Research Progress of Polysaccharide-Based Natural Polymer Hydrogels in Water Purification
Abstract
:1. Introduction
2. Preparation and Modification of Polysaccharide Hydrogels
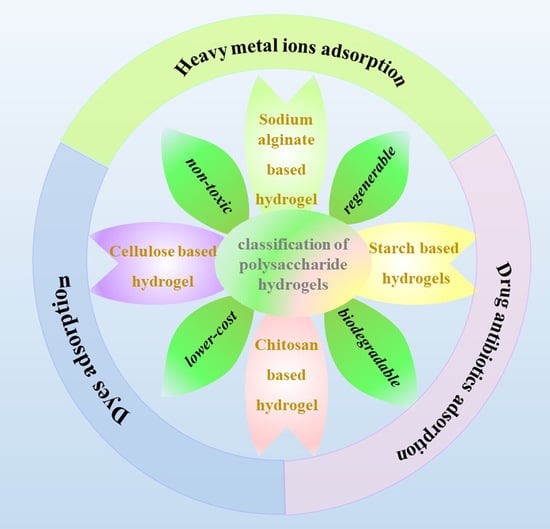
2.1. Cellulose-Based Hydrogels
2.2. Chitosan-Based Hydrogels
2.3. Starch-Based Hydrogels
2.4. Sodium-Based Hydrogels
3. Adsorption Mechanism and Kinetics of Polysaccharide-Based Hydrogels
3.1. Adsorption Mechanism
3.2. Adsorption Kinetics
3.2.1. Pseudo-First-Order Kinetic
3.2.2. Pseudo-Second-Order Kinetic
3.3. Adsorption Isotherms
3.3.1. Langmuir Isotherm Equation
3.3.2. Freundlich Isotherm Equation
4. Modification of Polysaccharide-Based Hydrogels
4.1. Functionalization of Nitrogen-Containing Groups
4.2. Functionalization of Oxygen-Containing Groups
4.3. Functionalization of Sulfur-Containing Groups
5. Adsorption Applications of Polysaccharide-Based Hydrogels
5.1. Heavy Metal Ion Adsorption
5.2. Dye Adsorption
5.3. Drug Antibiotics Adsorption
6. Perspectives and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Abbreviation | Full Name |
| AM | Acrylamide |
| AA | Acrylic acid |
| GO | Graphene oxide |
| PVA | Polyvinyl alcohol |
| SA | Salicylic acid |
| CS | Salicylic acid |
| PEI | Polyethyleneimine ethoxylated |
| PAM | Polyacrylamide |
| CNF | Cellulose nanofibril |
| HPCS | Hydroxypropyl chitosan |
| MB | Methylene blue |
| MG | Malachite green |
| MV | Methylrosanilinium chloride |
| EBT | Eriochrome black T |
| CR | Congo red |
References
- Godiya, C.B.; Martins Ruotolo, L.A.; Cai, W. Functional biobased hydrogels for the removal of aqueous hazardous pollutants: Current status, challenges, and future perspectives. J. Mater. Chem. A 2020, 8, 21585–21612. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, Q.; Song, X.; Wang, H.; Bian, Z. Recent electrochemical methods in electrochemical degradation of halogenated organics: A review. Environ. Sci. Pollut. Res. Int. 2019, 26, 10457–10486. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Gao, B.; Ren, J.; Li, A.; Yang, H. Coagulation/flocculation in dewatering of sludge: A review. Water Res. 2018, 143, 608–631. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamadani, Y.A.J.; Jun, B.M.; Yoon, M.; Taheri-Qazvini, N.; Snyder, S.A.; Jang, M.; Heo, J.; Yoon, Y. Applications of MXene-based membranes in water purification: A review. Chemosphere 2020, 254, 126821. [Google Scholar] [CrossRef] [PubMed]
- Hansima, M.; Makehelwala, M.; Jinadasa, K.; Wei, Y.; Nanayakkara, K.G.N.; Herath, A.C.; Weerasooriya, R. Fouling of ion exchange membranes used in the electrodialysis reversal advanced water treatment: A review. Chemosphere 2021, 263, 127951. [Google Scholar] [CrossRef]
- Badsha, M.A.H.; Khan, M.; Wu, B.; Kumar, A.; Lo, I.M.C. Role of surface functional groups of hydrogels in metal adsorption: From performance to mechanism. J. Hazard. Mater. 2021, 408, 124463. [Google Scholar] [CrossRef]
- Alsaid, Y.; Wu, S.; Wu, D.; Du, Y.; Shi, L.; Khodambashi, R.; Rico, R.; Hua, M.; Yan, Y.; Zhao, Y.; et al. Tunable Sponge-Like Hierarchically Porous Hydrogels with Simultaneously Enhanced Diffusivity and Mechanical Properties. Adv. Mater. 2021, 33, e2008235. [Google Scholar] [CrossRef]
- Kayan, G.Ö.; Kayan, A. Composite of Natural Polymers and Their Adsorbent Properties on the Dyes and Heavy Metal Ions. J. Polym. Environ. 2021, 29, 3477–3496. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Luo, Q.; Huang, X.; Luo, Y.; Yuan, H.; Ren, T.; Li, X.; Xu, D.; Guo, X.; Wu, Y. Fluorescent chitosan-based hydrogel incorporating titanate and cellulose nanofibers modified with carbon dots for adsorption and detection of Cr(VI). Chem. Eng. J. 2021, 407, 127050. [Google Scholar] [CrossRef]
- Lei, X.; Li, H.; Luo, Y.; Sun, X.; Guo, X.; Hu, Y.; Wen, R. Novel fluorescent nanocellulose hydrogel based on gold nanoclusters for the effective adsorption and sensitive detection of mercury ions. J. Taiwan Inst. Chem. Eng. 2021, 123, 79–86. [Google Scholar] [CrossRef]
- Li, Y.; Hou, X.; Pan, Y.; Wang, L.; Xiao, H. Redox-responsive carboxymethyl cellulose hydrogel for adsorption and controlled release of dye. Eur. Polym. J. 2020, 123, 109447. [Google Scholar] [CrossRef]
