The Effect of Sage (Salvia sclarea) Essential Oil on the Physiochemical and Antioxidant Properties of Sodium Alginate and Casein-Based Composite Edible Films
Abstract
:1. Introduction
2. Results and Discussion
2.1. GC–MS Analysis of Sage Essential Oil
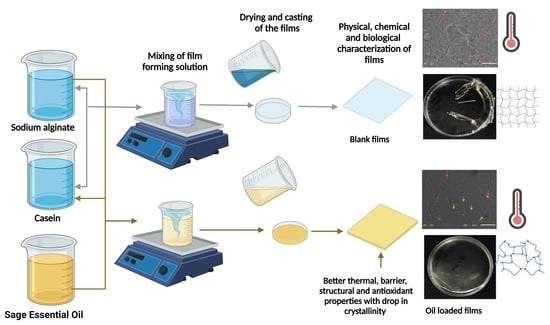
2.2. Visual Characterization
2.3. Thickness
2.4. Mechanical Properties
2.5. Moisture Content and Water Solubility
2.6. Water Vapor Permeability
2.7. Transparency
2.8. Color Parameters
2.9. TGA
2.10. XRD
2.11. SEM Analysis
2.12. Fourier Transform Infrared Spectroscopy Spectrum (FTIR) Analysis
2.13. Antioxidant Activity
3. Conclusions
4. Materials and Methods
4.1. Chemical Procurement
4.2. Sodium Alginate and Casein-Based Film Formation
4.3. GC–MS of the SEO
4.4. Thickness of the Films
4.5. Mechanical Properties of the Films
4.6. Moisture Content
4.7. Water Solubility
4.8. Water Vapor Permeability (WVP) of the Films
4.9. Transparency
4.10. Color Analysis
4.11. Thermogravimetric Analysis
4.12. XRD
4.13. Scanning Electron Microscopy
4.14. Fourier Transform Infrared Spectroscopy Analysis
4.15. Antioxidant Activity
4.16. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shivangi, S.; Dorairaj, D.; Negi, P.S.; Shetty, N.P. Development and characterisation of a pectin-based edible film that contains mulberry leaf extract and its bio-active components. Food Hydrocoll. 2021, 121, 107046. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Shendurse, A.; Gopikrishna, G.; Patel, A.; Pandya, A. Milk protein based edible films and coatings–preparation, properties and food applications. J. Nutr. Health Food Eng. 2018, 8, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Yerramathi, B.B.; Kola, M.; Muniraj, B.A.; Aluru, R.; Thirumanyam, M.; Zyryanov, G.V. Structural studies and bioactivity of sodium alginate edible films fabricated through ferulic acid crosslinking mechanism. J. Food Eng. 2021, 301, 110566. [Google Scholar] [CrossRef]
- Bhatia, S.; Al-Harrasi, A.; Al-Azri, M.S.; Ullah, S.; Bekhit, A.E.-D.A.; Pratap-Singh, A.; Chatli, M.K.; Anwer, M.K.; Aldawsari, M.F. Preparation and physiochemical characterization of bitter Orange oil loaded sodium alginate and casein based edible films. Polymers 2022, 14, 3855. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Q.; Critzer, F.; Davidson, P.M.; Zhong, Q. Organic thyme oil emulsion as an alternative washing solution to enhance the microbial safety of organic cantaloupes. Food Control 2016, 67, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Yazgan, H. Investigation of antimicrobial properties of sage essential oil and its nanoemulsion as antimicrobial agent. LWT 2020, 130, 109669. [Google Scholar] [CrossRef]
- Cai, J.; Lin, P.; Zhu, X.; Su, Q. Comparative analysis of clary sage (S. sclarea L.) oil volatiles by GC–FTIR and GC–MS. Food Chem. 2006, 99, 401–407. [Google Scholar] [CrossRef]
- Abu-Darwish, M.; Cabral, C.; Ferreira, I.; Gonçalves, M.; Cavaleiro, C.; Cruz, M.; Al-Bdour, T.; Salgueiro, L. Essential oil of common sage (Salvia officinalis L.) from Jordan: Assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential. BioMed Res. Int. 2013, 2013, 538940. [Google Scholar] [CrossRef] [Green Version]
- Fasseas, M.; Mountzouris, K.; Tarantilis, P.; Polissiou, M.; Zervas, G. Antioxidant activity in meat treated with oregano and sage essential oils. Food Chem. 2008, 106, 1188–1194. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef]
- Ehsani, A.; Hashemi, M.; Aminzare, M.; Raeisi, M.; Afshari, A.; Mirza Alizadeh, A.; Rezaeigolestani, M. Comparative evaluation of edible films impregnated with sage essential oil or lactoperoxidase system: Impact on chemical and sensory quality of carp burgers. J. Food Process. Preserv. 2019, 43, e14070. [Google Scholar] [CrossRef]
- Cozmuta, A.M.; Turila, A.; Apjok, R.; Ciocian, A.; Cozmuta, L.M.; Peter, A.; Nicula, C.; Galić, N.; Benković, T. Preparation and characterization of improved gelatin films incorporating hemp and sage oils. Food Hydrocoll. 2015, 49, 144–155. [Google Scholar] [CrossRef]
