Thermo-Responsive Injectable Hydrogels Formed by Self-Assembly of Alginate-Based Heterograft Copolymers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Heterograft Copolymer
2.2. Thermo-Gelling Behavior
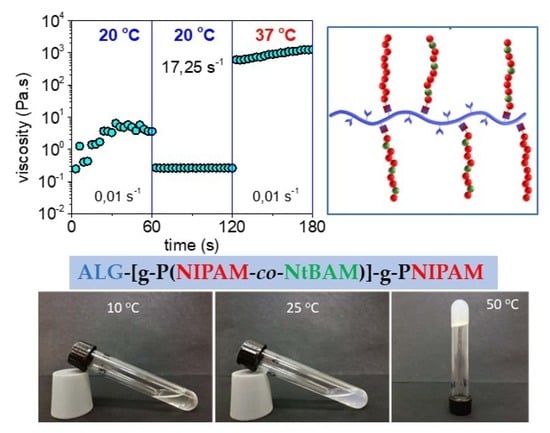
2.3. Rheological Properties
2.4. Injectability and Self-Healing
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of the ALG/HGC Heterograft Copolymer
4.3. Polymer Characterization
4.4. Preparation of Polymer Solutions
4.5. Rheological Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsitsilianis, C. Responsive reversible hydrogels from associative “smart” macromolecules. Soft Matter 2010, 6, 2372–2388. [Google Scholar] [CrossRef]
- Chassenieux, C.; Tsitsilianis, C. Recent trends on pH/thermo-responsive self-assembling hydrogels: From polyions to peptide-based polymeric gelators. Soft Matter 2016, 12, 1344–1359. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Soft materials by design: Unconventional polymer networks give extreme properties. Chem. Rev. 2021, 121, 4309–4372. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, A.P.; Wang, L.; Wang, S.; Georgiou, T.K. Thermoresponsive block copolymers of increasing architecture complexity: A review on structure- property relationships. Polym. Chem. 2023, 14, 223–247. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Engin. R 2015, 93, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Feng, Q.; Fang, Z.; Gu, L.; Bian, L. Structurally dynamic hydrogels for biomedical applications: Pursuing a fine balance between macroscopic stability and microscopic dynamics. Chem. Rev. 2021, 12, 11149–11193. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Heilshorn, S.C. Adaptable Hydrogel Networks with Reversible Linkages for Tissue Engineering. Adv. Mater. 2015, 27, 3717–3736. [Google Scholar] [CrossRef]
- Constantinou, A.P.; Georgiou, T.K. Pre-clinical and clinical applications of thermoreversible hydrogels in biomedical engineering: A review. Polym. Int. 2021, 70, 1433–1448. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric Hydrogel Systems as Emerging Biomaterial Platforms to Enable Hemostasis and Wound Healing. Adv. Healthc. Mater. 2020, 9, 2000905. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, E.; Schilling, A.L.; Little, S.R.; Decuzzi, P. Injectable thermoresponsive hydrogels as drug delivery system for the treatment of central nervous system disorders: A review. J. Control. Release 2021, 329, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, C.; Zhang, Z.; Zhao, J.; Yuan, Y.; Wang, S. Oxidation triggered formation of polydopamine methyl cellulose hydrogel for anti-recurrence of tumor. Colloids Surf. B 2021, 207, 112025. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yi, W.; Zhang, Y.; Wu, H.; Fan, H.; Zhao, J.; Wang, S. Sodium alginate hydrogel containing platelet-rich plasma for wound healing. Colloids Surf. B 2023, 222, 113096. [Google Scholar] [CrossRef]
- Wu, M.; Peng, Q.Y.; Han, L.B.; Zeng, H.B. Self-healing Hydrogels and Underlying Reversible Intermolecular Interactions. Chin. J. Polym. Sci. 2021, 39, 1246–1261. [Google Scholar] [CrossRef]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Peng, D.; Nie, X.; Wang, J.; Yu, C.-Y. Hyaluronic Acid-Based Injectable Hydrogels for Wound Dressing and Localized Tumor Therapy: A Review. Adv. NanoBiomed Res. 2022, 2, 2200124. [Google Scholar] [CrossRef]
- Cook, M.T.; Haddow, P.; Kirton, S.B.; McAuley, W.J. Polymers Exhibiting Lower Critical Solution Temperatures as a Route to Thermoreversible Gelators for Healthcare. Adv. Funct. Mater. 2020, 31, 2008123. [Google Scholar] [CrossRef]
