Amidoamine Oxide Surfactants as Low-Molecular-Weight Hydrogelators: Effect of Methylene Chain Length on Aggregate Structure and Rheological Behavior
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gelation Temperature (Tgel)
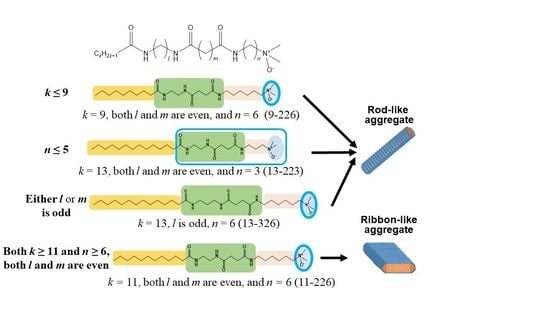
2.2. Effects of the Length of the Methylene Chain between the Amide and Amine Oxide Groups (n)
2.3. Effects of the Length of Methylene Chain in the Hydrophobic Part (k)
2.4. Effects of the Lengths of Methylene Chains between Amide Groups (l and m)
3. Conclusions
4. Materials and Methods
4.1. Materials
4.1.1. Synthesis of N-acylethlalkyldiamine (1a, 1b, 1c, 1d, 1e): General Procedure
4.1.2. Synthesis of N-acylaminoalkylsuccinimide (2a, 2b, 2c, 2d): General Procedure
4.1.3. Synthesis of N-myristoyletylglutarimide (2e)
4.1.4. Synthesis of N-(acylaminoalkyl)succinamoylaminohexylamine (3a, 3b, 3c, 3d) and [(N-myristoyl)glutaramoylaminohexyl]dimethylamine (3e): General Procedure
4.1.5. Synthesis of [(N-acylaminoalkyl)succinamoylaminohexyl]dimethylamine (4a, 4b, 4c, 4d) and [(N-myristoyl)glutaramoylaminohexyl]dimethylamine (4e): General Procedure
4.1.6. Synthesis of [(N-acylaminoalkyl)succinamoylaminohexyl]dimethylamine oxide (5a, 5b, 5c, 5d) and [(N-myristoylaminoetyl)glutaramoylaminohexyl]dimethylamine oxide (5e): General Procedure
4.2. Characterization
4.3. Viscosity Measurement
4.4. Rheological Measurement
4.5. Electron Microscopy
4.5.1. Quick-Freezing of Surfactant Aqueous Solutions
4.5.2. Cryo-Scanning Electron Microscopy (Cryo-SEM)
4.5.3. Freeze-Fracture Replica Transmission Electron Microscopy (TEM)
4.5.4. Negative-Staining TEM
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menger, F.M.; Yamasaki, Y.; Catlin, K.K.; Nishimi, T. X-ray structure of a self-assembled gelating fiber. Angew. Chem. Int. Ed. Engl. 1995, 34, 585–586. [Google Scholar] [CrossRef]
- Terech, P.; Weiss, R.G. Low molecular mass gelators of organic liquids and the properties of their gels. Chem. Rev. 1997, 97, 3133–3160. [Google Scholar] [CrossRef] [PubMed]
- Hafkamp, R.J.H.; Kokke, B.P.A.; Danke, I.M.; Geurts, H.P.M.; Rowan, A.E.; Feiters, M.C.; Nolte, R.J.M. Organogel formation and molecular imprinting by functionalized gluconamides and their metal complexes. Chem. Commun. 1997, 6, 545–546. [Google Scholar] [CrossRef] [Green Version]
- Van Esch, J.H.; Feringa, B.L. New functional materials based on self-assembling organogels: From serendipity towards design. Angew. Chem. Int. Ed. 2000, 39, 2263–2266. [Google Scholar] [CrossRef]
- Jung, J.H.; John, G.; Masuda, M.; Yoshida, K.; Shinkai, S.; Shimizu, T. Self-assembly of a sugar-based gelator in water: Its remarkable diversity in gelation ability and aggregate structure. Langmuir 2001, 17, 7229–7232. [Google Scholar] [CrossRef]
- Estroff, L.A.; Hamilton, A.D. Water gelation by small organic molecules. Chem. Rev. 2004, 104, 1201–1218. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Hanabusa, K. L-lysine-based low-molecular-weight gelators. Chem. Soc. Rev. 2009, 38, 967–975. [Google Scholar] [CrossRef]
- John, G.; Shankar, B.V.; Jadhav, S.R.; Vemula, P.K. Biorefinery: A design tool for molecular gelators. Langmuir 2010, 26, 17843–17851. [Google Scholar] [CrossRef] [PubMed]
