Molten Salts Approach of Poly(vinyl alcohol)-Derived Bimetallic Nickel–Iron Sheets Supported on Porous Carbon Nanosheet as an Effective and Durable Electrocatalyst for Methanol Oxidation
Abstract
:1. Introduction
2. Results and Discussion
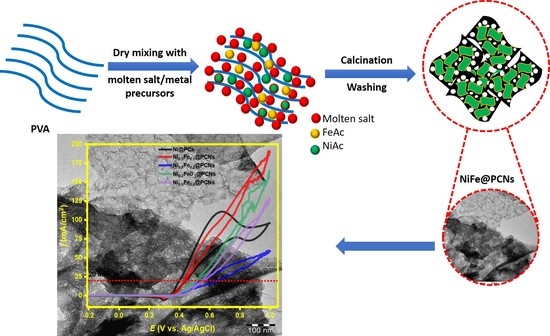
2.1. Structure and Morphology of the Catalysts
2.2. Electrocatalytic Activity and Durability of Catalysts
3. Conclusions
4. Materials and Methods
4.1. Materials and Synthesis Method
4.2. Preparation Method
4.3. Characterization
4.4. Electrochemical Measurements
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boudghene Stambouli, A.; Traversa, E. Fuel cells, an alternative to standard sources of energy. Renew. Sustain. Energy Rev. 2002, 6, 295–304. [Google Scholar] [CrossRef]
- Brouwer, J. On the role of fuel cells and hydrogen in a more sustainable and renewable energy future. Curr. Appl. Phys. 2010, 10, S9–S17. [Google Scholar] [CrossRef] [Green Version]
- Wilberforce, T.; Alaswad, A.; Palumbo, A.; Dassisti, M.; Olabi, A.G. Advances in stationary and portable fuel cell applications. Int. J. Hydrogen Energy 2016, 41, 16509–16522. [Google Scholar] [CrossRef] [Green Version]
- Kamarudin, S.K.; Achmad, F.; Daud, W.R.W. Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int. J. Hydrogen Energy 2009, 34, 6902–6916. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, X.; Sun, H.; Wang, S.; Sun, G. Recent advances in multi-scale design and construction of materials for direct methanol fuel cells. Nano Energy 2019, 65, 104048. [Google Scholar] [CrossRef]
- Alias, M.S.; Kamarudin, S.K.; Zainoodin, A.M.; Masdar, M.S. Active direct methanol fuel cell: An overview. Int. J. Hydrogen Energy 2020, 45, 19620–19641. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Singh, G.; Kim, K.S. Recent progress in the development of anode and cathode catalysts for direct methanol fuel cells. Nano Energy 2013, 2, 553–578. [Google Scholar] [CrossRef]
- Liu, H.; Song, C.; Zhang, L.; Zhang, J.; Wang, H.; Wilkinson, D.P. A review of anode catalysis in the direct methanol fuel cell. J. Power Sources 2006, 155, 95–110. [Google Scholar] [CrossRef]
- Lou, W.; Ali, A.; Shen, P.K. Recent development of Au arched Pt nanomaterials as promising electrocatalysts for methanol oxidation reaction. Nano Res. 2021, 15, 18–37. [Google Scholar] [CrossRef]
- Kakati, N.; Maiti, J.; Lee, S.H.; Jee, S.H.; Viswanathan, B.; Yoon, Y.S. Anode catalysts for direct methanol fuel cells in acidic media: Do we have any alternative for Pt or Pt-Ru? Chem. Rev. 2014, 114, 12397–12429. [Google Scholar] [CrossRef]
- Mansor, M.; Timmiati, S.N.; Lim, K.L.; Wong, W.Y.; Kamarudin, S.K.; Nazirah Kamarudin, N.H. Recent progress of anode catalysts and their support materials for methanol electrooxidation reaction. Int. J. Hydrogen Energy 2019, 44, 14744–14769. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.; Chen, Y.; Kim, H.; Xu, X.; Guan, D.; Hu, Z.; Zhang, L.; Shao, Z.; Jung, W.C. Boosting ethanol oxidation by NiOOH-CuO nano-heterostructure for energy-saving hydrogen production and biomass upgrading. Appl. Catal. B Environ. 2023, 325, 122388. [Google Scholar] [CrossRef]
- Chen, D.; Minteer, S.D. Mechanistic study of nickel based catalysts for oxygen evolution and methanol oxidation in alkaline medium. J. Power Sources 2015, 284, 27–37. [Google Scholar] [CrossRef]
