Microencapsulation of a Pickering Oil/Water Emulsion Loaded with Vitamin D3
Abstract
:1. Introduction
2. Results and Discussion
2.1. Vegetable Oil Blend Characterization: Evaluation of Fatty Acids Composition, ω6:ω3 Ratio and Peroxide Value
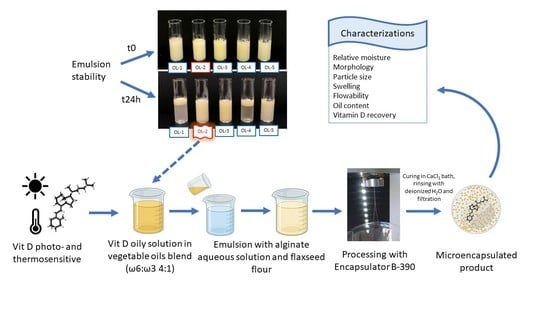
2.2. Preliminary Study
2.3. Placebo and vit D3-Loaded Microparticles
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Experimental Methods
4.2.1. Vegetable Oil Blend Characterization: Evaluation of ω6:ω3 Ratio
4.2.2. Determination of Peroxide Value of Vegetable Oil Blend
4.2.3. Preliminary Studies
4.2.4. Preparation of Placebo and vit D3-Loaded Microparticles
4.2.5. Determination of Residual Water Content of Dried Microparticles
4.2.6. Morphological and Particle Size Analysis
4.2.7. Swelling Test
4.2.8. Flowability Test
4.2.9. Content of Vegetable Oil Blend
4.2.10. Determination of Vegetable Oil Blend on the Microparticle Surface
4.2.11. Vitamin D3 Recovery
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taofiq, O.; Fernandes, Â.; Barros, L.; Barreiro, M.F.; Ferreira, I.C. UV-irradiated mushrooms as a source of vitamin D2: A review. Trends Food Sci. Technol. 2017, 70, 82–94. [Google Scholar] [CrossRef]
- Lee, J.H.; O’ Keefe, J.H.; Bell, D.; Hensrud, D.D.; Holick, M.F. Vitamin D deficiency: An important, common, and easily treatable cardiovascular risk factor? J. Am. Coll. Cardiol. 2008, 52, 1949–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Kostenberg, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutrition 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Bakaloudi, D.R.; Chourdakis, M. A critical update on the role of mild and serious vitamin D deficiency prevalence and the COVID-19 epidemic in Europe. Nutrition 2022, 93, 111441. [Google Scholar] [CrossRef] [PubMed]
- Glowka, E.; Stasiak, J.; Lulek, J. Drug delivery systems for vitamin D supplementation and therapy. Pharmaceutics 2019, 11, 347. [Google Scholar] [CrossRef] [Green Version]
- Jannasari, N.; Fathi, M.; Moshtaghian, S.J.; Abbaspourrad, A. Microencapsulation of vitamin D using gelatin and cress seed mucilage: Production, characterization and in vivo study. Int. J. Biol. Macromol. 2019, 129, 972–979. [Google Scholar] [CrossRef]
- Mitbumrung, W.; Suphantharika, M.; McClements, D.J.; Winuprasith, T. Encapsulation of vitamin D3 in Pickering emulsion stabilized by nanofibrillated mangosteen cellulose: Effect of environmental stresses. J. Food Sci. 2019, 84, 3213–3221. [Google Scholar] [CrossRef]
- Bajaj, S.R.; Marathe, S.J.; Singhal, R.S. Co-encapsulation of vitamins B12 and D3 using spray drying: Wall material optimization, product characterization, and release kinetics. Food Chem. 2021, 335, 127642. [Google Scholar] [CrossRef]
- Fuchs, M.; Turchiuli, C.; Bohin, M.; Cuvelier, M.E.; Ordonnaud, C.; Peyrat-Maillard, M.N.; Dumoulin, E. Encapsulation of oil in powder using spray drying and fluidized bed agglomeration. J. Food Eng. 2006, 75, 27–35. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, N.B.; Rocha, F. Microencapsulation of polyphenols—The specific case of the microencapsulation of Sambucus Nigra L. extracts—A review. Trends Food Sci. Technol. 2020, 105, 454–467. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Foglio Bonda, A.; Candiani, A.; Pertile, M.; Giovannelli, L.; Segale, L. Shellac gum/carrageenan alginate-based core–shell systems containing peppermint essential oil formulated by mixture design approach. Gels 2021, 7, 162. