reaction kinetics and structure-related characteristics. J. Appl. Polym. Sci. 2015, 132, 42256. [Google Scholar] [CrossRef]
- Koc, F.E.; Altıncekic, T.G. Investigation of gelatin/chitosan as potential biodegradable polymer films on swelling behavior and methylene blue release kinetics. Polym. Bull. 2021, 78, 3383–3398. [Google Scholar] [CrossRef]
- Gao, L.; Gan, H.; Meng, Z.; Gu, R.; Wu, Z.; Zhang, L.; Zhu, X.; Sun, W.; Li, J.; Zheng, Y.; et al. Effects of genipin cross-linking of chitosan hydrogels on cellular adhesion and viability. Colloids Surf. B Biointerfaces 2014, 117, 398–405. [Google Scholar] [CrossRef]
- Sukul, M.; Sahariah, P.; Lauzon, H.L.; Borges, J.; Másson, M.; Mano, J.F.; Haugen, H.J.; Reseland, J.E. In vitro biological response of human osteoblasts in 3D chitosan sponges with controlled degree of deacetylation and molecular weight. Carbohydr. Polym. 2021, 254, 117434. [Google Scholar] [CrossRef]
- Govindaraj, P.; Raghavachari, D. Fabrication of macroporous soft hydrogels of Chitosan scaffolds by hydrothermal reaction and cytotoxicity to 3T3 L1 cells. J. Polym. Res. 2021, 28, 86. [Google Scholar] [CrossRef]
- Yu, S.-H.; Wu, S.-J.; Wu, J.-Y.; Wen, D.-Y.; Mi, F.-L. Preparation of fucoidan-shelled and genipin-crosslinked chitosan beads for antibacterial application. Carbohydr. Polym. 2015, 126, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Pujana, M.A.; Pérez-Álvarez, L.; Iturbe, L.C.C.; Katime, I. Water soluble folate-chitosan nanogels crosslinked by genipin. Carbohydr. Polym. 2014, 101, 113–120. [Google Scholar] [CrossRef]
- Moura, M.J.; Gil, M.H.; Figueiredo, M.M. Cisplatin delivery systems based on different drug encapsulation techniques. Eur. Polym. J. 2019, 113, 357–364. [Google Scholar] [CrossRef]
- Rezaei, A.; Hooman Vahidi, S.; Nasrabadi, M.; Ali Beyramabadi, S.; Morsali, A. Quantum chemical study of 2-hydroxypropyl-β-cyclodextrin and genipin-crosslinked chitosan nanocarriers functionalized with cytarabine anticancer drug. J. Mol. Liq. 2022, 367, 120427. [Google Scholar] [CrossRef]
- Yin, R.; Wang, K.; Du, S.; Chen, L.; Nie, J.; Zhang, W. Design of genipin-crosslinked microgels from concanavalin A and glucosyloxyethyl acrylated chitosan for glucose-responsive insulin delivery. Carbohydr. Polym. 2014, 103, 369–376. [Google Scholar] [CrossRef]
- Zuo, R.; Shi, J.; Jiang, S.; Chu, M.; Wang, Q.; Kong, L.; Kang, Q.; Guo, Y.; Guan, J. Promotion of the genipin crosslinked chitosan-fiber hydrogel loaded with sustained release of clemastine fumarate in diabetic wound repair. Int. J. Biol. Macromol. 2023, 226, 900–914. [Google Scholar] [CrossRef]
- Jalani, G.; Rosenzweig, D.H.; Makhoul, G.; Abdalla, S.; Cecere, R.; Vetrone, F.; Haglund, L.; Cerruti, M. Tough, In-Situ Thermogelling, Injectable Hydrogels for Biomedical Applications. Macromol. Biosci. 2015, 15, 473–480. [Google Scholar] [CrossRef]
- Valmikinathan, C.M.; Mukhatyar, V.J.; Jain, A.; Karumbaiah, L.; Dasari, M.; Bellamkonda, R.V. Photocrosslinkable chitosan based hydrogels for neural tissue engineering. Soft Matter 2012, 8, 1964–1976. [Google Scholar] [CrossRef]
- Qi, Z.; Xu, J.; Wang, Z.; Nie, J.; Ma, G. Preparation and properties of photo-crosslinkable hydrogel based on photopolymerizable chitosan derivative. Int. J. Biol. Macromol. 2013, 53, 144–149. [Google Scholar] [CrossRef]
- Ahn, J.; Ryu, J.; Song, G.; Whang, M.; Kim, J. Network structure and enzymatic degradation of chitosan hydrogels determined by crosslinking methods. Carbohydr. Polym. 2019, 217, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-J.; Kim, S.H.; Choi, J.W.; Chun, H.J.; Yang, D.H. Guided cortical and cancellous bone formation using a minimally invasive technique of BMSC- and BMP-2-laden visible light-cured carboxymethyl chitosan hydrogels. Int. J. Biol. Macromol. 2023, 227, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, B.; Xu, F.; Xu, Z.; Wei, D.; Feng, Y.; Wang, Y.; Jia, D.; Zhou, Y. UV-crosslinkable and thermo-responsive chitosan hybrid hydrogel for NIR-triggered localized on-demand drug delivery. Carbohydr. Polym. 2017, 174, 904–914. [Google Scholar] [CrossRef]
