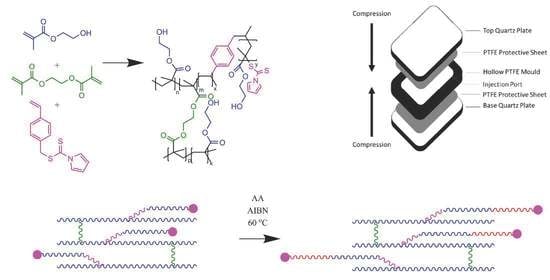
Chain-Extendable Crosslinked Hydrogels Using Branching RAFT Modification
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crosslinked Hydrogels
2.2. Grafting of Acrylic Acid onto PCN
2.3. RAFT–HEMA Copolymer Networks
2.4. Mechanical Analysis
2.5. Thermal Analysis
2.6. Interpolymer Complexation of Polymers from Solution
3. Conclusions
4. Materials and Methods
4.1. Materials and Equipment
4.2. Chemical Synthesis
4.2.1. Polymer Synthesis
4.2.2. RAFT Agent Synthesis
4.3. Solid Polymer Film Synthesis
4.3.1. UV Polymerization Using EGDMA as a Crosslinker
4.3.2. Thermal Polymerization with RAFT Functionality
4.4. Chain Extension—RAFT Polymerization of Acrylic Acid onto HEMA Film
4.5. Hydrogel Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chilkoti, A.; Lopez, G.P.; Ratner, B.D.; Hearn, M.J.; Briggs, D. Analysis of Polymer Surfaces by SIMS. 16. Investigation of Surface Cross-Linking in Polymer Gels of 2-Hydroxyethyl Methacrylate. Macromolecules 1993, 26, 4825–4832. [Google Scholar] [CrossRef]
- Billaud, C.; Sarakha, M.; Bolte, M. Polymerization of 2-Hydroxyethyl Methacrylate Initiated by Excitation of [Co(NH3)5N3]2+ at 365 and 546 nm. Eur. Polym. J. 2000, 36, 1401–1408. [Google Scholar] [CrossRef]
- Feng, M.; Morales, A.B.; Beugeling, T.; Bantjes, A.; Van Der Werf, K.; Gosselink, G.; De Grooth, B.; Greve, J. Adsorption of High Density Lipoproteins (HDL) on Solid Surfaces. J. Colloid Interface Sci. 1996, 177, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Smirnov, K.N.; Dyatchkov, I.A.; Pirogov, A.V.; Shpigun, O.A. The Influence of the Synthesis Pressure on the Chromatographic Properties of Poly(Divinylbenzene-Co-Ethylvinylbenzene-Co-2-Hydroxyethyl Methacrylate) Monolithic Columns. Mosc. Univ. Chem. Bull. 2011, 66, 351–355. [Google Scholar] [CrossRef]
- Dursch, T.J.; Taylor, N.O.; Liu, D.E.; Wu, R.Y.; Prausnitz, J.M.; Radke, C.J. Water-Soluble Drug Partitioning and Adsorption in HEMA/MAA Hydrogels. Biomaterials 2014, 35, 620–629. [Google Scholar] [CrossRef] [Green Version]
- Unruh, R.M.; Roberts, J.R.; Nichols, S.P.; Gamsey, S.; Wisniewski, N.A.; McShane, M.J. Preclinical Evaluation of Poly(HEMA-Co-Acrylamide) Hydrogels Encapsulating Glucose Oxidase and Palladium Benzoporphyrin as Fully Implantable Glucose Sensors. J. Diabetes Sci. Technol. 2015, 9, 985–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plenderleith, R.A.; Pateman, C.J.; Rodenburg, C.; Haycock, J.W.; Claeyssens, F.; Sammon, C.; Rimmer, S. Arginine–Glycine–Aspartic Acid Functional Branched Semi-Interpenetrating Hydrogels. Soft Matter 2015, 11, 7567–7578. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Feng, L.; Wei, W.; Xie, A.; Wang, S.; Zhang, J.; Dong, W. Synthesis and Characterization of a Novel Semi-IPN Hydrogel Based on Salecan and Poly(N,N-Dimethylacrylamide-Co-2-Hydroxyethyl Methacrylate). Carbohydr. Polym. 2014, 105, 135–144. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Chen, X.; Yang, Q.; Sun, T.; Zhou, J. Study on the Graft Reaction of Poly(Propylene) Fiber with Acrylic Acid. Macromol. Mater. Eng. 2006, 291, 173–180. [Google Scholar] [CrossRef]