- Pasparakis, G.; Tsitsilianis, C. LCST polymers: Thermoresponsive nanostructured assemblies towards bioapplications. Polymer 2020, 211, 123146. [Google Scholar] [CrossRef]
- Hogan, K.J.; Mikos, A.G. Biodegradable thermoresponsive polymers: Applications in drug delivery and tissue engineering. Polymer 2020, 211, 123063. [Google Scholar] [CrossRef]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Feng, Y.; Wei, K.; Xy, X.; Liu, R.; Chen, G. Carbohydrate-based Macromolecular Biomaterials. Chem. Rev. 2021, 121, 10950–11020. [Google Scholar] [CrossRef] [PubMed]
- D’Este, M.; Alini, M.; Eglin, D. Single step synthesis and characterization of thermoresponsive hyaluronan hydrogels. Carbohydr. Polym. 2012, 90, 1378–1385. [Google Scholar] [CrossRef]
- Muramatsu, K.; Saito, Y.; Wada, T.; Hirai, H.; Miyawaki, F. Poly(Nisopropylacrylamide-co-N-tert-butylacrylamide)- grafted hyaluronan as an injectable and self-assembling scaffold for cartilage tissue engineering. J. Biomed. Sci. Eng. 2012, 5, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Leal, D.; De Borggraeve, W.; Encinas, M.V.; Matsuhiro, B.; Muller, R. Preparation and characterization of hydrogels based on homopolymeric fractions of sodium alginate and PNIPAAm. Carbohydr. Polym. 2013, 92, 157–166. [Google Scholar] [CrossRef]
- Liu, M.; Song, X.; Wen, Y.; Zhu, J.-L.; Li, J. Injectable Thermoresponsive Hydrogel Formed by Alginate-g-Poly(N-isopropylacrylamide) Releasing Doxorubicin-Encapsulated Micelles as Smart Drug Delivery System. ACS Appl. Mater. Interfaces 2017, 9, 35673–35682. [Google Scholar] [CrossRef]
- Lencina, M.M.S.; Iatridi, Z.; Villar, M.A.; Tsitsilianis, C. Thermoresponsive hydrogels from alginate-based graft copolymers. Eur. Polym. J. 2014, 61, 33–44. [Google Scholar] [CrossRef]
- Martinez-Gomez, F.; Encinas, M.V.; Matsuhiro, B.; Pavez, J. Preparation and swelling properties of homopolymeric alginic acid fractions/poly(N-isopropyl acrylamide) graft copolymers. J. Appl. Polym. Sci. 2015, 132, 42398. [Google Scholar] [CrossRef]
- Lencina, S.M.M.; Ciolino, A.E.; Andreucetti, N.A.; Villar, M.A. Thermoresponsive hydrogels based on alginate-g-poly(N-isopropylacrylamide) copolymers obtained by low doses of gamma radiation. Eur. Polym. J. 2015, 68, 641–649. [Google Scholar] [CrossRef]
- Iatridi, Z.; Saravanou, S.F.; Tsitsilianis, C. Injectable self-assembling hydrogel from alginate grafted by P(N-isopropylacrylamide-co-N-tert-butylacrylamide) random copolymers. Carbohydr. Polym. 2019, 219, 344–352. [Google Scholar] [CrossRef]
- Theodorakis, N.; Saravanou, S.-F.; Kouli, N.-P.; Iatridi, Z.; Tsitsilianis, C. pH/Thermo-Responsive Grafted Alginate-Based SiO2 Hybrid Nanocarrier/Hydrogel Drug Delivery Systems. Polymers 2021, 13, 1228. [Google Scholar] [CrossRef]
- Karakasyan, C.; Legros, M.; Lack, S.; Brunel, F.; Maingault, P.; Ducouret, G.; Hourdet, D. Cold Gelation of Alginates Induced by Monovalent Cations. Biomacromolecules 2010, 11, 2966–2975. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; de Magalhaes Goncalves, M.; Ducouret, G.; Hourdet, D. Cold and Hot Gelling of Alginate-graft-PNIPAM: A Schizophrenic Behavior Induced by Potassium Salts. Biomacromolecules 2018, 12, 576–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalanqui, M.J.; Pentlavalli, S.; McCrudden, C.; Chambers, P.; Ziminska, M.; Dunne, N.; McCarthy, H.O. Influence of alginate backbone on efficacy of thermo-responsive alginate-g-P(NIPAAm) hydrogel as a vehicle for sustained and controlled gene delivery. Mater. Sci. Eng. C 2019, 95, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Safakas, K.; Saravanou, S.-F.; Iatridi, Z.; Tsitsilianis, C. Alginate-g-PNIPAM-based Thermo/shear-responsive Injectable Hydrogels: Tailoring the Rheological Properties by Adjusting the LCST of the Grafting Chains. Int. J. Mol. Sci. 2021, 22, 3824. [Google Scholar] [CrossRef] [PubMed]