- Godiya, C.B.; Cheng, X.; Li, D.; Chen, Z.; Lu, X. Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. J. Hazard. Mater. 2019, 364, 28–38. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y. Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors-A review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef]
- Lim, C.; Hwang, D.S.; Lee, D.W. Intermolecular interactions of chitosan: Degree of acetylation and molecular weight. Carbohydr. Polym. 2021, 259, 117782. [Google Scholar] [CrossRef]
- Blachnio, M.; Budnyak, T.M.; Derylo-Marczewska, A.; Marczewski, A.W.; Tertykh, V.A. Chitosan-Silica Hybrid Composites for Removal of Sulfonated Azo Dyes from Aqueous Solutions. Langmuir 2018, 34, 2258–2273. [Google Scholar] [CrossRef]
- Deng, H.; Wei, Z.; Wang, X. Enhanced adsorption of active brilliant red X-3B dye on chitosan molecularly imprinted polymer functionalized with Ti(IV) as Lewis acid. Carbohydr. Polym. 2017, 157, 1190–1197. [Google Scholar] [CrossRef]
- Affonso, L.N.; Marques, J.L., Jr.; Lima, V.V.C.; Goncalves, J.O.; Barbosa, S.C.; Primel, E.G.; Burgo, T.A.L.; Dotto, G.L.; Pinto, L.A.A.; Cadaval, T.R.S., Jr. Removal of fluoride from fertilizer industry effluent using carbon nanotubes stabilized in chitosan sponge. J. Hazard. Mater. 2020, 388, 122042. [Google Scholar] [CrossRef]
- Yang, S.C.; Liao, Y.; Karthikeyan, K.G.; Pan, X.J. Mesoporous cellulose-chitosan composite hydrogel fabricated via the co-dissolution-regeneration process as biosorbent of heavy metals. Environ. Pollut. 2021, 286, 117324. [Google Scholar] [CrossRef]
- Kekes, T.; Kolliopoulos, G.; Tzia, C. Hexavalent chromium adsorption onto crosslinked chitosan and chitosan/β-cyclodextrin beads: Novel materials for water decontamination. J. Environ. Chem. Eng. 2021, 9, 105581. [Google Scholar] [CrossRef]
- Kekes, T.; Tzia, C. Adsorption of indigo carmine on functional chitosan and beta-cyclodextrin/chitosan beads: Equilibrium, kinetics and mechanism studies. J. Environ. Manag. 2020, 262, 110372. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Jin, S.; Bao, F.; Tang, S.; Yang, J.; Peng, K.; Chen, Y. Construction of a physically cross-linked carrageenan/chitosan/calcium ion double-network hydrogel for 3-Nitro-1, 2, 4-triazole-5-one removal. J. Hazard. Mater. 2022, 424, 127510. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Zhao, H.; Gao, L.; Qin, Z.; Ma, J.; Han, Y.; Jiao, T. Preparation of carboxymethyl chitosan/phytic acid composite hydrogels for rapid dye adsorption in wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127355. [Google Scholar] [CrossRef]
- Tang, S.; Yang, J.; Lin, L.; Peng, K.; Chen, Y.; Jin, S.; Yao, W. Construction of physically crosslinked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem. Eng. J. 2020, 393, 124728. [Google Scholar] [CrossRef]
- Konstantakos, S.; Marinopoulou, A.; Papaemmanouil, S.; Emmanouilidou, M.; Karamalaki, M.; Kolothas, E.; Saridou, E.; Papastergiadis, E.; Karageorgiou, V. Preparation of model starch complex hydrogels. Food Hydrocoll. 2019, 96, 365–372. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, W.; Zhang, C.; Zhu, Y.; Xu, J.; Li, E.; Gilbert, R.G.; Liu, Q. New insights into amylose and amylopectin biosynthesis in rice endosperm. Carbohydr. Polym. 2020, 230, 115656. [Google Scholar] [CrossRef]
- Gupta, A.D.; Rawat, K.P.; Bhadauria, V.; Singh, H. Recent trends in the application of modified starch in the adsorption of heavy metals from water: A review. Carbohydr. Polym. 2021, 269, 117763. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Cui, Y.; Dai, R.; Shan, Z.; Chen, H. Fabrication of starch-based high-performance adsorptive hydrogels using a novel effective pretreatment and adsorption for cationic methylene blue dye: Behavior and mechanism. Chem. Eng. J. 2021, 405, 126953. [Google Scholar] [CrossRef]
- Fang, C.; Huang, J.; Yang, Q.; Pu, H.; Liu, S.; Zhu, Z. Adsorption capacity and cold-water solubility of honeycomb-like potato starch granule. Int. J. Biol. Macromol. 2020, 147, 741–749. [Google Scholar] [CrossRef]