- Currie, E.P.K.; Sieval, A.B.; Fleer, G.J.; Stuart, M.A.C. Polyacrylic Acid Brushes: Surface Pressure and Salt-Induced Swelling. Langmuir 2000, 16, 8324–8333. [Google Scholar] [CrossRef]
- Am Ende, M.T.; Peppas, N.A. Transport of Ionizable Drugs and Proteins in Crosslinked Poly(Acrylic Acid) and Poly(Acrylic Acid-Co-2-Hydroxyethyl Methacrylate) Hydrogels. II. Diffusion and Release Studies. J. Control. Release 1997, 48, 47–56. [Google Scholar] [CrossRef]
- Ende, M.T.A.; Peppas, N.A. Transport of Ionizable Drugs and Proteins in Crosslinked Poly(Acrylic Acid) and Poly(Acrylic Acid-co-2-hydroxyethyl Methacrylate) Hydrogels. I. Polymer Characterization. J. Appl. Polym. Sci. 1996, 59, 673–685. [Google Scholar] [CrossRef]
- Liu, D.E.; Dursch, T.J.; Taylor, N.O.; Chan, S.Y.; Bregante, D.T.; Radke, C.J. Diffusion of Water-Soluble Sorptive Drugs in HEMA/MAA Hydrogels. J. Control. Release 2016, 239, 242–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onuki, Y.; Hoshi, M.; Okabe, H.; Fujikawa, M.; Morishita, M.; Takayama, K. Formulation Optimization of Photocrosslinked Polyacrylic Acid Modified with 2-Hydroxyethyl Methacrylate Hydrogel as an Adhesive for a Dermatological Patch. J. Control. Release 2005, 108, 331–340. [Google Scholar] [CrossRef]
- Wang, J.; Ma, X.; Wei, L.; Zhu, X.; Zhu, Y.; Wang, G.; Mei, T.; Li, J.; Wang, X. Construction of High-Strength p(HEMA-Co-AA) Fluorescent Hydrogels Based on Modified Carbon Dots as Chemically Crosslinkers. Colloid Polym. Sci. 2018, 296, 745–752. [Google Scholar] [CrossRef]
- Akkahat, P.; Hoven, V.P. Introducing Surface-Tethered Poly(Acrylic Acid) Brushes as 3D Functional Thin Film for Biosensing Applications. Colloids Surf. B Biointerfaces 2011, 86, 198–205. [Google Scholar] [CrossRef]
- Akkahat, P.; Mekboonsonglarp, W.; Kiatkamjornwong, S.; Hoven, V.P. Surface-Grafted Poly(Acrylic Acid) Brushes as a Precursor Layer for Biosensing Applications: Effect of Graft Density and Swellability on the Detection Efficiency. Langmuir 2012, 28, 5302–5311. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yu, H.; Wang, L.; ul Abdin, Z.; Yang, X.; Wang, J.; Zhou, W.; Zhang, H.; Chen, X. Synthesis and Characterization of Amylose Grafted Poly(Acrylic Acid) and Its Application in Ammonia Adsorption. Carbohydr. Polym. 2016, 153, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Bujans, F.; Serna, R.; Sow, E.; Fierro, J.L.G.; Veith, M. Grafting of Poly(Acrylic Acid) onto an Aluminum Surface. Langmuir 2009, 25, 9094–9100. [Google Scholar] [CrossRef]
- Liu, Y.; Klep, V.; Zdyrko, B.; Luzinov, I. Polymer Grafting via ATRP Initiated from Macroinitiator Synthesized on Surface. Langmuir 2004, 20, 6710–6718. [Google Scholar] [CrossRef]