- Yang, J.; Liu, F.; Zhou, C.; Li, H.; Yang, G.; Fang, S.; Lee, I.-S.; Liu, Y.; Bai, H.; Chen, C. 3D printed porous titanium filled with mineralized UV-responsive chitosan hydrogel promotes cell proliferation and osteogenesis in vitro. J. Mater. Sci. Technol. 2023, 142, 34–44. [Google Scholar] [CrossRef]
- Zanon, M.; Chiappone, A.; Garino, N.; Canta, M.; Frascella, F.; Hakkarainen, M.; Pirri, C.F.; Sangermano, M. Microwave-assisted methacrylation of chitosan for 3D printable hydrogels in tissue engineering. Mater. Adv. 2022, 3, 514–525. [Google Scholar] [CrossRef]
- Li, B.; Wang, L.; Xu, F.; Gang, X.; Demirci, U.; Wei, D.; Li, Y.; Feng, Y.; Jia, D.; Zhou, Y. Hydrosoluble, UV-crosslinkable and injectable chitosan for patterned cell-laden microgel and rapid transdermal curing hydrogel in vivo. Acta Biomater. 2015, 22, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.; Howard, D.; Takae, S.; Wang, W.; Vermonden, T.; Hennink, W.E.; Stayton, P.S.; Hoffman, A.S.; Endruweit, A.; Alexander, C.; et al. Photo-Cross-Linked Hydrogels from Thermoresponsive PEGMEMA-PPGMA-EGDMA Copolymers Containing Multiple Methacrylate Groups: Mechanical Property, Swelling, Protein Release, and Cytotoxicity. Biomacromolecules 2009, 10, 2895–2903. [Google Scholar] [CrossRef]
- Seo, J.W.; Shin, S.R.; Lee, M.-Y.; Cha, J.M.; Min, K.H.; Lee, S.C.; Shin, S.Y.; Bae, H. Injectable hydrogel derived from chitosan with tunable mechanical properties via hybrid-crosslinking system. Carbohydr. Polym. 2021, 251, 117036. [Google Scholar] [CrossRef]
- Luo, X.; Liu, Y.; Pang, J.; Bi, S.; Zhou, Z.; Lu, Z.; Feng, C.; Chen, X.; Kong, M. Thermo/photo dual-crosslinking chitosan-gelatin methacrylate hydrogel with controlled shrinking property for contraction fabrication. Carbohydr. Polym. 2020, 236, 116067. [Google Scholar] [CrossRef]
- Seo, J.W.; Shin, S.R.; Park, Y.J.; Bae, H. Hydrogel Production Platform with Dynamic Movement Using Photo-Crosslinkable/Temperature Reversible Chitosan Polymer and Stereolithography 4D Printing Technology. Tissue Eng. Regen. Med. 2020, 17, 423–431. [Google Scholar] [CrossRef]
- Zhu, Y.; Qin, D.; Liu, J.; Wu, G.; Wang, H.; Wu, F.; Liu, Y.; Liu, Y.; Cheng, X.; Chen, X. Chitin whiskers enhanced methacrylated hydroxybutyl chitosan hydrogels as anti-deformation scaffold for 3D cell culture. Carbohydr. Polym. 2023, 304, 120483. [Google Scholar] [CrossRef]
- Che, Q.T.; Charoensri, K.; Seo, J.W.; Nguyen, M.H.; Jang, G.; Bae, H.; Park, H.J. Triple-conjugated photo-/temperature-/pH-sensitive chitosan with an intelligent response for bioengineering applications. Carbohydr. Polym. 2022, 298, 120066. [Google Scholar] [CrossRef]
- Pieklarz, K.; Jenczyk, J.; Modrzejewska, Z.; Owczarz, P.; Jurga, S. An Investigation of the Sol-Gel Transition of Chitosan Lactate and Chitosan Chloride Solutions via Rheological and NMR Studies. Gels 2022, 8, 670. [Google Scholar] [CrossRef] [PubMed]
- Songkroh, T.; Xie, H.; Yu, W.; Lv, G.; Liu, X.; Wang, L.; Sun, G.; Xu, X.; Ma, X. In situ forming chitosan-based hydrogel as a lung sealant for biological lung volume reduction. Sci. Bull. 2015, 60, 235–240. [Google Scholar] [CrossRef]
- Ailincai, D.; Marin, L.; Morariu, S.; Mares, M.; Bostanaru, A.-C.; Pinteala, M.; Simionescu, B.C.; Barboiu, M. Dual crosslinked iminoboronate-chitosan hydrogels with strong antifungal activity against Candida planktonic yeasts and biofilms. Carbohydr. Polym. 2016, 152, 306–316. [Google Scholar] [CrossRef]
- Taaca, K.L.M.; De Leon, M.J.D.; Thumanu, K.; Nakajima, H.; Chanlek, N.; Prieto, E.I.; Vasquez, M.R. Probing the structural features of a plasma-treated chitosan-acrylic acid hydrogel. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128233. [Google Scholar] [CrossRef]