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, E.; Poncelet, D.; Rodrigues, R.C.; Renard, D. Oil encapsulation in core–shell alginate capsules by inverse gelation II: Comparison between dripping techniques using W/O or O/W emulsions. J. Microencapsul. 2017, 34, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Yue, C.; Wusigale; Ni, Y.; Liang, L. Preparation and characterization of emulsion-filled gel beads for the encapsulation and protection of resveratrol and α-tocopherol. Food Res. Int. 2018, 108, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Foglio Bonda, A.; Regis, L.; Giovannelli, L.; Segale, L. Alginate/maltodextrin and alginate/shellac gum core-shell capsules for the encapsulation of peppermint essential oil. Int. J. Biol. Macromol. 2020, 162, 1293–1302. [Google Scholar] [CrossRef]
- Peito, S.; Peixoto, D.; Ferreira-Faria, I.; Martins, M.A.; Ribeiro, H.M.; Veiga, F.; Marto, J.; Paiva-Santos, A.C. Nano- and microparticle-stabilized Pickering emulsions designed for topical therapeutics and cosmetic applications. Int. J. Pharm. 2022, 615, 121455. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An overview of Pickering emulsions: Solid-particle materials, classification, morphology, and applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary polyunsaturated fatty acids (PUFAs): Uses and potential health benefits. Curr. Nutr. Rep. 2021, 10, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, M.-I. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef]
- Uyen, N.T.T.; Hamid, Z.A.A.; Tram, N.X.T.; Ahmad, N. Fabrication of alginate microsphere for drug delivery: A review. Int. J. Biol. Macromol. 2020, 153, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Sharma, S. Investigation of swelling/degradation behavior of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Locatelli, M.; Coïsson, J.D.; Travaglia, F.; Cereti, E.; Garino, C.; D’Andrea, M.; Martelli, A.; Arlorio, M. Chemotype and genotype chemometrical evaluation applied to authentication and traceability of “tonda Gentile Trilobata” hazelnuts from Piedmont (Italy). Food Chem. 2011, 129, 1865–1873. [Google Scholar] [CrossRef]
- Shantha, N.C.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Cirlini, M.; Caligiani, A.; Palla, G.; De Ascentiis, A.; Tortini, P. Stability Studies of Ozonized Sunflower Oil and Enriched Cosmetics with a Dedicated Peroxide Value Determination. Ozone Sci. Eng. 2012, 34, 293–299. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Prieto, S.; Blanco-Mendez, J.; Otero-Espinar, F.J. Image analysis of the shape of granulated powder grains. J. Pharm. Sci. 2004, 93, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Remunan-Lopez, C.; Bodmeier, R. Mechanical, water uptake and permeability properties of crosslinking chitosan glutamate and alginate films. J. Control. Release 1997, 44, 215–225. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2022.
- Amidon, G.E.; Meyer, P.J.; Mudie, D.M. Particle, Powder, and Compact Characterization. In Developing Solid Oral Dosage Forms, Pharmaceutical Theory and Practice, 2nd ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 271–293. [Google Scholar] [CrossRef]
- Bartolucci, G.; Giocaliere, E.; Boscaro, F.; Vannacci, A.; Gallo, E.; Pieraccini, G.; Moneti, G. Vitamin D3 quantification in a cod liver oil-based supplement. J. Pharm. Biomed. Anal. 2011, 55, 64–70. [Google Scholar] [CrossRef] [PubMed]






| Fatty Acid | Oil Blend (%) ± sd | Dried Microparticles (%) ± sd |
|---|---|---|
| C16:0 | 10.0 ± 0.18 | 10.30 ± 0.12 |
| C16:1 | 0.32 ± 0.01 | 0.33 ± 0.01 |