- Khabibullin, A.; Bhangaonkar, K.; Mahoney, C.; Lu, Z.; Schmitt, M.; Sekizkardes, A.K.; Bockstaller, M.R.; Matyjaszewski, K. Grafting PMMA Brushes from α-Alumina Nanoparticles via SI-ATRP. ACS Appl. Mater. Interfaces 2016, 8, 5458–5465. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Higaki, Y.; Kimura, T.; Boschet, F.; Takahara, A.; Ameduri, B. Direct Surface Modification of Poly(VDF-Co-TrFE) Films by Surface-Initiated ATRP without Pretreatment. RSC Adv. 2016, 6, 86373–86384. [Google Scholar] [CrossRef]
- Perruchot, C.; Khan, M.A.; Kamitsi, A.; Armes, S.P.; Von Werne, T.; Patten, T.E. Synthesis of Well-Defined, Polymer-Grafted Silica Particles by Aqueous ATRP. Langmuir 2001, 17, 4479–4481. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Dong, H.; Jakubowski, W.; Pietrasik, J.; Kusumo, A. Grafting from Surfaces for “Everyone”: ARGET ATRP in the Presence of Air. Langmuir 2007, 23, 4528–4531. [Google Scholar] [CrossRef] [PubMed]
- Treat, N.D.; Ayres, N.; Boyes, S.G.; Brittain, W.J. A Facile Route to Poly(Acrylic Acid) Brushes Using Atom Transfer Radical Polymerization. Macromolecules 2006, 39, 26–29. [Google Scholar] [CrossRef]
- Wu, T.; Gong, P.; Szleifer, I.; Vlček, P.; Šubr, V.; Genzer, J. Behavior of Surface-Anchored Poly(Acrylic Acid) Brushes with Grafting Density Gradients on Solid Substrates: 1. Experiment. Macromolecules 2007, 40, 8756–8764. [Google Scholar] [CrossRef]
- Roy, D.; Knapp, J.S.; Guthrie, J.T.; Perrier, S. Antibacterial Cellulose Fiber via RAFT Surface Graft Polymerization. Biomacromolecules 2008, 9, 91–99. [Google Scholar] [CrossRef]
- Ying, L.; Yu, W.H.; Kang, E.T.; Neoh, K.G. Functional and Surface-Active Membranes from Poly(Vinylidene Fluoride)-Graft-Poly(Acrylic Acid) Prepared via RAFT-Mediated Graft Copolymerization. Langmuir 2004, 20, 6032–6040. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.Y.; You, Y.Z.; Pan, C.Y. Synthesis of Water-Soluble Multiwalled Carbon Nanotubes with Grafted Temperature-Responsive Shells by Surface RAFT Polymerization. Chem. Mater. 2005, 17, 2247–2254. [Google Scholar] [CrossRef]
- Wang, W.C.; Neoh, K.G.; Kong, E.T. Surface Functionalization of Fe3O4 Magnetic Nanoparticles via RAFT-Mediated Graft Polymerization. Macromol. Rapid Commun. 2006, 27, 1665–1669. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, P.; Qing, A.; Lan, Y.; Lu, M. Poly(N-Isopropylacrylamide) Hydrogels with Improved Shrinking Kinetics by RAFT Polymerization. Polymer 2006, 47, 2330–2336. [Google Scholar] [CrossRef]
- Barlow (née Tan), K.J.; Hao, X.; Hughes, T.C.; Hutt, O.E.; Polyzos, A.; Turner, K.A.; Moad, G. Porous, Functional, Poly(Styrene-Co-Divinylbenzene) Monoliths by RAFT Polymerization. Polym. Chem. 2014, 5, 722–732. [Google Scholar] [CrossRef]
- Turson, M.; Zhou, M.; Jiang, P.; Dong, X. Monolithic Poly(Ethylhexyl Methacrylate-Co-Ethylene Dimethacrylate) Column with Restricted Access Layers Prepared via Reversible Addition-Fragmentation Chain Transfer Polymerization. J. Sep. Sci. 2011, 34, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, L.; Zhou, J.; Ye, L.; Hu, H.; Luo, Z.; Zhou, L. Surface Modification of PVA Hydrogel Membranes with Carboxybetaine Methacrylate via PET-RAFT for Anti-Fouling. Polymer 2019, 162, 80–90. [Google Scholar] [CrossRef]
