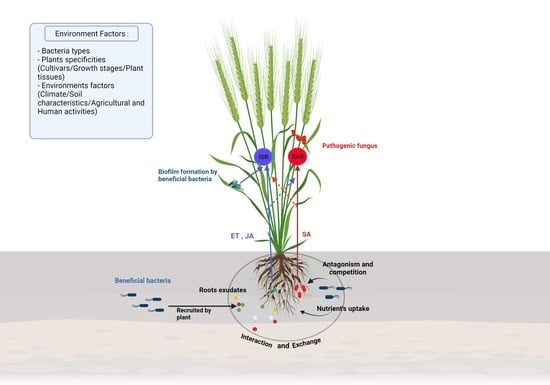
Plant Beneficial Bacteria as Bioprotectants against Wheat and Barley Diseases
Abstract
:1. Wheat and Barley: Duo Cereals at the Foundation of Global Food Stability
2. Main Diseases Affecting Barley and Wheat
2.1. Fusarium Diseases
2.2. Septoria tritici Blotch
2.3. Net Blotch
3. Current Control Strategies against Pathogens of Barley and Wheat
3.1. Chemical Control, Prophylactic Strategies and Genetics Selection
3.2. Biological Control
4. The Context of Plant Defense
5. First Steps of Interaction between Plants and Beneficial Bacteria
5.1. Bacteria Perception
5.2. Colonization and By-Passing of Plant Defense
6. Beneficial Bacteria Implicated in Plant Defense
6.1. Induction of Plant Defense Mechanisms
6.1.1. SAR and ISR Pathways
6.1.2. Priming Effect
6.2. Direct Antagonism
6.2.1. Space and Nutrients Competition and Plant Health
6.2.2. Secretion of Metabolites
Enzymes
Volatiles Compounds
Secondary Metabolites
6.2.3. Limitation of Mycotoxins Production
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/RP (accessed on 19 April 2022).
- Sharma, I.; Tyagi, B.; Singh, G. Enhancing Wheat Production—A Global Perspective. Indian J. Agric. Sci. 2015, 85, 3–13. [Google Scholar]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.-D.; Hatta, M.A.M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siou, D. Développement épidémique de la Fusariose des épis de blé et Conséquences des Intéractions Entre Espèces du Complexe Fusarien. Ph.D. Thesis, Université Paris Sud, Paris, France, 2013. Available online: https://tel.archives-ouvertes.fr/tel-00849969 (accessed on 15 December 2021).
- Feldman, M. Origin of Cultivated Wheat. In The World Wheat Book: A History of Wheat Breeding; Bonjean, A.P., Angus, W.J., Eds.; Intercept Ltd.: London, UK, 2001; pp. 3–56. [Google Scholar]
- Matsuoka, Y. Evolution of Polyploid Triticum Wheats under Cultivation: The Role of Domestication, Natural Hybridization and Allopolyploid Speciation in their Diversification. Plant Cell Physiol. 2011, 52, 750–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef] [PubMed]
- El-Haramein, F.J.; Grando, S. Determination of iron and zinc content in food barley. In Proceedings of the 10th International Barley Genetics Symposium, Alexandria, Egypt, 5–10 April 2008. [Google Scholar]
- Baum, B.B. Classification of cultivated barley (Hordeum vulgare). 1. Historical aspects, and phenetic character analysis of some characters by information theory and by spatial autocorrelation. Can. J. Bot. 1986, 64, 2769–2773. [Google Scholar] [CrossRef]
- Wiggans, R.-G.; Meunissier, A. Classification des variétés d’Orges cultivées. J. D’agriculture Tradit. Bot. Appliquée 1992, 14, 568–571. [Google Scholar] [CrossRef]
- Harwood, W.A. An Introduction to Barley: The Crop and the Model. In Barley: Methods and Protocols; Harwood, W.A., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; pp. 1–5. ISBN 978-1-4939-8944-7. [Google Scholar]
- Soltner, D. Les grandes Productions Végétales: Céréales, Plantes Sarclées, Prairies. In Sciences et Techniques Agricoles; Université de Lorraine: Nancy, France, 1995; Volume 18, p. 471. ISBN 2-907710-02-8. [Google Scholar]
- Meier, U. Stades phénologiques des mono-et dicotylédones cultivées. In BBCH Monographie; Open Agrar Repositorium: Greifswald, Germany, 2018; Volume 18. [Google Scholar] [CrossRef]
- Bingham, I.J.; Hoad, S.P.; Spink, J.H.; Blake, J.; Foulkes, J. The Barley Growth Guide; Agriculture and Horticulture Development Board: Kenilworth, UK, 2006; pp. 3–27.