| C18:0 | 2.79 ± 0.08 | 2.80 ± 0.02 |
| C18:1cis | 69.90 ± 0.15 | 69.90 ± 0.07 |
| C18:2n6cis | 12.3 ± 0.05 | 12.10 ± 0.06 |
| C18:3n6 | 0.49 ± 0.01 | 0.47 ± 0.01 |
| C18:3n3 | 2.88 ± 0.01 | 2.77 ± 0.01 |
| C20:0 | 0.56 ± 0.01 | 0.55 ± 0.01 |
| C20:1 | 0.44 ± 0.01 | 0.42 ± 0.01 |
| C22:0 | 0.17 ± 0.01 | 0.17 ± 0.01 |
| C24:0 | 0.07 ± 0.01 | 0.08 ± 0.01 |
| Fatty acid | Oil blend (%) ± sd | Dried microparticles (%) ± sd |
| ω6 | 12.80 ± 0.05 | 12.60 ± 0.05 |
| ω3 | 2.88 ± 0.01 | 2.77 ± 0.01 |
| ω6:ω3 | 4.45 ± 0.02 | 4.55 ± 0.02 |
| Iodometric Method | Spectrophotometric Method | |
|---|---|---|
| Fresh vegetable oil blend | 4.5 ± 0.1 | 8.4 ± 0.1 |
| Vegetable oil blend extracted from microparticles | --- | 43.0 ± 2.1 |
| Vegetable oil blend after solvent treatment | --- | 36.5 ± 3.0 |
| Emulsion Viscosity (cP) | Placebo Dried Microparticles Mean Diameter ± sd | Placebo Dried Microparticles Shape Factor ± sd | |
|---|---|---|---|
| OL-1 | 144 | 1212.05 ± 226.43 | 0.787 ± 0.145 |
| OL-2 | 956 | 1643.50 ± 87.28 | 0.923 ± 0.052 |
| OL-3 | 2298 | 1679.47 ± 75.38 | 0.901 ± 0.056 |
| OL-4 | 253 | 1515.34 ± 116.55 | 0.855 ± 0.101 |
| OL-5 | 1426 | 1448.49 ± 98.35 | 0.916 ± 0.053 |
| Product | Mean Diameter Wet (µm) ± sd | Mean Diameter Dried (µm) ± sd | Shape Factor Wet ± sd | Shape Factor Dried ± sd |
|---|---|---|---|---|
| Placebo microparticles | 1776 ± 460 | 1121 ± 240 | 0.932 ± 0.050 | 0.940 ± 0.036 |
| vit D3 microparticles | 1977 ± 240 | 1092 ± 163 | 0.938 ± 0.035 | 0.932 ± 0.052 |
| Product | Residual Water (%) | Angle of Repose (°) ± sd |
|---|---|---|
| Placebo microparticles | 6.42 | 27.40 ± 0.21 |
| vit D3 microparticles | 6.08 | 28.70 ± 1.47 |
| Components | OL-1 | OL-2 | OL-3 | OL-4 | OL-5 |
|---|---|---|---|---|---|
| Sodium alginate Protanal LF10/60 | 1.419 | 2.578 | 3.329 | --- | --- |
| Sodium Alginate Manucol | --- | --- | --- | 1.419 | --- |
| Sodium alginate Farmalabor | --- | --- | --- | --- | 1.419 |
| Water | 94.620 | 90.224 | 87.351 | 94.620 | 94.620 |
| Flaxseed flour | 0.236 | 0.429 | 0.555 | 0.236 | 0.236 |
| Vegetable oil blend | 3.725 | 6.769 | 8.765 | 3.725 | 3.725 |
| Components | OL-2 Placebo | OL-2 vit D3 |
|---|---|---|
| Sodium alginate Protanal LF10/60 | 2.578 | 2.578 |
| Water | 90.224 | 90.224 |
| Flaxseed flour | 0.429 | 0.429 |
| Vegetable oil blend | 6.769 | 6.7688 |
| Vitamin D3 | --- | 0.0002 |
| Range (ng) | R2 | Calibration Equation | Barlett Test (p < 0.05) | Shapiro–Wilk Test (p < 0.05) | Mandel Test (p < 0.05) | LOF (p < 0.05) | LOD (ng) | LOQ (ng) |
|---|---|---|---|---|---|---|---|---|
| 0.5–10 | 0.9977 | y = 5526.1x + 2439.7 | 0.162 | 0.609 | 0.137 | 0.162 | 2.71 | 8.22 |
| 20–75 | 0.9957 | y = 6730.7 − 48,679 | 0.629 | 0.322 | 0.131 | 0.259 | 20.50 | 62.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candiani, A.; Diana, G.; Martoccia, M.; Travaglia, F.; Giovannelli, L.; Coïsson, J.D.; Segale, L. Microencapsulation of a Pickering Oil/Water Emulsion Loaded with Vitamin D3. Gels 2023, 9, 255. https://doi.org/10.3390/gels9030255
Candiani A, Diana G, Martoccia M, Travaglia F, Giovannelli L, Coïsson JD, Segale L. Microencapsulation of a Pickering Oil/Water Emulsion Loaded with Vitamin D3. Gels. 2023; 9(3):255. https://doi.org/10.3390/gels9030255
Chicago/Turabian StyleCandiani, Alessandro, Giada Diana, Manuel Martoccia, Fabiano Travaglia, Lorella Giovannelli, Jean Daniel Coïsson, and Lorena Segale. 2023. "Microencapsulation of a Pickering Oil/Water Emulsion Loaded with Vitamin D3" Gels 9, no. 3: 255. https://doi.org/10.3390/gels9030255