- Collett, J.; Crawford, A.; Hatton, P.V.; Geoghegan, M.; Rimmer, S. Thermally Responsive Polymeric Hydrogel Brushes: Synthesis, Physical Properties and Use for the Culture of Chondrocytes. J. R. Soc. Interface 2006, 4, 117–126. [Google Scholar] [CrossRef]
- Carter, S.R.; England, R.M.; Hunt, B.J.; Rimmer, S. Functional Graft Poly(N-Isopropyl Acrylamide)s Using Reversible Addition-Fragmentation Chain Transfer (RAFT) Polymerisation. Macromol. Biosci. 2007, 7, 975–986. [Google Scholar] [CrossRef]
- Shallcross, L.; Roche, K.; Wilcock, C.J.; Stanton, K.T.; Swift, T.; Rimmer, S.; Hatton, P.V.; Spain, S.G. The Effect of Hyperbranched Poly(Acrylic Acid)s on the Morphology and Size of Precipitated Nanoscale (Fluor)Hydroxyapatite. J. Mater. Chem. B 2017, 5, 6027–6033. [Google Scholar] [CrossRef] [Green Version]
- Teratanatorn, P.; Hoskins, R.; Swift, T.; Douglas, C.W.I.I.; Shepherd, J.; Rimmer, S. Binding of Bacteria to Poly(N-Isopropylacrylamide) Modified with Vancomycin: Comparison of Behavior of Linear and Highly Branched Polymers. Biomacromolecules 2017, 18, 2887–2899. [Google Scholar] [CrossRef] [Green Version]
- Swift, T.; Katsikogianni, M.; Hoskins, R.; Teratarantorn, P.; Douglas, I.; MacNeil, S.; Rimmer, S. Highly-Branched Poly(N-Isopropyl Acrylamide) Functionalised with Pendant Nile Red and Chain End Vancomycin for the Detection of Gram-Positive Bacteria. Acta Biomater. 2019, 87, 197–206. [Google Scholar] [CrossRef]
- Swift, T.; Swanson, L.; Bretherick, A.; Rimmer, S. Measuring Poly(Acrylamide) Flocculants in Fresh Water Using Inter-Polymer Complex Formation. Environ. Sci. Water Res. Technol. 2015, 1, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Das, D.; Das, R.; Ghosh, P.; Dhara, S.; Panda, A.B.; Pal, S. Dextrin Cross Linked with Poly(HEMA): A Novel Hydrogel for Colon Specific Delivery of Ornidazole. RSC Adv. 2013, 3, 25340–25350. [Google Scholar] [CrossRef]
- Taleb, M.A.; Hegazy, D.E.; Mahmoud, G.A. Characterization and in Vitro Drug Release Behavior of (2-Hydroxyethyl Methacrylate)-Co-(2-Acrylamido-2-Methyl-1-Propanesulfonic Acid) Crosslinked Hydrogels Prepared by Ionizing Radiation. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 840–845. [Google Scholar] [CrossRef]
- Podkościelna, B.; Bartnicki, A.; Gawdzik, B. New Crosslinked Hydrogels Derivatives of 2-Hydroxyethyl Methacrylate: Synthesis, Modifications and Properties. Express Polym. Lett. 2012, 6, 759–771. [Google Scholar] [CrossRef]
- Swift, T.; Swanson, L.; Rimmer, S. Poly(Acrylic Acid) Interpolymer Complexation: Use of a Fluorescence Time Resolved Anisotropy as a Poly(Acrylamide) Probe. RSC Adv. 2014, 4, 57991–57995. [Google Scholar] [CrossRef] [Green Version]