- Rahim, M.A.A.; Hameed, R.M.A.; Khalil, M.W. Nickel as a catalyst for the electro-oxidation of methanol in alkaline medium. J. Power Sources 2004, 134, 160–169. [Google Scholar] [CrossRef]
- Dubale, A.A.; Zheng, Y.; Wang, H.; Hübner, R.; Li, Y.; Yang, J.; Zhang, J.; Sethi, N.K.; He, L.; Zheng, Z.; et al. High-Performance Bismuth-Doped Nickel Aerogel Electrocatalyst for the Methanol Oxidation Reaction. Angew. Chem. 2020, 132, 13995–14003. [Google Scholar] [CrossRef]
- Zhang, H.; Gu, C.D.; Huang, M.L.; Wang, X.L.; Tu, J.P. Anchoring three-dimensional network structured Ni–P nanowires on reduced graphene oxide and their enhanced electrocatalytic activity towards methanol oxidation. Electrochem. Commun. 2013, 35, 108–111. [Google Scholar] [CrossRef]
- Wang, J.; Teschner, D.; Yao, Y.; Huang, X.; Willinger, M.; Shao, L.; Schlögl, R. Fabrication of nanoscale NiO/Ni heterostructures as electrocatalysts for efficient methanol oxidation. J. Mater. Chem. A 2017, 5, 9946–9951. [Google Scholar] [CrossRef] [Green Version]
- Araujo, R.B.; Martín-Yerga, D.; dos Santos, E.C.; Cornell, A.; Pettersson, L.G.M. Elucidating the role of Ni to enhance the methanol oxidation reaction on Pd electrocatalysts. Electrochim. Acta 2020, 360, 136954. [Google Scholar] [CrossRef]
- Li, Y.; Bao, X.; Chen, D.; Wang, Z.; Dewangan, N.; Li, M.; Xu, Z.; Wang, J.; Kawi, S.; Zhong, Q. A Minireview on Nickel-Based Heterogeneous Electrocatalysts for Water Splitting. ChemCatChem 2019, 11, 5913–5928. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, J.; Liu, X.; Zhu, X. Three-dimensional porous graphene supported Ni nanoparticles with enhanced catalytic performance for Methanol electrooxidation. Int. J. Hydrogen Energy 2017, 42, 11206–11214. [Google Scholar] [CrossRef]
- Altaleb, H.A.; Salah, A.; Thamer, B.M. Hydrogel Nanocomposite-Derived Nickel Nanoparticles/Porous Carbon Frameworks as Non-Precious and Effective Electrocatalysts for Methanol Oxidation. Gels 2022, 8, 542. [Google Scholar] [CrossRef] [PubMed]
- Thamer, B.M.; El-Newehy, M.H.; Barakat, N.A.M.; Abdelkareem, M.A.; Al-Deyab, S.S.; Kim, H.Y. Influence of Nitrogen doping on the Catalytic Activity of Ni-incorporated Carbon Nanofibers for Alkaline Direct Methanol Fuel Cells. Electrochim. Acta 2014, 142, 228–239. [Google Scholar] [CrossRef]
- Thamer, B.M.; Moydeen Abdulhameed, M.; El-Newehy, M.H. Tragacanth Gum Hydrogel-Derived Trimetallic Nanoparticles Supported on Porous Carbon Catalyst for Urea Electrooxidation. Gels 2022, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Abdel Hameed, R.M.; Al-Enizi, A.M.; Thamer, B.M.; Yousef, A.; El-Newehy, M.H. Decorated carbon nanofibers with mixed nickel−manganese carbides for methanol electro-oxidation in alkaline solution. Int. J. Hydrogen Energy 2021, 46, 6494–6512. [Google Scholar] [CrossRef]
- Xiu, R.; Zhang, F.; Wang, Z.; Yang, M.; Xia, J.; Gui, R.; Xia, Y. Electrodeposition of PtNi bimetallic nanoparticles on three-dimensional graphene for highly efficient methanol oxidation. RSC Adv. 2015, 5, 86578–86583. [Google Scholar] [CrossRef]
- Guan, H.; Wang, N.; Feng, X.; Bian, S.; Li, W.; Chen, Y. A high-efficiency oxygen evolution electrode material of a carbon material containing a NiCo bimetal. RSC Adv. 2021, 11, 16461–16467. [Google Scholar] [CrossRef]