- Piepenbrock, M.O.M.; Lloyd, G.O.; Clarke, N.; Steed, J.W. Metal-and anion-binding supramolecular gels. Chem. Rev. 2010, 110, 1960–2004. [Google Scholar] [CrossRef]
- Buerkle, L.E.; Rowan, S.J. Supramolecular gels formed from multi-component low molecular weight species. Chem. Soc. Rev. 2012, 41, 6089–6102. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, H.; Noponen, V.; Kolehmainen, E.; Sievänen, E. Recent advances in steroidal supramolecular gels. RSC Adv. 2012, 2, 4985–5007. [Google Scholar] [CrossRef]
- Minakuchi, N.; Hoe, K.; Yamaki, D.; Ten-no, S.; Nakashima, K.; Goto, M.; Mizuhata, M.; Maruyama, T. Versatile supramolecular gelators that can harden water, organic solvents and ionic liquids. Langmuir 2012, 28, 9259–9266. [Google Scholar] [CrossRef] [PubMed]
- Raeburn, J.; Cardoso, A.Z.; Adams, D.J. The importance of the self-assembly process to control mechanical properties of low molecular weight hydrogels. Chem. Soc. Rev. 2013, 42, 5143–5156. [Google Scholar] [CrossRef] [PubMed]
- Collin, D.; Covis, R.; Allix, F.; Jamart-Grégoire, B.; Martinoty, P. Jamming transition in solutions containing organogelator molecules of amino-acid type: Rheological and calorimetry experiments. Soft Matter 2013, 9, 2947–2958. [Google Scholar] [CrossRef]
- Ishioka, Y.; Minakuchi, N.; Mizuhata, M.; Maruyama, T. Supramolecular gelators based on benzenetricarboxamides for ionic liquids. Soft Matter 2014, 10, 965–971. [Google Scholar] [CrossRef] [Green Version]
- Guenet, J.M. Organogels: Thermodynamics, Structure, Solvent Role, and Properties; Springer: New York, NY, USA, 2016. [Google Scholar]
- Hanabusa, K.; Suzuki, M. Physical gelation by low-molecular-weight compounds and development of gelators. Bull. Chem. Soc. Jpn. 2016, 89, 174–182. [Google Scholar] [CrossRef]
- Wu, A.; Lu, F.; Sun, P.; Qiao, X.; Gao, X.; Zheng, L. Low-molecular-weight supramolecular ionogel based on host-guest interaction. Langmuir 2017, 33, 13982–13989. [Google Scholar] [CrossRef]
- Kocasoy, V.; Dedeoglu, B.; Demir-Ordu, O.; Aviyente, V. Influence of odd-even effect and intermolecular interactions in 2D molecular layers of bisamide organogelators. RSC Adv. 2018, 8, 35195–35204. [Google Scholar] [CrossRef] [Green Version]
- Weiss, R.G. (Ed.) Molecular Gels, Structure and Dynamics; Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Chivers, P.R.; Smith, D.K. Shaping and structuring supramolecular gels. Nat. Rev. Mater. 2019, 4, 463–478. [Google Scholar] [CrossRef] [Green Version]
- Draper, E.R.; Adams, D.J. Controlling the assembly and properties of low-molecular-weight hydrogelators. Langmuir 2019, 35, 6506–6521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Ni, R.; Chau, Y. A self-assembled peptidic nanomillipede to fabricate a tuneable hybrid hydrogel. Chem. Commun. 2019, 55, 7093–7096. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Luo, Y.; Li, F.; Jian, X.; Liu, Y. Development of natural-drugs-based low-molecular-weight supramolecular gels. Gels 2021, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yan, T.; Zhang, J.; Pei, X.; Cui, Z.; Song, B. Formation of asymmetric belt-like aggregates from a bio-based surfactant derived from dehydroabietic acid. Soft Matter 2021, 17, 9950–9956. [Google Scholar] [CrossRef]