- Iglesias, N.; Galbis, E.; Valencia, C.; Díaz-Blanco, M.J.; Lacroix, B.; de-Paz, M.V. Biodegradable double cross-linked chitosan hydrogels for drug delivery: Impact of chemistry on rheological and pharmacological performance. Int. J. Biol. Macromol. 2020, 165, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Krishna Murthy, S.; Veerabhadraiah Basavaraj, B.; Srinivasan, B. Microwave assisted vanillin crosslinked chitosan/polycarbophil superporous hydrogels for biomedical application: Optimization and characterization. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Liu, C.; Liu, C.; Liu, Z.; Shi, Z.; Liu, S.; Wang, X.; Wang, X.; Huang, F. Injectable thermogelling bioadhesive chitosan-based hydrogels for efficient hemostasis. Int. J. Biol. Macromol. 2023, 224, 1091–1100. [Google Scholar] [CrossRef]
- Tao, Q.; Zhong, J.; Wang, R.; Huang, Y. Ionic and Enzymatic Multiple-Crosslinked Nanogels for Drug Delivery. Polymers 2021, 13, 3565. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Cui, Y.; Guo, Y.; Zhao, P.; Wang, J.; Zhang, R.; Wang, X. Design of carboxymethyl chitosan-reinforced pH-responsive hydrogels for on-demand release of carvacrol and simulation of release kinetics. Food Chem. 2023, 405, 134856. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, W.; Zhang, M.; Ma, Z.; Zhao, T.; Zhang, Y. Preparation and characterization of Panax notoginseng saponins loaded hyaluronic acid/carboxymethyl chitosan hydrogel for type o diabetic wound healing. Mater. Today Commun. 2023, 34, 105284. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, F.; Pang, Q.; Hu, Y.; Wu, H.; Yu, X.; Chen, X.; Shi, L.; Wen, B.; Xu, R.; et al. The osteogenesis and the biologic mechanism of thermo-responsive injectable hydrogel containing carboxymethyl chitosan/sodium alginate nanoparticles towards promoting osteal wound healing. Int. J. Biol. Macromol. 2023, 224, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, Q.; Chen, Q.; Ma, Y.; Wang, Z.; Luo, L.; Ding, Q.; Li, H.; Tang, S. Carboxymethyl Chitosan/Tannic Acid Hydrogel with Antibacterial, Hemostasis, and Antioxidant Properties Promoting Skin Wound Repair. ACS Biomater. Sci. Eng. 2023, 9, 437–448. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, J.; Zhao, J.; Wang, S. Injectable wound dressing based on carboxymethyl chitosan triple-network hydrogel for effective wound antibacterial and hemostasis. Int. J. Biol. Macromol. 2023, 225, 1235–1245. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, J.; Wu, H.; Wang, H.; Lu, X.; Shahbazi, M.-A.; Wang, S. A triple-network carboxymethyl chitosan-based hydrogel for hemostasis of incompressible bleeding on wet wound surfaces. Carbohydr. Polym. 2023, 303, 120434. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Yang, J.; Zhou, S.; Situ, W. Plasma etching effect on the molecular structure of chitosan-based hydrogels and its biological properties. Int. J. Biol. Macromol. 2023, 230, 123257. [Google Scholar] [CrossRef] [PubMed]
- Engkagul, V.; Sereemaspun, A.; Chirachanchai, S. One pot preparation of chitosan/hyaluronic acid-based triple network hydrogel via in situ click reaction, metal coordination and polyion complexation in water. Carbohydr. Polym. 2018, 200, 616–623. [Google Scholar] [CrossRef]
- Dahan, W.M.; Mohammad, F.; Ezzat, A.O.; Atta, A.M.; Al-Tilasi, H.H.; Al-Lohedan, H.A. Enhanced Delivery of Insulin through Acrylamide-Modified Chitosan Containing Smart Carrier System. Gels 2022, 8, 701. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Mathiyalagan, R.; Nguyen, M.-T.; Tran, T.-T.; Murugesan, M.; Ho, T.-N.; Huong, H.; Yang, D.C.; Li, Y.; Thambi, T. Ionically cross-linked alginate-chitosan core-shell hydrogel beads for oral delivery of insulin. Int. J. Biol. Macromol. 2022, 222, 262–271. [Google Scholar] [CrossRef]
- Yari, K.; Gharati, G.; Akbari, I. Evaluating effect of salt leaching method on release and swelling rate of metformin nanoparticles loaded-chitosan/polyvinyl alcohol porous composite. Int. J. Biol. Macromol. 2023, 227, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, N.; Youseftabar-Miri, L.; Divsar, F.; Hallajian, S.; Hafezi, K. Development and evaluation of chitosan-g-poly(acrylic acid-co-acrylamide) hydrogel composite containing gabapentin for in vitro controlled release. J. Mol. Struct. 2022, 1270, 133934. [Google Scholar] [CrossRef]