- Vleeshouwers, V.G.A.A.; Oliver, R.P. Effectors as Tools in Disease Resistance Breeding Against Biotrophic, Hemibiotrophic, and Necrotrophic Plant Pathogens. Mol. Plant Microbe Interact. 2014, 27, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases-A field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef]
- BASF Maladies du Blé. Available online: https://www.agro.basf.fr/fr/cultures/ble/maladies_du_ble/ (accessed on 7 December 2021).
- Ludovic, B.; Giles, C.; Philippe, C.; Denis, G.; Lise, G.V.; Eric, M.; Jean-Yves, M.; Claude, M.; Pierre, T.; Romain, V.; et al. Céréales à Paille: Interventions de Printemps; Arvalis—Institut du vegetal: Paris, France, 2019; pp. 1–172. [Google Scholar]
- Karlsson, I.; Persson, P.; Friberg, H. Fusarium Head Blight from a Microbiome Perspective. Front. Microbiol. 2021, 12, 371. [Google Scholar] [CrossRef]
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight (scab) in small grain cereals? a review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comby, M.; Gacoin, M.; Robineau, M.; Rabenoelina, F.; Ptas, S.; Dupont, J.; Profizi, C.; Baillieul, F. Screening of wheat endophytes as biological control agents against Fusarium head blight using two different in vitro tests. Microbiol. Res. 2017, 202, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Nicholson, P. Community Ecology of Fungal Pathogens Causing Wheat Head Blight. Annu. Rev. Phytopathol. 2009, 47, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Bal, G. Scab of Wheat: Prospects For Control. Plant Dis. 1994, 78, 760–766. [Google Scholar] [CrossRef]
- Kazan, K.; Gardiner, D.M.; Manners, J.M. On the trail of a cereal killer: Recent advances in Fusarium graminearum pathogenomics and host resistance. Mol. Plant Pathol. 2012, 13, 399–413. [Google Scholar] [CrossRef]
- Champeil, A.; Doré, T.; Fourbet, J. Fusarium head blight: Epidemiological origin of the effects of cultural practices on head blight attacks and the production of mycotoxins by Fusarium in wheat grains. Plant Sci. 2004, 166, 1389–1415. [Google Scholar] [CrossRef]
- Trail, F. For Blighted Waves of Grain: Fusarium graminearum in the Postgenomics Era. Plant Physiol. 2009, 149, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W. Zearalenone as an endocrine disruptor in humans. Environ. Toxicol. Pharmacol. 2016, 48, 141–149. [Google Scholar] [CrossRef]
- Miller, S.S.; Watson, E.M.; Lazebnik, J. Characterization of an Alien Source of Resistance to Fusarium Head Blight Transferred to Chinese Spring Wheat; Canadian Science Publishing: Ottawa, ON, Canada, 2011; pp. 301–311. [Google Scholar]
- Desmond, O.J.; Manners, J.M.; Stephens, A.E.; Maclean, D.J.; Schenk, P.M.; Gardiner, D.M.; Munn, A.L.; Kazan, K. The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defence responses in wheat. Mol. Plant Pathol. 2008, 9, 435–445. [Google Scholar] [CrossRef] [Green Version]
- Shaw, M.W.; Royle, D.J. Airborne inoculum as a major source of Septoria tritici (Mycosphaerella graminicola) infections in winter wheat crops in the UK. Plant Pathol. 1989, 38, 35–43. [Google Scholar] [CrossRef]