- Peana, A.T.; D'Aquila, P.S.; Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D.L. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 2002, 9, 721–726. [Google Scholar] [CrossRef]
- Wu, P.-S.; Kuo, Y.-T.; Chen, S.-M.; Li, Y.; Lou, B.-S. Gas chromatography-mass spectrometry analysis of photosensitive characteristics in citrus and herb essential oils. J. Chromatogr. Sep. Tech. 2014, 6, 1–9. [Google Scholar]
- Al-Harrasi, A.; Bhtaia, S.; Al-Azri, M.S.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Mohan, S.; Sharma, A.; Behl, T. Development and characterization of chitosan and porphyran based composite edible films containing ginger essential oil. Polymers 2022, 14, 1782. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Khaneghah, A.M. Characterization of novel basil-seed gum active edible films and coatings containing oregano essential oil. Prog. Org. Coat. 2017, 110, 35–41. [Google Scholar] [CrossRef]
- Jamróz, E.; Juszczak, L.; Kucharek, M. Development of starch-furcellaran-gelatin films containing tea tree essential oil. J. Appl. Polym. Sci. 2018, 135, 46754. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Prodpran, T.; Agustini, T.W. Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leatherjacket incorporated with essential oils. Food Hydrocoll. 2012, 28, 189–199. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T.; Nilsuwan, K. Emulsion film based on fish skin gelatin and palm oil: Physical, structural and thermal properties. Food Hydrocoll. 2015, 48, 248–259. [Google Scholar] [CrossRef]
- Scartazzini, L.; Tosati, J.; Cortez, D.; Rossi, M.; Flôres, S.; Hubinger, M.; Di Luccio, M.; Monteiro, A. Gelatin edible coatings with mint essential oil (Mentha arvensis): Film characterization and antifungal properties. J. Food Sci. Technol. 2019, 56, 4045–4056. [Google Scholar] [CrossRef] [PubMed]
- Galus, S.; Kadzińska, J. Moisture sensitivity, optical, mechanical and structural properties of whey protein-based edible films incorporated with rapeseed oil. Food Technol. Biotechnol. 2016, 54, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Bora, A.; Mishra, P. Characterization of casein and casein-silver conjugated nanoparticle containing multifunctional (pectin–sodium alginate/casein) bilayer film. J. Food Sci. Technol. 2016, 53, 3704–3714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, M.; Mahajan, H.; Joshi, R.; Gupta, M. Development and structural characterization of edible films for improving fruit quality. Food Packag. Shelf Life 2017, 12, 42–50. [Google Scholar] [CrossRef]
- Pereda, M.; Amica, G.; Marcovich, N.E. Development and characterization of edible chitosan/olive oil emulsion films. Carbohydr. Polym. 2012, 87, 1318–1325. [Google Scholar] [CrossRef]
- Namratha, S.; Sreejit, V.; Preetha, R. Fabrication and evaluation of physicochemical properties of probiotic edible film based on pectin–alginate–casein composite. Int. J. Food Sci. Technol. 2020, 55, 1497–1505. [Google Scholar] [CrossRef]
- Ptiček Siročić, A.; Kratofil Krehula, L.; Katančić, Z.; Hrnjak-Murgić, Z. Characterization of casein fractions–Comparison of commercial casein and casein extracted from cow’s milk. Chem. Biochem. Eng. Q 2016, 30, 501–509. [Google Scholar] [CrossRef]
- Tabatabaei, S.D.; Ghiasi, F.; Gahruie, H.H.; Hosseini, S.M.H. Effect of emulsified oil droplets and glycerol content on the physicochemical properties of Persian gum-based edible films. Polym. Test. 2022, 106, 107427. [Google Scholar] [CrossRef]
- Khan, A.; Sliem, M.H.; Arif, A.; Salih, M.A.; Shakoor, R.; Montemor, M.; Kahraman, R.; Mansour, S.; Abdullah, A.M.; Hasan, A. Designing and performance evaluation of polyelectrolyte multilayered composite smart coatings. Prog. Org. Coat. 2019, 137, 105319. [Google Scholar] [CrossRef]
- Pirouzifard, M.; Yorghanlu, R.A.; Pirsa, S. Production of active film based on potato starch containing Zedo gum and essential oil of Salvia officinalis and study of physical, mechanical, and antioxidant properties. J. Thermoplast. Compos. Mater. 2020, 33, 915–937. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Chi, F.; Tan, Z.; Liu, L. Development and characterization of novel active chitosan films containing fennel and peppermint essential oils. Coatings 2020, 10, 936. [Google Scholar] [CrossRef]