- Omrani, M.; Naimi-Jamal, M.R.; Far, B.F. The design of multi-responsive nanohydrogel networks of chitosan for controlled drug delivery. Carbohydr. Polym. 2022, 298, 120143. [Google Scholar] [CrossRef] [PubMed]
- Aslzad, S.; Savadi, P.; Abdolahinia, E.D.; Omidi, Y.; Fathi, M.; Barar, J. Chitosan/dialdehyde starch hybrid in situ forming hydrogel for ocular delivery of betamethasone. Mater. Today Commun. 2022, 33, 104873. [Google Scholar] [CrossRef]
- Ortega, A.; da Silva, A.B.; da Costa, L.M.; Zatta, K.C.; Onzi, G.R.; da Fonseca, F.N.; Guterres, S.S.; Paese, K. Thermosensitive and mucoadhesive hydrogel containing curcumin-loaded lipid-core nanocapsules coated with chitosan for the treatment of oral squamous cell carcinoma. Drug Deliv. Transl. Res. 2023, 13, 642–657. [Google Scholar] [CrossRef]
- Tran Vo, T.M.; Piroonpan, T.; Preuksarattanawut, C.; Kobayashi, T.; Potiyaraj, P. Characterization of pH-responsive high molecular-weight chitosan/poly (vinyl alcohol) hydrogel prepared by gamma irradiation for localizing drug release. Bioresour. Bioprocess. 2022, 9, 89. [Google Scholar] [CrossRef]
- Shilova, S.V.; Mirgaleev, G.M.; Barabanov, V.P. pH-Responsive Calcium Alginate Microspheres Modified with Chitosan for Immobilization of Antibiotic Cefotaxime. Polym. Sci. Ser. A 2022, 64, 447–455. [Google Scholar] [CrossRef]
- Batool, N.; Sarfraz, R.M.; Mahmood, A.; Zafar, N.; Minhas, M.U.; Hussain, Z.; Rehman, U. Biocompatible polymeric blend for pH driven delivery of cytarabine: Effect of feed contents on swelling and release kinetics. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1545–1562. [Google Scholar] [CrossRef]
- Barkhordari, S.; Alizadeh, A. Fabrication of pH-sensitive chitosan/layered double hydroxide (LDH)/Fe3O4 nanocomposite hydrogel beads for controlled release of diclofenac. Polym. Bull. 2022, 79, 5533–5548. [Google Scholar] [CrossRef]
- Jiang, K.; Zhou, X.; He, T. The synthesis of bacterial cellulose-chitosan zwitterionic hydrogels with pH responsiveness for drug release mechanism of the naproxen. Int. J. Biol. Macromol. 2022, 209, 814–824. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhai, Z.; Yao, Y.; Stant, J.C.; Landrum, S.L.; Bortner, M.J.; Frazier, C.E.; Edgar, K.J. Oxidized hydroxypropyl cellulose/carboxymethyl chitosan hydrogels permit pH-responsive, targeted drug release. Carbohydr. Polym. 2023, 300, 120213. [Google Scholar] [CrossRef] [PubMed]
- Al homsi, R.; Eltahir, S.; Jagal, J.; Ali Abdelkareem, M.; Ghoneim, M.M.; Rawas-Qalaji, M.M.; Greish, K.; Haider, M. Thermosensitive injectable graphene oxide/chitosan-based nanocomposite hydrogels for controlling the in vivo release of bupivacaine hydrochloride. Int. J. Pharm. 2022, 621, 121786. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, G.; Wang, Y.; Tang, M.; Ma, X.; Chang, X.; Guo, J.; Gui, S. Thermosensitive acetylated carboxymethyl chitosan gel depot systems sustained release caffeic acid phenethyl ester for periodontitis treatment. Polym. Adv. Technol. 2023, 34, 155–165. [Google Scholar] [CrossRef]
- Kaul, L.; Grundmann, C.E.; Köll-Weber, M.; Löffler, H.; Weiz, A.; Zannettino, A.C.W.; Richter, K.; Süss, R. A Thermosensitive, Chitosan-Based Hydrogel as Delivery System for Antibacterial Liposomes to Surgical Site Infections. Pharmaceutics 2022, 14, 2841. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lin, J.; Liu, C.; Zhang, Q.; Li, R.; Wang, C.; Zhao, C.; Lu, L.; Zhou, C.; Tian, J. Temperature-and pH-responsive injectable chitosan hydrogels loaded with doxorubicin and curcumin as long-lasting release platforms for the treatment of solid tumors. Front. Bioeng. Biotechnol. 2022, 10, 1043939. [Google Scholar] [CrossRef] [PubMed]
- García-Couce, J.; Schomann, T.; Chung, C.K.; Que, I.; Jorquera-Cordero, C.; Fuentes, G.; Almirall, A.; Chan, A.; Cruz, L.J. Thermosensitive Injectable Hydrogels for Intra-Articular Delivery of Etanercept for the Treatment of Osteoarthritis. Gels 2022, 8, 488. [Google Scholar] [CrossRef]