- Bao, L.; Zhu, X.; Dai, H.; Tao, Y.; Zhou, X.; Liu, W.; Kong, Y. Synthesis of porous starch xerogels modified with mercaptosuccinic acid to remove hazardous gardenia yellow. Int. J. Biol. Macromol. 2016, 89, 389–395. [Google Scholar] [CrossRef]
- Chen, Q.J.; Zheng, X.M.; Zhou, L.L.; Zhang, Y.F. Adsorption of Cu(II) and Methylene Blue by Succinylated Starch Nanocrystals. Starch-Stärke 2019, 71, 1800266. [Google Scholar] [CrossRef]
- Guo, L.; Li, J.; Gui, Y.; Zhu, Y.; Yu, B.; Tan, C.; Fang, Y.; Cui, B. Porous starches modified with double enzymes: Structure and adsorption properties. Int. J. Biol. Macromol. 2020, 164, 1758–1765. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, X.; Han, J.; Yu, L.; Chen, J.; Wu, Q.; Jiang, J. Effects of nanocellulose on sodium alginate/polyacrylamide hydrogel: Mechanical properties and adsorption-desorption capacities. Carbohydr. Polym. 2019, 206, 289–301. [Google Scholar] [CrossRef]
- Zhao, D.; Ye, W.; Cui, W. Fabrication of novel bio-adsorbent and its application for the removal of Cu(II) from aqueous solution. Environ. Sci. Pollut. Res. Int. 2021. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Wang, X.; Yan, B.; Peng, Y.; Ran, R. Fe(3+)-citric acid/sodium alginate hydrogel: A photo-responsive platform for rapid water purification. Carbohydr. Polym. 2021, 269, 118269. [Google Scholar] [CrossRef]
- Mozaffari, T.; Keshtkar Vanashi, A.; Ghasemzadeh, H. Nanocomposite hydrogel based on sodium alginate, poly (acrylic acid), and tetraamminecopper (II) sulfate as an efficient dye adsorbent. Carbohydr. Polym. 2021, 267, 118182. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, Z.; Cao, J.; Yu, F. Enhanced adsorption for the removal of antibiotics by carbon nanotubes/graphene oxide/sodium alginate triple-network nanocomposite hydrogels in aqueous solutions. Chemosphere 2020, 242, 125188. [Google Scholar] [CrossRef]
- Zhang, M.-K.; Zhang, X.-H.; Han, G.-Z. Magnetic alginate/PVA hydrogel microspheres with selective adsorption performance for aromatic compounds. Sep. Purif. Technol. 2021, 278, 119547. [Google Scholar] [CrossRef]
- Can, V.; Kochovski, Z.; Reiter, V.; Severin, N.; Siebenbürger, M.; Kent, B.; Just, J.; Rabe, J.P.; Ballauff, M.; Okay, O. Nanostructural Evolution and Self-Healing Mechanism of Micellar Hydrogels. Macromolecules 2016, 49, 2281–2287. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. New models for kinetics and equilibrium homogeneous adsorption. Chem. Eng. Res. Des. 2016, 112, 289–297. [Google Scholar] [CrossRef]
- Rodríguez-Narciso, S.; Lozano-Álvarez, J.A.; Salinas-Gutiérrez, R.; Castañeda-Leyva, N.; Bonilla-Petriciolet, A. A Stochastic Model for Adsorption Kinetics. Adsorpt. Sci. Technol. 2021, 2021, 1–21. [Google Scholar] [CrossRef]
- Arroyave, J.M.; Avena, M.; Tan, W.; Wang, M. The two-species phosphate adsorption kinetics on goethite. Chemosphere 2022, 307, 135782. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Wen, J.; Chen, J.; Xue, Y.; Feng, Y.; Duan, H.; Ji, B.; Li, R. Diameter dependent thermodynamics of adsorption on nanowires: A theoretical and experimental study. Chem. Eng. Sci. 2022, 247, 117061. [Google Scholar] [CrossRef]
- Latour, R.A. Fundamental Principles of the Thermodynamics and Kinetics of Protein Adsorption to Material Surfaces. Colloids Surf. B Biointerfaces 2020, 191, 110992. [Google Scholar] [CrossRef]
- Lombardo, S.; Thielemans, W. Thermodynamics of adsorption on nanocellulose surfaces. Cellulose 2019, 26, 249–279. [Google Scholar] [CrossRef]
- Ali, H.E.; Nasef, S.M.; Gad, Y.H. Remediation of Astrazon blue and Lerui acid brilliant blue dyes from waste solutions using amphoteric superparamagnetic nanocomposite hydrogels based on chitosan prepared by gamma rays. Carbohydr. Polym. 2022, 283, 119149. [Google Scholar] [CrossRef]
- Godiya, C.B.; Xiao, Y.; Lu, X. Amine functionalized sodium alginate hydrogel for efficient and rapid removal of methyl blue in water. Int. J. Biol. Macromol. 2020, 144, 671–681. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, C.; Zhang, Y.; Wang, X.; Duan, Y.; Peng, J.; Luo, P.; Peng, Y.; Wang, X.; Wang, S.; et al. Copolymerization of Aniline, Melamine and p-Phenylenediamine for Enhanced Pseudocapacitance Hydrogel Supercapacitor Electrodes. Macromol. Mater. Eng. 2022, 307, 2200180. [Google Scholar] [CrossRef]