- Yang, H.; Bradley, S.J.; Chan, A.; Waterhouse, G.I.N.; Nann, T.; Kruger, P.E.; Telfer, S.G. Catalytically Active Bimetallic Nanoparticles Supported on Porous Carbon Capsules Derived from Metal-Organic Framework Composites. J. Am. Chem. Soc. 2016, 138, 11872–11881. [Google Scholar] [CrossRef]
- Gupta, S.K.; Mao, Y. Recent Developments on Molten Salt Synthesis of Inorganic Nanomaterials: A Review. J. Phys. Chem. C 2021, 125, 6508–6533. [Google Scholar] [CrossRef]
- Yu, H.; Sun, X.; Tang, D.; Huang, Y.; Zhang, W.; Miao, S.; Qiao, Z.A.; Wang, J.; Zhao, Z. Molten salt strategy to synthesize alkali metal-doped Co9S8 nanoparticles embedded, N, S co-doped mesoporous carbon as hydrogen evolution electrocatalyst. Int. J. Hydrogen Energy 2020, 45, 6006–6014. [Google Scholar] [CrossRef]
- Yi, L.; Chen, J.; Shao, P.; Huang, J.; Peng, X.; Li, J.; Wang, G.; Zhang, C.; Wen, Z. Molten-Salt-Assisted Synthesis of Bismuth Nanosheets for Long-term Continuous Electrocatalytic Conversion of CO2 to Formate. Angew. Chem. Int. Ed. 2020, 59, 20112–20119. [Google Scholar] [CrossRef]
- Gupta, S.K.; Mao, Y. A review on molten salt synthesis of metal oxide nanomaterials: Status, opportunity, and challenge. Prog. Mater. Sci. 2021, 117, 100734. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, C.; Shi, Z.; Chen, D.; Chen, X.; Zhang, Q.; Pang, B.; Feng, J.; Yu, L.; Dong, L. Molten-salt-assisted synthesis of onion-like Co/CoO@FeNC materials with boosting reversible oxygen electrocatalysis for rechargeable Zn-air battery. J. Colloid Interface Sci. 2021, 596, 206–214. [Google Scholar] [CrossRef]
- Aldalbahi, A.; El-Newehy, M.H.; El-Hamshary, H.; Samuel, E.Y.S. Facile Preparation of Porous Carbon Flake-Supported Nickel Nanoplates as Effective Catalysts for Methanol Electrooxidation. Catalysts 2022, 12, 556. [Google Scholar] [CrossRef]
- Lokanathan, M.; Patil, I.M.; Mukherjee, P.; Swami, A.; Kakade, B. Molten-Salt Synthesis of Pt3Co Binary Alloy Nanoplates as Excellent and Durable Electrocatalysts toward Oxygen Electroreduction. ACS Sustain. Chem. Eng. 2020, 8, 986–993. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Y.; Sun, F.; Chen, G.; Wang, J.; Peng, L.; Chen, W.T.; Shang, L.; Zhao, J.; Sun-Waterhouse, D.; et al. Molten NaCl-Assisted Synthesis of Porous Fe-N-C Electrocatalysts with a High Density of Catalytically Accessible FeN4 Active Sites and Outstanding Oxygen Reduction Reaction Performance. Adv. Energy Mater. 2021, 11, 2100219. [Google Scholar] [CrossRef]
- Sun, X.; Tang, D.; Zhang, W.; Li, K.; Qiao, Z.A.; Liu, Y.; Liang, D.; Zhu, J.; Zhao, Z. Molten salt synthesis of Co-entrapped, N-doped porous carbon from various nitrogen precursors as efficient electrocatalysts for hydrogen evolution. J. Mater. Sci. 2019, 54, 638–647. [Google Scholar] [CrossRef]
- Hu, J.; Wu, D.; Zhu, C.; Hao, C.; Xin, C.; Zhang, J.; Guo, J.; Li, N.; Zhang, G.; Shi, Y. Melt-salt-assisted direct transformation of solid oxide into atomically dispersed FeN4 sites on nitrogen-doped porous carbon. Nano Energy 2020, 72, 104670. [Google Scholar] [CrossRef]