- Qiao, N.; Zhang, Y.; Fang, Y.; Deng, H.; Zhang, D.; Lin, H.; Chen, Y.; Yong, K.T.; Xiong, J. Silk Fabric Decorated with Thermo-Sensitive Hydrogel for Sustained Release of Paracetamol. Macromol. Biosci. 2022, 22, 2200029. [Google Scholar] [CrossRef]
- Ren, L.J.; Zhou, H.Y.; Hao, P.Y.; Zheng, H.J.; Tong, J.N.; Chen, Y.W.; Park, H.J. Amino acids grafted-chitosan/glycerophosphate hydrogel for controlled release of berberine hydrochloride. J. Appl. Polym. Sci. 2023, 140, e53632. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, T.; Xiong, M.; Li, S.; Li, W.-X.; Liu, J.; Zhou, X.; Qi, J.; Jiang, G.-B. Cannabidiol-loaded injectable chitosan-based hydrogels promote spinal cord injury repair by enhancing mitochondrial biogenesis. Int. J. Biol. Macromol. 2022, 221, 1259–1270. [Google Scholar] [CrossRef]
- Kurtulbaş, E.; Albarri, R.; Torun, M.; Şahin, S. Encapsulation of Moringa oleifera leaf extract in chitosan-coated alginate microbeads produced by ionic gelation. Food Biosci. 2022, 50, 102158. [Google Scholar] [CrossRef]
- Guan, Y.; Yu, C.; Zang, Z.; Wan, X.; Naeem, A.; Zhang, R.; Zhu, W. Chitosan/xanthan gum-based (Hydroxypropyl methylcellulose-co-2-Acrylamido-2-methylpropane sulfonic acid) interpenetrating hydrogels for controlled release of amorphous solid dispersion of bioactive constituents of Pueraria lobatae. Int. J. Biol. Macromol. 2023, 224, 380–395. [Google Scholar] [CrossRef]
- Sabzini, M.; Pourmadadi, M.; Yazdian, F.; Khadiv-Parsi, P.; Rashedi, H. Development of chitosan/halloysite/graphitic-carbon nitride nanovehicle for targeted delivery of quercetin to enhance its limitation in cancer therapy: An in vitro cytotoxicity against MCF-7 cells. Int. J. Biol. Macromol. 2023, 226, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Issarachot, O.; Bunlung, S.; Kaewkroek, K.; Wiwattanapatapee, R. Superporous hydrogels based on blends of chitosan and polyvinyl alcohol as a carrier for enhanced gastric delivery of resveratrol. Saudi Pharm. J. 2023, 31, 335–347. [Google Scholar] [CrossRef]
- Kamaci, M.; Kaya, I. Chitosan based hybrid hydrogels for drug delivery: Preparation, biodegradation, thermal, and mechanical properties. Polym. Adv. Technol. 2023, 34, 779–788. [Google Scholar] [CrossRef]
- Rui, Q.; Gao, J.; Yin, Z.-Z.; Li, J.; Cai, W.; Wu, D.; Kong, Y. A biodegradable pH and glutathione dual-triggered drug delivery system based on mesoporous silica, carboxymethyl chitosan and oxidized pullulan. Int. J. Biol. Macromol. 2023, 224, 1294–1302. [Google Scholar] [CrossRef]
- Rajabzadeh-Khosroshahi, M.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Navaei-Nigjeh, M.; Rasekh, B. Chitosan/agarose/graphitic carbon nitride nanocomposite as an efficient pH-sensitive drug delivery system for anticancer curcumin releasing. J. Drug Deliv. Sci. Technol. 2022, 74, 103443. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Ahmadi, M.; Abdouss, M.; Yazdian, F.; Rashedi, H.; Navaei-Nigjeh, M.; Hesari, Y. The synthesis and characterization of double nanoemulsion for targeted Co-Delivery of 5-fluorouracil and curcumin using pH-sensitive agarose/chitosan nanocarrier. J. Drug Deliv. Sci. Technol. 2022, 70, 102849. [Google Scholar] [CrossRef]
- Leipzig, N.D.; Wylie, R.G.; Kim, H.; Shoichet, M.S. Differentiation of neural stem cells in three-dimensional growth factor-immobilized chitosan hydrogel scaffolds. Biomaterials 2011, 32, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, X.; Huang, L. Feasibility of chitosan-alginate (Chi-Alg) hydrogel used as scaffold for neural tissue engineering: A pilot study in vitro. Biotechnol. Biotechnol. Equip. 2017, 31, 766–773. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Chen, M.; Bai, J.; Chen, T.; He, S.; Peng, W.; Wang, J.; Zhi, W.; Weng, J. A bionic composite hydrogel with dual regulatory functions for the osteochondral repair. Colloids Surf. B Biointerfaces 2022, 219, 112821. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, H.; Yao, J.; Yang, L.; Zhang, X.; Wang, J.; Li, L.; Li, M.; Liu, Z. 3D printing of “green” thermo-sensitive chitosan-hydroxyapatite bone scaffold based on lyophilized platelet-rich fibrin. Biomed. Mater. 