- Yusof, N.H.; Foo, K.Y.; Hameed, B.H.; Hussin, M.H.; Lee, H.K.; Sabar, S. One-step synthesis of chitosan-polyethyleneimine with calcium chloride as effective adsorbent for Acid Red 88 removal. Int. J. Biol. Macromol. 2020, 157, 648–658. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y.; Wu, Y.; Li, S.; Yin, H.; Wang, J. α-ketoglutaric acid modified chitosan/polyacrylamide semi-interpenetrating polymer network hydrogel for removal of heavy metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127262. [Google Scholar] [CrossRef]
- Badsha, M.A.H.; Lo, I.M.C. An innovative pH-independent magnetically separable hydrogel for the removal of Cu(II) and Ni(II) ions from electroplating wastewater. J. Hazard. Mater. 2020, 381, 121000. [Google Scholar] [CrossRef]
- Pirgalıoğlu, S.; Özbelge, T.A.; Özbelge, H.Ö.; Bicak, N. Crosslinked polyDADMAC gels as highly selective and reusable arsenate binding materials. Chem. Eng. J. 2015, 262, 607–615. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Morajkar, P.P.; Alhassan, S.M. GO crosslinked hydrogel nanocomposites of chitosan/carboxymethyl cellulose-A versatile adsorbent for the treatment of dyes contaminated wastewater. Int. J. Biol. Macromol. 2021, 167, 1248–1261. [Google Scholar] [CrossRef]
- Zhu, F.; Huang, H.; Yang, Z.; Xu, M. Dual-responsive copolymer hydrogel as broad-spectrum adsorbents for metal ions. Polym. Test. 2019, 77, 105887. [Google Scholar] [CrossRef]
- He, J.; Ni, F.; Cui, A.; Chen, X.; Deng, S.; Shen, F.; Huang, C.; Yang, G.; Song, C.; Zhang, J.; et al. New insight into adsorption and co-adsorption of arsenic and tetracycline using a Y-immobilized graphene oxide-alginate hydrogel: Adsorption behaviours and mechanisms. Sci. Total Environ. 2020, 701, 134363. [Google Scholar] [CrossRef]
- Lai, H.; Liu, S.; Yan, J.; Xing, F.; Xiao, P. Facile Fabrication of Biobased Hydrogel from Natural Resources: L-Cysteine, Itaconic Anhydride, and Chitosan. ACS Sustain. Chem. Eng. 2020, 8, 4941–4947. [Google Scholar] [CrossRef]
- Omer, A.M.; Sadik, W.A.A.; El-Demerdash, A.G.M.; Tamer, T.M.; Khalifa, R.E.; Mohyeldin, M.S.; Abdelwahed, N.A. Fabrication of semi-interpenetrated PVA/PAMPS hydrogel as a reusable adsorbent for cationic methylene blue dye: Isotherms, kinetics and thermodynamics studies. Polym. Bull. 2020, 78, 6649–6673. [Google Scholar] [CrossRef]
- Aflaki Jalali, M.; Dadvand Koohi, A.; Sheykhan, M. Experimental study of the removal of copper ions using hydrogels of xanthan, 2-acrylamido-2-methyl-1-propane sulfonic acid, montmorillonite: Kinetic and equilibrium study. Carbohydr. Polym. 2016, 142, 124–132. [Google Scholar] [CrossRef]
- He, S.; Zhang, F.; Cheng, S.; Wang, W. Synthesis of Sodium Acrylate and Acrylamide Copolymer/GO Hydrogels and Their Effective Adsorption for Pb2+ and Cd2+. ACS Sustain. Chem. Eng. 2016, 4, 3948–3959. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; He, S.; Hou, H.; Hao, C. Ultrasonic-assisted synthesis of superabsorbent hydrogels based on sodium lignosulfonate and their adsorption properties for Ni(2). Ultrason. Sonochem. 2018, 40, 221–229. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Wang, G.; Hao, C.; Li, X.; Li, T. Adsorption performance of a polysaccharide composite hydrogel based on crosslinked glucan/chitosan for heavy metal ions. Compos. Part B Eng. 2019, 169, 45–54. [Google Scholar] [CrossRef]
- Wu, D.; Gao, Y.; Li, W.; Zheng, X.; Chen, Y.; Wang, Q. Selective Adsorption of La3+ Using a Tough Alginate-Clay-Poly(n-isopropylacrylamide) Hydrogel with Hierarchical Pores and Reversible Re-Deswelling/Swelling Cycles. ACS Sustain. Chem. Eng. 2016, 4, 6732–6743. [Google Scholar] [CrossRef]