- Wang, A.; Zhao, Z.; Hu, D.; Niu, J.; Zhang, M.; Yan, K.; Lu, G. Tuning the oxygen evolution reaction on a nickel–iron alloy via active straining. Nanoscale 2019, 11, 426–430. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Li, X.; Li, Y.; Yang, T.; Liang, Y. Facile Synthesis of Nickel-Iron/Nanocarbon Hybrids as Advanced Electrocatalysts for Efficient Water Splitting. ACS Catal. 2016, 6, 580–588. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, M.; Li, Z.; Zhu, X.; Tian, C.; Xia, C.; He, L.; Dai, S. Coupling FeNi alloys and hollow nitrogen-enriched carbon frameworks leads to high-performance oxygen electrocatalysts for rechargeable zinc–air batteries. Sustain. Energy Fuels 2018, 3, 136–141. [Google Scholar] [CrossRef]
- Qazi, U.Y.; Yuan, C.Z.; Ullah, N.; Jiang, Y.F.; Imran, M.; Zeb, A.; Zhao, S.J.; Javaid, R.; Xu, A.W. One-Step Growth of Iron-Nickel Bimetallic Nanoparticles on FeNi Alloy Foils: Highly Efficient Advanced Electrodes for the Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 28627–28634. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, H.; Thamer, B.M.; Sedky, A.; El-Newehy, M.H. Facile two-step synthesis of nickel nanoparticles supported on 3D porous carbon frameworks as an effective electrocatalyst for urea and methanol oxidation. Mater. Chem. Phys. 2023, 297, 127361. [Google Scholar] [CrossRef]
- Xiang, D.; Bo, X.; Gao, X.; Zhang, C.; Du, C.; Zheng, F.; Zhuang, Z.; Li, P.; Zhu, L.; Chen, W. Novel one-step synthesis of core@shell iron–nickel alloy nanoparticles coated by carbon layers for efficient oxygen evolution reaction electrocatalysis. J. Power Sources 2019, 438, 226988. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, J.; Ai, L. Layered Bimetallic Iron-Nickel Alkoxide Microspheres as High-Performance Electrocatalysts for Oxygen Evolution Reaction in Alkaline Media. ACS Sustain. Chem. Eng. 2018, 6, 6117–6125. [Google Scholar] [CrossRef]
- Wang, B.; Pan, J.; Jiang, Z.; Dong, Z.; Zhao, C.; Wang, J.; Song, C.; Zheng, Y.; Li, C. The bimetallic iron−nickel sulfide modified g-C3N4 nano-heterojunction and its photocatalytic hydrogen production enhancement. J. Alloys Compd. 2018, 766, 421–428. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, T.; Liu, Q.; Wang, Z.; Gu, R.; Guo, L.; Liu, Y. Three-Dimensional Flower-Like Bimetallic Nickel-Iron Selenide for Efficient Oxygen Evolution Reaction. J. Phys. Chem. C 2022, 126, 5131–5137. [Google Scholar] [CrossRef]
- Leonard, N.; Nallathambi, V.; Barton, S.C. Carbon Supports for Non-Precious Metal Oxygen Reducing Catalysts. J. Electrochem. Soc. 2013, 160, F788–F792. [Google Scholar] [CrossRef]
- De Jesus, J.C.; González, I.; Quevedo, A.; Puerta, T. Thermal decomposition of nickel acetate tetrahydrate: An integrated study by TGA, QMS and XPS techniques. J. Mol. Catal. A Chem. 2005, 228, 283–291. [Google Scholar] [CrossRef]
- Thamer, B.M.; Al-Sabri, A.E.; Almansob, A.; El-Newehy, M.H. Fabrication of Biohybrid Nanofibers by the Green Electrospinning Technique and Their Antibacterial Activity. ACS Omega 2022, 7, 7311–7319. [Google Scholar] [CrossRef]
- Hoekstra, J.; Versluijs-Helder, M.; Vlietstra, E.J.; Geus, J.W.; Jenneskens, L.W. Carbon-Supported Base Metal Nanoparticles: Cellulose at Work. ChemSusChem 2015, 8, 985–989. [Google Scholar] [CrossRef]
- Candelaria, S.L.; Bedford, N.M.; Woehl, T.J.; Rentz, N.S.; Showalter, A.R.; Pylypenko, S.; Bunker, B.A.; Lee, S.; Reinhart, B.; Ren, Y.; et al. Multi-Component Fe-Ni Hydroxide Nanocatalyst for Oxygen Evolution and Methanol Oxidation Reactions under Alkaline Conditions. ACS Catal. 2017, 7, 365–379. [Google Scholar] [CrossRef]