2023, 18, 025022. [Google Scholar] [CrossRef] [PubMed]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Crosslinking of hybrid scaffolds produced from collagen and chitosan. Int. J. Biol. Macromol. 2019, 139, 262–269. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, W.; Zhang, H.; Zheng, W.; Zhou, Q. Carboxymethyl chitosan-based hydrogels containing fibroblast growth factors for triggering diabetic wound healing. Carbohydr. Polym. 2022, 287, 119336. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, Y.; Li, H.; Zhou, L.; Shi, H.; Feng, S.; Chen, J.; Mei, Z. Effect of natural-based biological hydrogels combined with growth factors on skin wound healing. Nanotechnol. Rev. 2022, 11, 2493–2512. [Google Scholar] [CrossRef]
- Lavanya, K.; Balagangadharan, K.; Chandran, S.V.; Selvamurugan, N. Chitosan-coated and thymol-loaded polymeric semi-interpenetrating hydrogels: An effective platform for bioactive molecule delivery and bone regeneration in vivo. Biomater. Adv. 2023, 146, 213305. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Liu, Y.; Yan, H.; Rafique, M.; Li, S.; Shan, X.; Wu, L.; Qiao, M.; Kong, D.; Wang, L. Anti-Infective and Pro-Coagulant Chitosan-Based Hydrogel Tissue Adhesive for Sutureless Wound Closure. Biomacromolecules 2020, 21, 1243–1253. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Xia, W.; Cao, D.; Wang, X.; Kuang, Y.; Luo, Y.; Yuan, C.; Lu, J.; Liu, X. Application of Hydrogels as Carrier in Tumor Therapy: A Review. Chem. Asian J. 2022, 17, e202200740. [Google Scholar] [CrossRef]
- Schneible, J.D.; Young, A.T.; Daniele, M.A.; Menegatti, S. Chitosan Hydrogels for Synergistic Delivery of Chemotherapeutics to Triple Negative Breast Cancer Cells and Spheroids. Pharm. Res. 2020, 37, 142. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Mohammed, A.M.; Saleem, I.; Alsharidah, M.; Al Rugaie, O.; Ahmed, F.; Osman, S.K. Smart Injectable Chitosan Hydrogels Loaded with 5-Fluorouracil for the Treatment of Breast Cancer. Pharmaceutics 2022, 14, 661. [Google Scholar] [CrossRef]
- Karimi, Z.; Taymouri, S.; Minaiyan, M.; Mirian, M. Evaluation of thermosensitive chitosan hydrogel containing gefitinib loaded cellulose acetate butyrate nanoparticles in a subcutaneous breast cancer model. Int. J. Pharm. 2022, 624, 122036. [Google Scholar] [CrossRef]
- Abbasalizadeh, F.; Alizadeh, E.; Bagher Fazljou, S.M.; Torbati, M.; Akbarzadeh, A. Anticancer Effect of Alginate-chitosan Hydrogel Loaded with Curcumin and Chrysin on Lung and Breast Cancer Cell Lines. Curr. Drug Deliv. 2022, 19, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Revkova, V.A.; Grebenik, E.A.; Kalsin, V.A.; Demina, T.S.; Bardakova, K.N.; Shavkuta, B.S.; Melnikov, P.A.; Samoilova, E.M.; Konoplyannikov, M.A.; Efremov, Y.M.; et al. Chitosan-g-oligo(L,L-lactide) Copolymer Hydrogel Potential for Neural Stem Cell Differentiation. Tissue Eng. Part A 2020, 26, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Cardia, M.C.; Carta, A.R.; Caboni, P.; Maccioni, A.M.; Erbi, S.; Boi, L.; Meloni, M.C.; Lai, F.; Sinico, C. Trimethyl Chitosan Hydrogel Nanoparticles for Progesterone Delivery in Neurodegenerative Disorders. Pharmaceutics 2019, 11, 657. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Chen, T.-Y.; Tai, C.-H.; Hsu, S.-H. Bioactive self-healing hydrogel based on tannic acid modified gold nano-crosslinker as an injectable brain implant for treating Parkinson’s disease. Biomater. Res. 2023, 27, 8. [Google Scholar] [CrossRef]
- Qin, H.; Ji, Y.; Li, G.; Xu, X.; Zhang, C.; Zhong, W.; Xu, S.; Yin, Y.; Song, J. MicroRNA-29b/graphene oxide–polyethyleneglycol–polyethylenimine complex incorporated within chitosan hydrogel promotes osteogenesis. Front. Chem. 2022, 10, 958561. [Google Scholar] [CrossRef] [PubMed]