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of acrylamide/acrylic acid cellulose hydrogels for the adsorption of heavy metal ions. Carbohydr. Polym. 2019, 224, 115022. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Peng, J.; Ren, T.; Luo, Q.; Luo, Y.; Zhang, N.; Huang, Y.; Guo, X.; Wu, Y. Novel fluorescent lignin-based hydrogel with cellulose nanofibers and carbon dots for highly efficient adsorption and detection of Cr(VI). Sci Total Environ. 2021, 760, 143395. [Google Scholar] [CrossRef]
- Zhang, W.; Song, J.; He, Q.; Wang, H.; Lyu, W.; Feng, H.; Xiong, W.; Guo, W.; Wu, J.; Chen, L. Novel pectin based composite hydrogel derived from grapefruit peel for enhanced Cu(II) removal. J. Hazard. Mater. 2020, 384, 121445. [Google Scholar] [CrossRef]
- Zhao, H.; Ouyang, X.-K.; Yang, L.-Y. Adsorption of lead ions from aqueous solutions by porous cellulose nanofiber–sodium alginate hydrogel beads. J. Mol. Liq. 2021, 324, 115122. [Google Scholar] [CrossRef]
- Li, C.; Yan, Y.; Zhang, Q.; Zhang, Z.; Huang, L.; Zhang, J.; Xiong, Y.; Tan, S. Adsorption of Cd(2+) and Ni(2+) from Aqueous Single-Metal Solutions on Graphene Oxide-Chitosan-Poly(vinyl alcohol) Hydrogels. Langmuir 2019, 35, 4481–4490. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Huang, Z.; Luo, Z.; Qian, C.; Li, Y.; Duan, Y. Preparation of low molecular chitosan by microwave-induced plasma desorption/ionization technology. Int. J. Biol. Macromol. 2021, 187, 441–450. [Google Scholar] [CrossRef]
- Mei, J.; Zhang, H.; Li, Z.; Ou, H. A novel tetraethylenepentamine crosslinked chitosan oligosaccharide hydrogel for total adsorption of Cr(VI). Carbohydr. Polym. 2019, 224, 115154. [Google Scholar] [CrossRef]
- Cao, J.; He, G.; Ning, X.; Wang, C.; Fan, L.; Yin, Y.; Cai, W. Hydroxypropyl chitosan-based dual self-healing hydrogel for adsorption of chromium ions. Int. J. Biol. Macromol. 2021, 174, 89–100. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Z.; Luo, S.-Y.; Zong, M.-H.; Lou, W.-Y. Multi-functional magnetic hydrogels based on Millettia speciosa Champ residue cellulose and Chitosan: Highly efficient and reusable adsorbent for Congo red and Cu2+ removal. Chem. Eng. J. 2021, 423, 130198. [Google Scholar] [CrossRef]
- Melilli, G.; Yao, J.; Chiappone, A.; Sangermano, M.; Hakkarainen, M. Photocurable “all-lignocellulose” derived hydrogel nanocomposites for adsorption of cationic contaminants. Sustain. Mater. Technol. 2021, 27, e00243. [Google Scholar] [CrossRef]
- Liu, F.; Hua, S.; Wang, C.; Qiu, M.; Jin, L.; Hu, B. Adsorption and reduction of Cr(VI) from aqueous solution using cost-effective caffeic acid functionalized corn starch. Chemosphere 2021, 279, 130539. [Google Scholar] [CrossRef]
- Xu, F.; Chen, H.; Dai, Y.; Wu, S.; Tang, X. Arsenic adsorption and removal by a new starch stabilized ferromanganese binary oxide in water. J. Environ. Manag. 2019, 245, 160–167. [Google Scholar] [CrossRef]
- Ibrahim, B.M.; Fakhre, N.A. Crown ether modification of starch for adsorption of heavy metals from synthetic wastewater. Int. J. Biol. Macromol. 2019, 123, 70–80. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Wang, X.; Zong, L.; Kang, Y.; Wang, A. Fast and Highly Efficient Adsorption Removal of Toxic Pb(II) by a Reusable Porous Semi-IPN Hydrogel Based on Alginate and Poly(Vinyl Alcohol). Front. Chem. 2021, 9, 662482. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Z.; Zhang, T.; Li, Q.; Chen, J.; Ji, W.; Liu, C.; Wei, Y. Fabrication of in situ ZIF-67 grown on alginate hydrogels and its application for enhancing Cu (II) adsorption from aqueous solutions. Colloids Surf. B Biointerfaces 2021, 207, 112036. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, Y.; Lin, Z.; Zhao, B.; Wang, J.; Xie, J.; Zhang, A. Preparation of a novel bio-adsorbent of sodium alginate grafted polyacrylamide/graphene oxide hydrogel for the adsorption of heavy metal ion. Sci. Total Environ. 2020, 744, 140653. [Google Scholar] [CrossRef]
- Isawi, H. Using Zeolite/Polyvinyl alcohol/sodium alginate nanocomposite beads for removal of some heavy metals from wastewater. Arab. J. Chem. 2020, 13, 5691–5716. [Google Scholar] [CrossRef]
- Dai, M.; Liu, Y.; Ju, B.; Tian, Y. Preparation of thermoresponsive alginate/starch ether composite hydrogel and its application to the removal of Cu(II) from aqueous solution. Bioresour. Technol. 2019, 294, 122192. [Google Scholar] [CrossRef]