- Louie, M.W.; Bell, A.T. An investigation of thin-film Ni-Fe oxide catalysts for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 2013, 135, 12329–12337. [Google Scholar] [CrossRef] [Green Version]
- Vedharathinam, V.; Botte, G.G. Understanding the electro-catalytic oxidation mechanism of urea on nickel electrodes in alkaline medium. Electrochim. Acta 2012, 81, 292–300. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, Y.; Yang, L.; Zhao, Y.; Zhu, Y.; Jiang, H.; Li, C. Insights into the activity of nickel boride/nickel heterostructures for efficient methanol electrooxidation. Nat. Commun. 2022, 13, 4602. [Google Scholar] [CrossRef]
- Thamer, B.M.; El-Newehy, M.H.; Al-Deyab, S.S.; Abdelkareem, M.A.; Kim, H.Y.; Barakat, N.A.M. Cobalt-incorporated, nitrogen-doped carbon nanofibers as effective non-precious catalyst for methanol electrooxidation in alkaline medium. Appl. Catal. A Gen. 2015, 498, 230–240. [Google Scholar] [CrossRef]
- Gu, L.; Qian, L.; Lei, Y.; Wang, Y.; Li, J.; Yuan, H.; Xiao, D. Microwave-assisted synthesis of nanosphere-like NiCo2O4 consisting of porous nanosheets and its application in electro-catalytic oxidation of methanol. J. Power Sources 2014, 261, 317–323. [Google Scholar] [CrossRef]
- Cui, X.; Xiao, P.; Wang, J.; Zhou, M.; Guo, W.; Yang, Y.; He, Y.; Wang, Z.; Yang, Y.; Zhang, Y.; et al. Highly Branched Metal Alloy Networks with Superior Activities for the Methanol Oxidation Reaction. Angew. Chem. 2017, 129, 4559–4564. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Motlak, M. CoxNiy-decorated graphene as novel, stable and super effective non-precious electro-catalyst for methanol oxidation. Appl. Catal. B Environ. 2014, 154–155, 221–231. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, W.; Cheng, D. Facile Synthesis of Cu/NiCu Electrocatalysts Integrating Alloy, Core-Shell, and One-Dimensional Structures for Efficient Methanol Oxidation Reaction. ACS Appl. Mater. Interfaces 2017, 9, 19843–19851. [Google Scholar] [CrossRef]
- Thamer, B.M.; El-Newehy, M.H.; Barakat, N.A.M.; Al-Deyab, S.S.; Kim, H.Y. Preparation of zero-valent Co/N-CNFs as an immobilized thin film onto graphite disc for methanol electrooxidation. Fibers Polym. 2017, 18, 696–705. [Google Scholar] [CrossRef]
- Rahmani, K.; Habibi, B. NiCo alloy nanoparticles electrodeposited on an electrochemically reduced nitrogen-doped graphene oxide/carbon-ceramic electrode: A low cost electrocatalyst towards methanol and ethanol oxidation. RSC Adv. 2019, 9, 34050–34064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, N.; Bernaurdshaw, N. Reduced Graphene Oxide Supported NiCo2O4 Nano-Rods: An Efficient, Stable and Cost-Effective Electrocatalyst for Methanol Oxidation Reaction. ChemCatChem 2020, 12, 771–780. [Google Scholar] [CrossRef]
- Rezaee, S.; Shahrokhian, S. Facile synthesis of petal-like NiCo/NiO-CoO/nanoporous carbon composite based on mixed-metallic MOFs and their application for electrocatalytic oxidation of methanol. Appl. Catal. B Environ. 2019, 244, 802–813. [Google Scholar] [CrossRef]