- Arpornmaeklong, P.; Jaiman, N.; Apinyauppatham, K.; Fuongfuchat, A.; Boonyuen, S. Effects of Calcium Carbonate Microcapsules and Nanohydroxyapatite on Properties of Thermosensitive Chitosan/Collagen Hydrogels. Polymers 2023, 15, 416. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, J.; Song, W.; Zhao, C. An injectable hydrogel to disrupt neutrophil extracellular traps for treating rheumatoid arthritis. Drug Deliv. 2023, 30, 2173332. [Google Scholar] [CrossRef]
- Haloi, P.; Chawla, S.; Konkimalla, V.B. Thermosensitive smart hydrogel of PEITC ameliorates the therapeutic efficacy in rheumatoid arthritis. Eur. J. Pharm. Sci. 2023, 181, 106367. [Google Scholar] [CrossRef]
- Kim, M.A.; Shin, S.R.; Kim, H.J.; Lee, J.S.; Lee, C.M. Chemo-photothermal therapeutic effect of chitosan-gelatin hydrogels containing methotrexate and melanin on a collagen-induced arthritis mouse model. Int. J. Biol. Macromol. 2022, 218, 1013–1020. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Ye, H.; Yu, Z.; Feng, L.; Zhou, J.; Che, Y. Fe(III)@TA@IGF-2 microspheres loaded hydrogel for liver injury treatment. Int. J. Biol. Macromol. 2020, 159, 183–193. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, L.; Hu, Z.; Kong, S.; Zhang, Z.; Li, G. Optimized preparation of gastric acid-response sulfhydryl functionalized chitosan/alginate/tilapia peptide hydrogel and its protective effects on alcohol-induced liver and brain injury. RSC Adv. 2021, 11, 34544–34557. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, W.; Cheng, S.; Liu, J.; Wang, S. Novel fabrication of bioengineered injectable chitosan hydrogel loaded with conductive nanoparticles to improve therapeutic potential of mesenchymal stem cells in functional recovery after ischemic myocardial infarction. Nanomedicine 2023, 47, 102616. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Wang, X.; Chen, Z.; Jiang, N.; Guo, Z.; Zhang, Y.; Zhang, S.; Liu, X.; Liu, L. Improved myocardial performance in infarcted rat heart by injection of disulfide-cross-linked chitosan hydrogels loaded with basic fibroblast growth factor. J. Mater. Chem. B 2022, 10, 656–665. [Google Scholar] [CrossRef]
- Jiang, Y.-L.; Niu, S.; Lin, Z.; Li, L.; Yang, P.; Rao, P.; Yang, L.; Jiang, L.; Sun, L. Injectable hydrogel with dual-sensitive behavior for targeted delivery of oncostatin M to improve cardiac restoration after myocardial infarction. J. Mater. Chem. B 2022, 10, 6514–6531. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, M.; Zhao, K.; Ma, C.; Tang, H.; Li, Y. Evaluation of an Injectable Hydrogel Based on Hyaluronic Acid–Chitosan/β-Glycerophosphate-Loaded Mesenchymal Stem Cells in Enhancing the Therapeutic Efficacy of Myocardial Infarction. Macromol. Biosci. 2022, 22, 2100286. [Google Scholar] [CrossRef]
- Domenge, O.; Ragot, H.; Deloux, R.; Crepet, A.; Revet, G.; Boitard, S.E.; Simon, A.; Mougenot, N.; David, L.; Delair, T.; et al. Efficacy of epicardial implantation of acellular chitosan hydrogels in ischemic and nonischemic heart failure: Impact of the acetylation degree of chitosan. Acta Biomater. 2021, 119, 125–139. [Google Scholar] [CrossRef]
- Wang, L.; Gong, T.; Brown, Z.; Gu, Y.; Teng, K.; Ye, W.; Ming, W. Preparation of Ascidian-Inspired Hydrogel Thin Films to Selectively Induce Vascular Endothelial Cell and Smooth Muscle Cell Growth. ACS Appl. BioMater. 2020, 3, 2068–2077. [Google Scholar] [CrossRef]
- Sundaram, M.N.; Mony, U.; Varma, P.K.; Rangasamy, J. Vasoconstrictor and coagulation activator entrapped chitosan based composite hydrogel for rapid bleeding control. Carbohydr. Polym. 2021, 258, 117634. [Google Scholar] [CrossRef]
- Flores, E.E.E.; Cardoso, F.D.; Siqueira, L.B.; Ricardi, N.C.; Costa, T.H.; Rodrigues, R.C.; Klein, M.P.; Hertz, P.F. Influence of reaction parameters in the polymerization between genipin and chitosan for enzyme immobilization. Process Biochem. 2019, 84, 73–80. [Google Scholar] [CrossRef]
- de Matos Morawski, F.; Dias, G.B.M.; Sousa, K.A.P.; Formiga, R.; Spiller, F.; Parize, A.L.; Báfica, A.; Jost, C.L. Chitosan/genipin modified electrode for voltammetric determination of interleukin-6 as a biomarker of sepsis. Int. J. Biol. Macromol. 2023, 224, 1450–1459. [Google Scholar] [CrossRef]