- Wang, F.; Chang, P.R.; Zheng, P.; Ma, X. Monolithic porous rectorite/starch composites: Fabrication, modification and adsorption. Appl. Surf. Sci. 2015, 349, 251–258. [Google Scholar] [CrossRef]
- Chen, X.; Song, Z.; Yuan, B.; Li, X.; Li, S.; Thang Nguyen, T.; Guo, M.; Guo, Z. Fluorescent carbon dots crosslinked cellulose Nanofibril/Chitosan interpenetrating hydrogel system for sensitive detection and efficient adsorption of Cu (II) and Cr (VI). Chem. Eng. J. 2022, 430, 133154. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, Y.; Liang, Z.; Zeng, L.; Zhang, A. Preparation of Chitosan/Calcium Alginate/Bentonite Composite Hydrogel and Its Heavy Metal Ions Adsorption Properties. Polymers 2021, 13, 1891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ou, J.; Wang, B.; Wang, H.; He, Q.; Song, J.; Zhang, H.; Tang, M.; Zhou, L.; Gao, Y.; et al. Efficient heavy metal removal from water by alginate-based porous nanocomposite hydrogels: The enhanced removal mechanism and influencing factor insight. J. Hazard. Mater. 2021, 418, 126358. [Google Scholar] [CrossRef] [PubMed]
- Veregue, F.R.; de Lima, H.H.C.; Ribeiro, S.C.; Almeida, M.S.; da Silva, C.T.P.; Guilherme, M.R.; Rinaldi, A.W. MCM-41/chondroitin sulfate hybrid hydrogels with remarkable mechanical properties and superabsorption of methylene blue. Carbohydr. Polym. 2020, 247, 116558. [Google Scholar] [CrossRef]
- Beyranvand, N.S.; Samiey, B.; Tehrani, A.D.; Soleimani, K. Graphene Oxide–Cellulose Nanowhisker Hydrogel Nanocomposite as a Novel Adsorbent for Methylene Blue. J. Chem. Eng. Data 2019, 64, 5558–5570. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Ray, S.K. Enhanced adsorption of synthetic dyes from aqueous solution by a semi-interpenetrating network hydrogel based on starch. J. Ind. Eng. Chem. 2014, 20, 3714–3725. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Q.; Zhu, S. Selective biosorption mechanism of methylene blue by a novel and reusable sugar beet pulp cellulose/sodium alginate/iron hydroxide composite hydrogel. Int. J. Biol. Macromol 2021, 188, 993–1002. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Zhang, Y.; Zhang, H.; Huang, H. Green and facile fabrication of pineapple peel cellulose/magnetic diatomite hydrogels in ionic liquid for methylene blue adsorption. Cellulose 2019, 26, 3825–3844. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Huang, H. Eco-friendly polyvinyl alcohol/carboxymethyl cellulose hydrogels reinforced with graphene oxide and bentonite for enhanced adsorption of methylene blue. Carbohydr. Polym. 2018, 185, 1–11. [Google Scholar] [CrossRef]
- Lu, W.; Dai, Z.; Li, L.; Liu, J.; Wang, S.; Yang, H.; Cao, C.; Liu, L.; Chen, T.; Zhu, B.; et al. Preparation of composite hydrogel (PCG) and its adsorption performance for uranium(VI). J. Mol. Liq. 2020, 303, 112604. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, Y.; Wang, W.; Zhang, T.; Chen, T.; Yi, H.; Rao, F.; Song, S. Removal of methylene blue from water with montmorillonite nanosheets/chitosan hydrogels as adsorbent. Appl. Surf. Sci. 2018, 448, 203–211. [Google Scholar] [CrossRef]
- Jamali, M.; Akbari, A. Facile fabrication of magnetic chitosan hydrogel beads and modified by interfacial polymerization method and study of adsorption of cationic/anionic dyes from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 105175. [Google Scholar] [CrossRef]
- Hossain, S.; Shahruzzaman, M.; Kabir, S.F.; Rahman, M.S.; Sultana, S.; Mallik, A.K.; Haque, P.; Takafuji, M.; Rahman, M.M. Jute cellulose nanocrystal/poly(N,N-dimethylacrylamide-co-3-methacryloxypropyltrimethoxysilane) hybrid hydrogels for removing methylene blue dye from aqueous solution. J. Sci. Adv. Mater. Devices 2021, 6, 254–263. [Google Scholar] [CrossRef]
- Junlapong, K.; Maijan, P.; Chaibundit, C.; Chantarak, S. Effective adsorption of methylene blue by biodegradable superabsorbent cassava starch-based hydrogel. Int. J. Biol. Macromol. 2020, 158, 258–264. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Xu, J.-Y.; Yang, X.-L. MXene/sodium alginate gel beads for adsorption of methylene blue. Mater. Chem. Phys. 2021, 260, 124123. [Google Scholar] [CrossRef]