- Mino, G.; Kaizerman, S. A New Method for the Preparation of Graft Copolymers. Polymerization Initiated by Ceric Ion Redox Systems. J. Polym. Sci. 1958, 31, 242–243. [Google Scholar] [CrossRef]
- Reyes, Z.; Rist, C.E.; Russell, C.R. Grafting Vinyl Monomers to Starch by Ceric Ion. I. Acrylonitrile and Acrylamide. J. Polym. Sci. Part A-1 Polym. Chem. 1966, 4, 1031–1043. [Google Scholar] [CrossRef]
- Athawale, V.D.; Lele, V. Recent Trends in Hydrogels Based on Starchgraft-Acrylic Acid: A Review. Starch/Staerke 2001, 53, 7–13. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cho, S.M.; Lee, Y.M.; Kim, S.J. Thermo- and PH-Responsive Behaviors of Graft Copolymer and Blend Based on Chitosan and N-Isopropylacrylamide. J. Appl. Polym. Sci. 2000, 78, 1381–1391. [Google Scholar] [CrossRef]
- Joshi, J.M.; Sinha, V.K. Ceric Ammonium Nitrate Induced Grafting of Polyacrylamide onto Carboxymethyl Chitosan. Carbohydr. Polym. 2007, 67, 427–435. [Google Scholar] [CrossRef]
- McCormick, C.L.; Park, L.S. Water-Soluble Copolymers. III. Dextran-g-Poly(Acrylamides) Control of Grafting Sites and Molecular Weight by Ce(IV)-Induced Initiation in Homogeneous Solutions. J. Polym. Sci. Polym. Chem. Ed. 1981, 19, 2229–2241. [Google Scholar] [CrossRef]
- Hritcu, D.; Müller, W.; Brooks, D.E. Poly(Styrene) Latex Carrying Cerium(IV)-Initiated Terminally Attached Cleavable Chains: Analysis of Grafted Chains and Model of the Surface Layer. Macromolecules 1999, 32, 565–573. [Google Scholar] [CrossRef]
- Ramos, V.D.; Derouet, D.; Visconte, L.L.Y. Addition of 2-Hydroxyethyl Methacrylate upon 4,5-Epoxy-4-Methyloctane Catalyzed by Ceric Ammonium Nitrate 1: Identification of the Reaction Products. Polym. Test. 2003, 22, 297–304. [Google Scholar] [CrossRef]
- Ramos, V.D.; Da Costa, H.M.; Derouet, D.; Visconte, L.L.Y. Addition of 2-Hydroxyethyl Methacrylate upon 4,5-Epoxy-4-Methyloctane Catalyzed by Ceric Ammonium Nitrate: Part 2. Kinetic Study. Polym. Test. 2004, 23, 387–395. [Google Scholar] [CrossRef]
- Gupta, K.C.; Khandekar, K. Graft Copolymerization of Acrylamide-Methylacrylate Comonomers onto Cellulose Using Ceric Ammonium Nitrate. J. Appl. Polym. Sci. 2002, 86, 2631–2642. [Google Scholar] [CrossRef]
- Chansook, N.; Kiatkamjornwong, S. Ce(IV)-Initiated Graft Polymerization of Acrylic Acid onto Poly(Ethylene Terephthalate) Fiber. J. Appl. Polym. Sci. 2003, 89, 1952–1958. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Mitra, B.C.; Palit, S.R. Grafting Acrylic Acid Monomer to Poly-(Vinyl Alcohol) and Methyl Cellulose by Ceric Ion. J. Polym. Sci. Part A-1 Polym. Chem. 1969, 7, 2079–2086. [Google Scholar] [CrossRef]
- Misra, B.N.; Mehta, I.K.; Dogra, R. Grafting onto Wool. VII. Ceric Ion-initiated Graft Copolymerization of Vinyl Monomers. Comparison of Monomer Reactivities. J. Appl. Polym. Sci. 1980, 25, 235–241. [Google Scholar] [CrossRef]
- Vargün, E.; Usanmaz, A. Degradation of Poly(2-Hydroxyethyl Methacrylate) Obtained by Radiation in Aqueous Solution. J. Macromol. Sci. Part A 2010, 47, 882–891. [Google Scholar] [CrossRef]