- Elshamy, S.; Khadizatul, K.; Uemura, K.; Nakajima, M.; Neves, M.A. Chitosan-based film incorporated with essential oil nanoemulsion foreseeing enhanced antimicrobial effect. J. Food Sci. Technol. 2021, 58, 3314–3327. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, C.; Xie, Y.; Mei, J.; Xie, J. Effect of Melissa officinalis L. essential oil nanoemulsions on structure and properties of carboxymethyl chitosan/locust bean gum composite films. Membranes 2022, 12, 568. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Kim, S.; Song, K.B. Antimicrobial activity of buckwheat starch films containing zinc oxide nanoparticles against Listeria monocytogenes on mushrooms. Int. J. Food Sci. Technol. 2018, 53, 1549–1557. [Google Scholar] [CrossRef]
- Erdem, B.G.; Dıblan, S.; Kaya, S. Development and structural assessment of whey protein isolate/sunflower seed oil biocomposite film. Food Bioprod. Process. 2019, 118, 270–280. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Liu, C. Film Transparency and Opacity Measurements. Food Anal. Methods 2022, 15, 2840–2846. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]







| No. | Name | R. Time | Composition % |
|---|---|---|---|
| 1 | Linalool | 13.78 | 28.51 |
| 2 | Terpineol | 17.822 | 8.09 |
| 3 | Linalyl acetate | 20.580 | 43.32 |
| 4 | Geranyl acetate | 25.653 | 4.04 |
| 5 | Neryl acetate | 24.772 | 2.85 |
| 6 | Caryophyllene | 27.006 | 2.44 |
| 7 | Myrcene | 8.548 | 1.85 |
| Sample Code | TS (Mpa) | EAB (%) | Thickness (mm) | WVP (×10−12 g·cm/cm2·s·Pa) | Moisture Content (%) |
|---|---|---|---|---|---|
| SE-1 | 1.022 ± 0.051 a | 28.2 ± 0.643 a | 0.053 ± 0.007 a | 0.427 ± 0.009 a | 25.04 ± 0.47 a |
| SE-2 | 0.823 ± 0.043 b | 21.9 ± 1.30 b | 0.050 ± 0.014 a | 0.485 ± 0.007 b | 22.53 ± 1.03 b |
| SE-3 | 0.576 ± 0.029 c | 14.8 ± 0.412 c | 0.045 ± 0.015 a | 0.537 ± 0.034 c | 18.38 ± 1.30 c |
| SE-4 | 0.140 ± 0.014 d | 14.6 ± 0.06 c | 0.053 ± 0.007 a | 0.667 ± 0.022 d | 14.70 ± 0.37 d |
| Sample Code | L | a* | b* | ΔE* | Transparency % |
|---|---|---|---|---|---|
| SE-1 | 97.50 ± 0.11 a | −0.13 ± 0.02 a | 2.63 ± 0.11 a | 2.28 ± 0.15 a | 86.100 ± 0.561 a |
| SE-2 | 97.12 ± 0.14 a | −0.26 ± 0.03 b | 4.35 ± 0.37 b | 3.63 ± 0.36 b | 82.319 ± 0.402 b |
| SE-3 | 97.65 ± 0.14 a | −0.28 ± 0.01 b | 4.62 ± 0.20 b | 4.07 ± 0.14 c | 74.560 ± 0.607 c |
| SE-4 | 97.59 ± 0.56 a | −0.26 ± 0.04 b | 5.55 ± 0.14 c | 4.93 ± 0.24 d | 56.299 ± 0.092 d |
| Film Sample | The Composition of the Film-Forming Solution |
|---|---|
| SE-1/Control | SA (1.5%) + CA (1%) + Gly (0.5%) + Sorb (0.1%) |
| SE-2 | SA (1.5%) + CA (1%) + Gly (0.5%) + Sorb (0.1%) + SEO (0.025%) |
| SE-3 | SA (1.5%) + CA (1%) + Gly (0.5%) + Sorb (0.1%) + SEO (0.050%) |
| SE-4 | SA (1.5%) + CA (1%) + Gly (0.5%) + Sorb (0.1%) + SEO (0.075%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatia, S.; Al-Harrasi, A.; Shah, Y.A.; Jawad, M.; Al-Azri, M.S.; Ullah, S.; Anwer, M.K.; Aldawsari, M.F.; Koca, E.; Aydemir, L.Y. The Effect of Sage (Salvia sclarea) Essential Oil on the Physiochemical and Antioxidant Properties of Sodium Alginate and Casein-Based Composite Edible Films. Gels 2023, 9, 233. https://doi.org/10.3390/gels9030233
Bhatia S, Al-Harrasi A, Shah YA, Jawad M, Al-Azri MS, Ullah S, Anwer MK, Aldawsari MF, Koca E, Aydemir LY. The Effect of Sage (Salvia sclarea) Essential Oil on the Physiochemical and Antioxidant Properties of Sodium Alginate and Casein-Based Composite Edible Films. Gels. 2023; 9(3):233. https://doi.org/10.3390/gels9030233
Chicago/Turabian StyleBhatia, Saurabh, Ahmed Al-Harrasi, Yasir Abbas Shah, Muhammad Jawad, Mohammed Said Al-Azri, Sana Ullah, Md Khalid Anwer, Mohammed F. Aldawsari, Esra Koca, and Levent Yurdaer Aydemir. 2023. "The Effect of Sage (Salvia sclarea) Essential Oil on the Physiochemical and Antioxidant Properties of Sodium Alginate and Casein-Based Composite Edible Films" Gels 9, no. 3: 233. https://doi.org/10.3390/gels9030233