- Verma, A.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int. J. Biol. Macromol. 2020, 148, 1130–1139. [Google Scholar] [CrossRef]
- Isik, B.; Ugraskan, V. Adsorption of methylene blue on sodium alginate-flax seed ash beads: Isotherm, kinetic and thermodynamic studies. Int. J. Biol. Macromol. 2021, 167, 1156–1167. [Google Scholar] [CrossRef]
- Malekzadeh, M.; Nejaei, A.; Baneshi, M.M.; Kokhdan, E.P.; Bardania, H. The use of starch-modified magnetic Fe0. nanoparticles for naphthalene adsorption from water samples: Adsorption isotherm, kinetic and thermodynamic studies. Appl. Organomet. Chem. 2018, 32, e4434. [Google Scholar] [CrossRef]
- Guan, Q.F.; Yang, H.B.; Han, Z.M.; Ling, Z.C.; Yin, C.H.; Yang, K.P.; Zhao, Y.X.; Yu, S.H. Sustainable Cellulose-Nanofiber-Based Hydrogels. ACS Nano 2021, 15, 7889–7898. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, T.; Li, W.; Yu, F.; Ma, J. Amino-functionalized alginate/graphene double-network hydrogel beads for emerging contaminant removal from aqueous solution. Chemosphere 2020, 241, 125110. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.Z.; Yue, R.; Sun, X.F.; Song, C.; Wang, S.G. Enhanced removal of ciprofloxacin using humic acid modified hydrogel beads. J. Colloid Interface Sci. 2019, 543, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Phuong, V.N.; Van, T.N.; Thi, P.N.; Dinh Thi Lan, P.; Pham, H.T.; Cao, H.T. Low-cost hydrogel derived from agro-waste for veterinary antibiotic removal: Optimization, kinetics, and toxicity evaluation. Environ. Technol. Innov. 2020, 20, 101098. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Singh, A.; Carabineiro, S.A.C. Methionine-Functionalized Graphene Oxide/Sodium Alginate Bio-Polymer Nanocomposite Hydrogel Beads: Synthesis, Isotherm and Kinetic Studies for an Adsorptive Removal of Fluoroquinolone Antibiotics. Nanomaterials 2021, 11, 568. [Google Scholar] [CrossRef]
- Karimi, S.; Namazi, H. Magnetic alginate/glycodendrimer beads for efficient removal of tetracycline and amoxicillin from aqueous solutions. Int. J. Biol. Macromol. 2022, 205, 128–140. [Google Scholar] [CrossRef]
- Ranjbari, S.; Tanhaei, B.; Ayati, A.; Khadempir, S.; Sillanpaa, M. Efficient tetracycline adsorptive removal using tricaprylmethylammonium chloride conjugated chitosan hydrogel beads: Mechanism, kinetic, isotherms and thermodynamic study. Int. J. Biol. Macromol. 2020, 155, 421–429. [Google Scholar] [CrossRef]
- Liao, Q.; Rong, H.; Zhao, M.; Luo, H.; Chu, Z.; Wang, R. Strong adsorption properties and mechanism of action with regard to tetracycline adsorption of double-network polyvinyl alcohol-copper alginate gel beads. J. Hazard. Mater. 2022, 422, 126863. [Google Scholar] [CrossRef]





| Polysaccharide Hydrogel Adsorption | Adsorbates | Adsorption Capacity (mg/g) | Adsorption Isotherm | Adsorption Kinetics | Ref. |
|---|---|---|---|---|---|
| AM/AA | Cu2+ | 157.51 | Langmuir | PSO | [63] |
| Pd2+ | 393.28 | Langmuir | PSO | ||
| Cd2+ | 289.97 | Langmuir | PSO | ||
| Nanocellulose/Carbon dot | Cr6+ | 599.9 | Freundlich | PSO | [64] |
| Straw cellulose | Cd2+ | 95.62 | Langmuir | PSO | [65] |
| Nanocellulose/SA | Pb2+ | 318.47 | Langmuir | PSO | [66] |
| GO-PVA-CS | Cd2+ | 172.11 | Langmuir | PSO | [67] |
| Ni2+ | 70.37 | Langmuir | PSO | ||
| CYCS/CNC | Pb2+ | 334.92 | Langmuir | PSO | [68] |
| Chitosan oligosaccharide | Cr6+ | 148.1 | Langmuir | PSO | [69] |
| α-ketoglutaric acid–PAM-CS | Cu2+ | 72.39 | Langmuir | PSO | [50] |
| Pd2+ | 61.41 | Langmuir | PSO | ||
| Zn2+ | 51.89 | Langmuir | PSO | ||
| CPCS-PAM-PVA | Cr6+ | 95.31 | Langmuir | PFO | [70] |
| Millettia speciosa Champ cellulose-CS | Cu2+ | 23.37 | Freundlich | PFO | [71] |
| All-lignocellulose | Cu2+ | 350 | Langmuir | PSO | [72] |
| Caffeic acid starch | Cr6+ | 96.45 | Langmuir | PSO | [73] |
| Starch-FMBO | As3+ | 161.29 | Langmuir | PSO | [74] |
| Starch nanoparticle | Pb2+ | 40.52 | Langmuir | PSO | [27] |
| Cu2+ | 32.88 | Langmuir | PSO | ||
| dibenzo-18-crown-6 starch | Cd2+ | 368.5 | Freundlich | PSO | [75] |