- Li, J.; Luo, Z.; Zuo, Y.; Liu, J.; Zhang, T.; Tang, P.; Arbiol, J.; Llorca, J.; Cabot, A. NiSn bimetallic nanoparticles as stable electrocatalysts for methanol oxidation reaction. Appl. Catal. B Environ. 2018, 234, 10–18. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; El-Newehy, M.; Al-Deyab, S.S.; Kim, H.Y. Cobalt/copper-decorated carbon nanofibers as novel non-precious electrocatalyst for methanol electrooxidation. Nanoscale Res. Lett. 2014, 9, 2. [Google Scholar] [CrossRef] [Green Version]













| Sample | 2θ | FWHM | Size of Crystallite (D) (nm) | Average of D (nm) | d-Space (nm) | PCNs Number |
|---|---|---|---|---|---|---|
| Ni@PCs | 44.40 | 0.2681 | 32.00 | 30.59 | 0.2038 | |
| 51.76 | 0.2770 | 31.87 | 0.1765 | |||
| 76.31 | 0.3622 | 27.89 | 0.1247 | |||
| 26.05 | 1.02 | 8.007 | 0.3417 | 23.43 | ||
| Ni0.9Fe0.1@PCNs | 44.32 | 0.3007 | 28.52 | 27.73 | 0.2042 | |
| 51.65 | 0.3186 | 27.70 | 0.1768 | |||
| 76.08 | 0.3740 | 26.97 | 0.1250 | |||
| 24.66 | 14.24 | 0.5711 | 0.3607 | 1.58 | ||
| Ni0.8Fe0.2@PCNs | 44.14 | 0.4019 | 21.33 | 21.69 | 0.20501 | |
| 51.43 | 0.4078 | 21.62 | 0.1775 | |||
| 75.76 | 0.4548 | 22.13 | 0.1254 | |||
| 25.11 | 16.16 | 0.5035 | 0.3543 | 1.42 | ||
| Ni0.7Fe0.3@PCNs | 44.04 | 0.3653 | 23.46 | 23.55 | 0.2055 | |
| 51.30 | 0.3769 | 23.38 | 0.1779 | |||
| 75.48 | 0.4221 | 23.80 | 0.1258 | |||
| 24.73 | 15.32 | 0.5307 | 0.3597 | 1.74 | ||
| Ni0.6Fe0.4@PCNs | 43.85 | 0.5289 | 16.19 | 15.51 | 0.2063 | |
| 51.08 | 0.5473 | 16.09 | 0.1787 | |||
| 75.07 | 0.7033 | 14.24 | 0.1264 | |||
| 23.65 | 10.39 | 0.7810 | 0.3758 | 2.08 |
| Electrocatalyst | Synthesis Method | j (mA/cm2) | Catalytic Activity Retention (%) | Ref. |
|---|---|---|---|---|
| NiCo2O4/Ni foam | Microwave-assisted synthesis | 10 | 91.7% at 1000 s | [56] |
| Ni0.75Cu0.25 | Electrodeposition | 84 | ~91% at 1200 s | [57] |
| Ni0.2Co0.2/Gr | Impregnation/calcination | 75 | - | [58] |
| Cu/NiCu nanowires | Wet synthesis | 34.9 | ~95% at 10,000 s | [59] |
| Co/NCNFs/graphite | Electrospinning/calcination | 90 | ~80% at 1000 s | [60] |
| NiCo/N-doped graphene | Electrodeposition | 88.04 | ~70% at 2000 s | [61] |
| NiCo2O4/rGO | Hydrothermal/calcination | 78 | ~19.2% at 3000 s | [62] |
| NiCo/NiO-CoO | Hydrothermal/carbonization | 178 | ~38% at 3570s | [63] |
| NiSn NPs | Coreduction | 50 | ~79% at 5000 s | [64] |
| Co-Cu/CNFs | Electrospinning/calcination | 17 | ~70% at 900 s | [65] |
| Ni0.9Fe0.1@PCNs | Molten salt | 191.3 | 97.1% at 1000 s | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thamer, B.M.; Abdul Hameed, M.M.; El-Newehy, M.H. Molten Salts Approach of Poly(vinyl alcohol)-Derived Bimetallic Nickel–Iron Sheets Supported on Porous Carbon Nanosheet as an Effective and Durable Electrocatalyst for Methanol Oxidation. Gels 2023, 9, 238. https://doi.org/10.3390/gels9030238
Thamer BM, Abdul Hameed MM, El-Newehy MH. Molten Salts Approach of Poly(vinyl alcohol)-Derived Bimetallic Nickel–Iron Sheets Supported on Porous Carbon Nanosheet as an Effective and Durable Electrocatalyst for Methanol Oxidation. Gels. 2023; 9(3):238. https://doi.org/10.3390/gels9030238
Chicago/Turabian StyleThamer, Badr M., Meera Moydeen Abdul Hameed, and Mohamed H. El-Newehy. 2023. "Molten Salts Approach of Poly(vinyl alcohol)-Derived Bimetallic Nickel–Iron Sheets Supported on Porous Carbon Nanosheet as an Effective and Durable Electrocatalyst for Methanol Oxidation" Gels 9, no. 3: 238. https://doi.org/10.3390/gels9030238