- Moghaddam, M.S.; Latifi, H.; Abedini, E.; Ghazanfar, M.; Behroodi, E.; Sadeghi, M.S. Label-Free Optical pH Measurement Based on Chitosan-TEOS-PDMS Hydrogel Layer for Microfluidic Applications. IEEE Sens. J. 2023, 23, 97–103. [Google Scholar] [CrossRef]
- Shekh, M.I.; Zhu, G.; Xiong, W.; Wu, W.; Stadler, F.J.; Patel, D.; Zhu, C. Dynamically bonded, tough, and conductive MXene@oxidized sodium alginate: Chitosan based multi-networked elastomeric hydrogels for physical motion detection. Int. J. Biol. Macromol. 2023, 224, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Maimaitiyiming, X.; Sun, S. 3D Printed Multifunctional Self-Adhesive and Conductive Polyacrylamide/Chitosan/Sodium Carboxymethyl Cellulose/CNT Hydrogels as Flexible Sensors. Macromol. Chem. Phys. 2023, 224, 2200272. [Google Scholar] [CrossRef]




| First Crosslinking | Second Crosslinking | Properties | Ref. | |
|---|---|---|---|---|
| Chitosan | sodium orthophosphate hydrate | genipin | 8 min gelation time, 195 Pa storage modulus, ~100% swelling degree, and ~80% 3T3 cell viability | [144] |
| 2-formylphenylboronic acid | aldehyde | 5 min gelation time, 648 Pa storage modulus, 110% swelling degree, and antifungal activity against Candida planktonic yeast | [145] | |
| acrylic acid | plasma treatment | 5 min gelation time, effective surface functionalization | [146] | |
| disaccharide α,α′-trehalose derivative | citric acid | 1 min gelation time, 100 Pa storage modulus. | [147] | |
| vanillin | microwave irradiation | 2 min gelation time, 1.17 void fraction, 0.43 apparent density | [148] | |
| dihydrocaffeic acid | βGP | 4 min gelation time, 500 Pa storage modulus, 30 kPa adhesive strength, 12% swelling degree, no cytotoxicity against 3T3 mouse fibroblasts, 100% lysozyme-containing simulated body fluid, hemostatic ability, and in vivo biocompatibility | [149] | |
| Phenolic hydroxyl Chitosan | sodium tripolyphosphate | horseradish peroxidase | Fast gelation, 35% drug (5-FU) loading and 66.8% release | [150] |
| Carboxymethyl chitosan | citric acid | sodium alginate | ~50% drug (carvacrol) loading and ~80% release, 20–100% swelling degree, 77% DPPH radical scavenging activity, antibacterial activities against Escherichia coli and Staphylococcus aureus, and ~80% HepG2 and HFL1 cell viability | [151] |
| hyaluronic acid | EDC and NHS | ~400 Pa storage modulus, 60% gel fraction, ~60% panax notoginseng saponins release, 90% L929 cell viability, anti-inflammatory property (30–40 μM iNOS secretion concentration), anti-oxidant property, and in vivo diabetic wound healing ability | [152] | |
| sodium alginate | sodium tripolyphosphate | 6 min gelation time, 3–10 kPa storage modulus 100% MC3T3-E1 cell viability, in vivo biocompatibility, and in vivo bone healing | [153] | |
| sodium bicarbonate | tannic acid | 100–200 Pa storage modulus, 80% DPPH radical scavenging activity, antibacterial activities against Escherichia coli and Staphylococcus aureus, 100% 3T3 cell viability, in vivo hemostatic ability, and in vivo wound healing ability | [154] | |
| oxidized dextran | poly-γ-glutamic acid | 30 s gelation time, 2 Pa storage modulus, ~40% swelling degree, antibacterial activities against Escherichia coli and Staphylococcus aureus, 100% L929 cell viability, in vivo biodegradation, in vivo hemostatic ability, and in vivo wound healing ability | [155,156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taokaew, S.; Kaewkong, W.; Kriangkrai, W. Recent Development of Functional Chitosan-Based Hydrogels for Pharmaceutical and Biomedical Applications. Gels 2023, 9, 277. https://doi.org/10.3390/gels9040277
Taokaew S, Kaewkong W, Kriangkrai W. Recent Development of Functional Chitosan-Based Hydrogels for Pharmaceutical and Biomedical Applications. Gels. 2023; 9(4):277. https://doi.org/10.3390/gels9040277
Chicago/Turabian StyleTaokaew, Siriporn, Worasak Kaewkong, and Worawut Kriangkrai. 2023. "Recent Development of Functional Chitosan-Based Hydrogels for Pharmaceutical and Biomedical Applications" Gels 9, no. 4: 277. https://doi.org/10.3390/gels9040277