| Ni2+ | 182.5 | Freundlich | PSO | ||
| Zn2+ | 377.5 | Freundlich | PSO | ||
| Cu2+ | 385 | Freundlich | PSO | ||
| Succinic anhydride-SNCs | Cu2+ | 84.07 | Freundlich | PSO | [31] |
| PVA-SA | Pb2+ | 784.97 | Langmuir | PSO | [76] |
| ZIF-67-SA | Cu2+ | 153.63 | Langmuir | PSO | [77] |
| AM-GO-SA | Cu2+ | 68.76 | Langmuir | PSO | [78] |
| Pb2+ | 240.69 | Langmuir | PSO | ||
| Zeolite-PVA-SA | Pb2+ | 99.5 | Langmuir | PFO | [79] |
| Cd2+ | 99.2 | Langmuir | PFO | ||
| Sr2+ | 98.8 | Langmuir | PFO | ||
| Cu2+ | 97.2 | Langmuir | PFO | ||
| Zn2+ | 95.6 | Langmuir | PFO | ||
| Ni2+ | 93.1 | Langmuir | PFO | ||
| Mn2+ | 92.4 | Langmuir | PFO | ||
| Starch ether-SA | Cu2+ | 25.81 | Langmuir | PSO | [80] |
| Reptilite-Starch | Pb2+ | 180.8 | Langmuir | PSO | [81] |
| NCDs-CNF/CS | Cu2+ | 148.3 | Langmuir | PSO | [82] |
| Cr6+ | 294.46 | Langmuir | PSO | ||
| CTS/CA/BT | Pb2+ | 434.89 | Freundlich | PSO | [83] |
| Cu2+ | 115.30 | Freundlich | PSO | ||
| Cd2+ | 102.38 | Freundlich | PSO | ||
| GO-SA | As5+ | 277.39 | Langmuir | PSO | [55] |
| PAN-PPY-SA-GO | Cu2+ | 133.7 | Redlich–Peterson | PFO | [84] |
| Cr6+ | 87.2 | Redlich–Peterson | PFO |
| Polysaccharide Hydrogels Adsorption | Adsorbates | Adsorption Capacity (mg/g) | Adsorption Isotherm | Adsorption Kinetics | Ref. |
|---|---|---|---|---|---|
| C/SA/Fe | MB | 105.93 | Langmuir | PSO | [88] |
| Carboxymethylcellulose | MB | 756 | Freundlich | PSO | [12] |
| Pineapple peel cellulose/diatomite | MB | 101.94 | Langmuir | PSO | [89] |
| PCMC-PVA | MB | 172.14 | Langmuir | PSO | [90] |
| All-lignocellulose | MB | 145 | Langmuir | PSO | [72] |
| Millettia speciosa Champ cellulose-CS | CR | 221.43 | Freundlich | PSO | [71] |
| PAM-Fe3O4-CS | MB | 1603 | Langmuir | PFO | [91] |
| Montmorillonite-CS | MB | 530 | Langmuir | PSO | [92] |
| GO-CS-Fe3O4 | MB | 289 | Langmuir | PSO | [93] |
| EBT | 292 | Langmuir | PSO | [94] | |
| Jute cellulose nanocrystal | MB | 131.58 | Langmuir | PSO | |
| Succinic anhydride-SNCs | MB | 84.00 | Freundlich | PSO | [31] |
| Reptilite-Starch | MB | 277.0 | Langmuir | PSO | [81] |
| PAM-cassava starch | MB | 2000 | Langmuir | PSO | [95] |
| MXene-SA | MB | 92.17 | Langmuir | PSO | [96] |
| AA-GO-SA | MG | 628.93 | Langmuir | PSO | [97] |
| Flax seed ash-SA | MB | 333.3 | Langmuir | PSO | [98] |
| PEI-SA | MB | 400 | Langmuir | PSO | [47] |
| AM-HEMA-Starch | MG | 164 | Langmuir | PFO | [71] |
| MV | 156 | Freundlich | PFO |
| Polysaccharide Hydrogels Adsorption | Adsorbates | Adsorption Capacity (mg/g) | Adsorption Isotherm | Adsorption Kinetics | Ref. |
|---|---|---|---|---|---|
| Fe3O4-Starch | Naphthalene | 24.752 | Langmuir | PSO | [99] |
| CS-Chitosan film | Cefotaxime Sodium | 1003.64 | Freundlich | PSO | [100] |
| GO-SA | Tetracycline | 477.9 | Freundlich | PSO | [55] |
| Amino/GO-SA | Ciprofloxacin | 301.36 | Langmuir | PSO | [101] |
| Humicacid-CS-Biochar | Ciprofloxacin | 154.89 | Langmuir | PSO | [102] |
| Biochar-CS | Ciprofloxacin | 106.038 | Langmuir | PSO | [103] |
| Enrofloxacin | 100.433 | Langmuir | PSO | ||
| GO-SA | Fluoxacin | 4.11 | Langmuir | PSO | [104] |
| Moxifloxacin | 3.43 | Langmuir | PSO | ||
| Fe3O4-SA | Tetracycline | 454.54 | Langmuir | PSO | [105] |
| Amoxicillin | 400 | Langmuir | PSO | ||
| Trimethylammonium chloride-CS | Tetracycline | 22.42 | Langmuir | PFO | [106] |
| PVA-SA-Cu2+ | Tetracycline | 231.431 | Langmuir | PSO | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Xu, Y.; Mu, X.; Li, S.; Liu, X.; Lei, Z. Research Progress of Polysaccharide-Based Natural Polymer Hydrogels in Water Purification. Gels 2023, 9, 249. https://doi.org/10.3390/gels9030249
Zhang W, Xu Y, Mu X, Li S, Liu X, Lei Z. Research Progress of Polysaccharide-Based Natural Polymer Hydrogels in Water Purification. Gels. 2023; 9(3):249. https://doi.org/10.3390/gels9030249
Chicago/Turabian StyleZhang, Wenxu, Yan Xu, Xuyang Mu, Sijie Li, Xiaoming Liu, and Ziqiang Lei. 2023. "Research Progress of Polysaccharide-Based Natural Polymer Hydrogels in Water Purification" Gels 9, no. 3: 249. https://doi.org/10.3390/gels9030249