- Barbier, L.; Protat, M.; Pipart, P.; Marcellan, A.; Tran, Y.; Hourdet, D. Sol/gel transition of thermoresponsive Hyaluronan: From liquids to elastic and sticky materials. Carbohydr. Polym. 2023, 310, 120715. [Google Scholar] [CrossRef]
- Atanase, L.I.; Desbrieres, J.; Riess, G. Micellization of synthetic and polysaccharides-based graft copolymers in aqueous media. Prog. Polym. Sci. 2017, 73, 32–60. [Google Scholar] [CrossRef]
- Krause, W.E.; Bellomo, E.G.; Colby, R.H. Rheology of sodium hyaluronate under physiological conditions. Biomacromolecules 2001, 2, 65–69. [Google Scholar] [CrossRef]
- Onoda, M.; Ueki, T.; Tamate, R.; Akimoto, A.M.; Hall, C.C.; Lodge, T.P.; Yoshida, R. Precisely Tunable Sol−Gel Transition Temperature by Blending Thermoresponsive ABC Triblock Terpolymers. ACS Macro Lett. 2018, 7, 950–955. [Google Scholar] [CrossRef]
- Tsitsilianis, C.; Serras, G.; Ko, C.-H.; Jung, F.; Papadakis, C.M.; Rikkou-Kalourkoti, M.; Patrickios, C.S.; Schweins, R.; Chassenieux, C. Thermoresponsive Hydrogels Based on Telechelic Polyelectrolytes: From Dynamic to “Frozen” Networks. Macromolecules 2018, 51, 2169–2179. [Google Scholar] [CrossRef]
- Aguado, B.A. Improving Viability of Stem Cells During Syringe Needle Flow Through the Design of Hydrogel Cell Carriers. Tissue Eng. Part A 2011, 18, 806–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Dewi, R.E.; Heilshorn, S.C. Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Adv. Funct. Mater. 2015, 25, 1344–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iatridi, Z.; Lencina, M.M.S.; Tsitsilianis, C. PNIPAM-based heteroarm star-graft quarterpolymers: Synthesis, characterization and pH-dependent thermoresponsiveness in aqueous media. Polym. Chem. 2015, 6, 3942–3955. [Google Scholar] [CrossRef]
- Yang, J.; Wang, S. Polysaccharide-Based Multifunctional Hydrogel Bio-Adhesives for Wound Healing: A Review. Gels 2023, 9, 138. [Google Scholar] [CrossRef] [PubMed]








| Grafting Chain | Mn a (g/mol) | NIPAM/NtBAM Molar Ratio b | Tcp c (°C) |
|---|---|---|---|
| NH2-PNIPAM | 22,700 | 100/0 | 32 |
| NH2-P(NIPAM86-co-NtBAM14) | 17,000 | 86/14 | 22 |
| Graft Copolymer | Mw d (×103 g/mol) | Number of P(NIPAM86-co-NtBAM14) chains per ALG backbone e | |
| ALG-g-P(NIPAM86-co-NtBAM14) | 222 | 3.5 | |
| ALG/HGC | 262 | 91/9 f | |
| Average number of P(NIPAM86-co-NtBAM14) chains per ALG backbone e | Average number of PNIPAM chains per ALG backbone e | ||
| 3.5 | 1.8 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safakas, K.; Saravanou, S.-F.; Iatridi, Z.; Tsitsilianis, C. Thermo-Responsive Injectable Hydrogels Formed by Self-Assembly of Alginate-Based Heterograft Copolymers. Gels 2023, 9, 236. https://doi.org/10.3390/gels9030236
Safakas K, Saravanou S-F, Iatridi Z, Tsitsilianis C. Thermo-Responsive Injectable Hydrogels Formed by Self-Assembly of Alginate-Based Heterograft Copolymers. Gels. 2023; 9(3):236. https://doi.org/10.3390/gels9030236
Chicago/Turabian StyleSafakas, Konstantinos, Sofia-Falia Saravanou, Zacharoula Iatridi, and Constantinos Tsitsilianis. 2023. "Thermo-Responsive Injectable Hydrogels Formed by Self-Assembly of Alginate-Based Heterograft Copolymers" Gels 9, no. 3: 236. https://doi.org/10.3390/gels9030236