- Alonso, T.; Irigoyen, J.; Iturri, J.J.; Larena, I.L.; Moya, S.E. Study of the Multilayer Assembly and Complex Formation of Poly(Diallyldimethylammonium Chloride) (PDADMAC) and Poly(Acrylic Acid) (PAA) as a Function of pH. Soft Matter 2013, 9, 1920–1928. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Fatehi, P. Flocculation of Thermomechanical Pulping Spent Liquor with Polydiallyldimethylammonium Chloride. J. Environ. Manag. 2017, 200, 275–282. [Google Scholar] [CrossRef]
- Bolto, B.; Xie, Z. The Use of Polymers in the Flotation Treatment of Wastewater. Processes 2019, 76, 374. [Google Scholar] [CrossRef] [Green Version]







| Solvent | HEMA | EGDMA | D117 | BP | TEHA | 4-VPC | ACVA | |
|---|---|---|---|---|---|---|---|---|
| PCG 1 | 0.49 a | 1.00 | 0.07 | 0.49 | 0.05 | 0.09 | - | - |
| PCG 2 | 0.43 b | 1.00 | 0.09 | 0.03 | 0.03 | 0.09 | 0.01 | 0.01 |
| Precursor Film | ACVA/g | AA/g | DMSO/g | IEC | Abs 1 | |
|---|---|---|---|---|---|---|
| PCG 2A | PCG 2 | 1.5 | 11.96 | 15.85 | 0.76 | 0.21 |
| PCG 2B | PCG 2 | 0.4 | 14.36 | 10.0 | 0.37 | 0.18 |
| PCG 2BB | PCG 2B | 0.4 | 14.36 | 10.0 | 0.68 | 0.34 |
| PCG 2BBB | PCG 3B | 0.4 | 14.36 | 10.0 | 1.23 | 0.46 |
| HEMA | AA | DMSO | EGDMA | BP | TEHA | RAFT | ACVA | Results | IEC | |
|---|---|---|---|---|---|---|---|---|---|---|
| PCG 3 | 1.00 | 0.00 | 0.53 | 0.14 | 0.03 | 0.14 | 0.003 | 0.01 | Yellow Film | −0.11 |
| PCG 3A | 1.00 | 0.06 | 0.69 | 0.19 | 0.04 | 0.19 | 0.003 | 0.02 | Yellow Film | 0.26 |
| PCG 3B | 1.00 | 0.11 | 0.72 | 0.20 | 0.04 | 0.20 | 0.004 | 0.02 | Yellow Film | 0.79 |
| PCG 3C | 1.00 | 0.19 | 0.80 | 0.22 | 0.05 | 0.22 | 0.004 | 0.02 | Yellow Film | 1.76 |
| PCG 3D | 1.00 | 0.27 | 0.86 | 0.23 | 0.05 | 0.23 | 0.004 | 0.02 | No Film | - |
| PCG 3E | 1.00 | 0.54 | 0.93 | 0.25 | 0.05 | 0.25 | 0.005 | 0.02 | No Film | - |
| PCG 3F | 1.00 | 0.72 | 0.88 | 0.24 | 0.05 | 0.24 | 0.004 | 0.002 | No Film | - |
| HEMA Gel Properties | n | E (MPa) | Max Stress (MPa) | Breaking Strain | |
|---|---|---|---|---|---|
| PCG 1 | EGDMA Crosslinked | 5 | 100 ± 80 | 9 ± 6 | 0.23 ± 0.09 |
| PCG 2 | RAFT Crosslinked | 4 | 400 ± 100 | 24 ± 7 | 0.24 ± 0.09 |
| PCG 2B | RAFT Graft | 4 | 100 ± 40 | 11± 4 | 0.21 ± 0.05 |
| PCG 3A | HEMA-stat-AA | 3 | 80 ± 20 | 3.1 ± 0.3 | 0.06 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimmer, S.; Spencer, P.; Nocita, D.; Sweeney, J.; Harrison, M.; Swift, T. Chain-Extendable Crosslinked Hydrogels Using Branching RAFT Modification. Gels 2023, 9, 235. https://doi.org/10.3390/gels9030235
Rimmer S, Spencer P, Nocita D, Sweeney J, Harrison M, Swift T. Chain-Extendable Crosslinked Hydrogels Using Branching RAFT Modification. Gels. 2023; 9(3):235. https://doi.org/10.3390/gels9030235
Chicago/Turabian StyleRimmer, Stephen, Paul Spencer, Davide Nocita, John Sweeney, Marcus Harrison, and Thomas Swift. 2023. "Chain-Extendable Crosslinked Hydrogels Using Branching RAFT Modification" Gels 9, no. 3: 235. https://doi.org/10.3390/gels9030235