- Majumdar, S.; Pal, B.; Sahu, R.; Das, K.S.; Ray, P.P.; Dey, B. A croconate-directed supramolecular self-healable Cd (ii)-metallogel with dispersed 2D-nanosheets of hexagonal boron nitride: A comparative outcome of the charge-transport phenomena and non-linear rectifying behaviour of semiconducting diodes. Dalton Trans. 2022, 51, 9007–9016. [Google Scholar] [CrossRef]
- Tripathy, D.; Gadtya, A.S.; Moharana, S. Supramolecular gel, its classification, preparation, properties, and applications: A review. Polym. Plast. Technol. Mater. 2023, 62, 306–326. [Google Scholar] [CrossRef]
- Kakehashi, R.; Tokai, N.; Yamamura, S. Solution behavior of long-alkyl-chain amide amine oxide surfactants having multiple hydrogen-bonding sites. Chem. Lett. 2012, 41, 1050–1051. [Google Scholar] [CrossRef] [Green Version]
- Kakehashi, R.; Tokai, N.; Maeda, H. Effects of the spacer length on the aggregate formation and the gelation of alkylamide amine oxides. Colloid Polym. Sci. 2015, 293, 3157–3165. [Google Scholar] [CrossRef]
- Kakehashi, R. Development from long chain alkylamine oxides to amidoamine oxide based low molecular weight gelators. Acc. Mater. Surf. Res. 2022, 7, 67–76. [Google Scholar]
- Kakehashi, R.; Tokai, N.; Maeda, H.; Yamamura, S. Protonation behavior and stability of micelles of N-lauroylaminoalkyl-dimethylamine oxides–effects of added salt concentration and spacer length. J. Oleo Sci. 2009, 58, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Tomioka, K.; Sumiyoshi, T.; Narui, S.; Nagaoka, Y.; Iida, A.; Miwa, Y.; Taga, T.; Nakano, M.; Handa, T. Molecular assembly and gelating behavior of didodecanoylamides of α,ω-alkylidenediamines. J. Am. Chem. Soc. 2001, 123, 11817–11818. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, T.; Nishimura, K.; Nakano, M.; Handa, T.; Miwa, Y.; Tomioka, K. Molecular assembly of C2-symmetric bis-(2S)-2-methyldodecanoylamides of α,ω-alkylidenediamines into coiled coil and twisted ribbon aggregates. J. Am. Chem. Soc. 2003, 125, 12137–12142. [Google Scholar] [CrossRef] [PubMed]












| k | l | m | n | Tgel/°C |
|---|---|---|---|---|
| 13 | 2 | 2 | 6 | 77 * |
| 13 | 2 | 3 | 6 | 53 |
| 13 | 3 | 2 | 6 | 45 * |
| 13 | 4 | 2 | 6 | 83 * |
| 13 | 5 | 2 | 6 | 59 * |
| 15 | 2 | 2 | 6 | >80 |
| 15 | 3 | 2 | 6 | 57 |
| 17 | 2 | 2 | 6 | >80 |
| 17 | 3 | 2 | 6 | 69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakehashi, R.; Tokai, N.; Nakagawa, M.; Kawasaki, K.; Horiuchi, S.; Yamamoto, A. Amidoamine Oxide Surfactants as Low-Molecular-Weight Hydrogelators: Effect of Methylene Chain Length on Aggregate Structure and Rheological Behavior. Gels 2023, 9, 261. https://doi.org/10.3390/gels9030261
Kakehashi R, Tokai N, Nakagawa M, Kawasaki K, Horiuchi S, Yamamoto A. Amidoamine Oxide Surfactants as Low-Molecular-Weight Hydrogelators: Effect of Methylene Chain Length on Aggregate Structure and Rheological Behavior. Gels. 2023; 9(3):261. https://doi.org/10.3390/gels9030261
Chicago/Turabian StyleKakehashi, Rie, Naoji Tokai, Makoto Nakagawa, Kazunori Kawasaki, Shin Horiuchi, and Atsushi Yamamoto. 2023. "Amidoamine Oxide Surfactants as Low-Molecular-Weight Hydrogelators: Effect of Methylene Chain Length on Aggregate Structure and Rheological Behavior" Gels 9, no. 3: 261. https://doi.org/10.3390/gels9030261