- Nasraoui, B. Les Champignons Parasites des Plantes Cultivées (Biologie, Systématique, Pathologie, Maladies); Centre de Publication Universitaire: Manouba, Tunisia, 2006; Volume 1, ISBN 978-9973-37-302-1. [Google Scholar]
- El Chartouni, L.; Tisserant, B.; Siah, A.; Duyme, F.; Leducq, J.-B.; Deweer, C.; Fichter-Roisin, C.; Sanssené, J.; Durand, R.; Halama, P.; et al. Genetic diversity and population structure in French populations of Mycosphaerella graminicola. Mycologia 2011, 103, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Brokenshire, T. Wheat Debris as an Inoculum Source for Seedling Infection by Septoria tritici. Plant Pathol. 1975, 24, 202–207. [Google Scholar] [CrossRef]
- Suffert, F.; Sache, I.; Lannou, C. Early stages of septoria tritici blotch epidemics of winter wheat: Build-up, overseasoning, and release of primary inoculum. Plant Pathol. 2010, 60, 166–177. [Google Scholar] [CrossRef]
- Palma-Guerrero, J.; Ma, X.; Torriani, S.F.F.; Zala, M.; Francisco, C.S.; Hartmann, F.E.; Croll, D.; McDonald, B.A. Comparative Transcriptome Analyses in Zymoseptoria tritici Reveal Significant Differences in Gene Expression Among Strains during Plant Infection. Mol. Plant Microbe Interact. 2017, 30, 231–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morais, D. Les Déterminants des Phases épidémiques Précoces de la Septoriose du Blé (Zymoseptoria tritici): Quantité, Efficacité et Origine de L’inoculum Primaire. Ph.D. Thesis, AgroParisTech, Paris, France, 2015. Available online: hal.archives-ouvertes.fr/tel-01142864 (accessed on 15 December 2021).
- Downie, R.C.; Lin, M.; Corsi, B.; Ficke, A.; Lillemo, M.; Oliver, R.P.; Phan, H.T.T.; Tan, K.-C.; Cockram, J. Septoria Nodorum Blotch of Wheat: Disease Management and Resistance Breeding in the Face of Shifting Disease Dynamics and a Changing Environment. Phytopathology 2021, 111, 906–920. [Google Scholar] [CrossRef]
- Ronen, M.; Sela, H.; Fridman, E.; Perl-Treves, R.; Kopahnke, D.; Moreau, A.; Ben-David, R.; Harel, A. Characterization of the Barley Net Blotch Pathosystem at the Center of Origin of Host and Pathogen. Pathogens 2019, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Backes, A.; Guerriero, G.; Barka, E.A.; Jacquard, C. Pyrenophora teres: Taxonomy, Morphology, Interaction with Barley, and Mode of Control. Front. Plant Sci. 2021, 12, 614951. [Google Scholar] [CrossRef]
- Moolhuijzen, P.; Lawrence, J.A.; Ellwood, S.R. Potentiators of Disease during Barley Infection by Pyrenophora teres f. teres in a Susceptible Interaction. Mol. Plant Microbe Interact. 2021, 34, 779–792. [Google Scholar] [CrossRef]
- Rau, D.; Attene, G.; Brown, A.H.D.; Nanni, L.; Maier, F.J.; Balmas, V.; Saba, E.; Schäfer, W.; Papa, R. Phylogeny and evolution of mating-type genes from Pyrenophora teres, the causal agent of barley “net blotch” disease. Curr. Genet. 2007, 51, 377–392. [Google Scholar] [CrossRef]
- McLean, M.S.; Martin, A.; Gupta, S.; Sutherland, M.W.; Hollaway, G.J.; Platz, G.J. Validation of a new spot form of net blotch differential set and evidence for hybridisation between the spot and net forms of net blotch in Australia. Australas. Plant Pathol. 2014, 43, 223–233. [Google Scholar] [CrossRef]
- Sarpeleh, A.; Wallwork, H.; Catcheside, D.E.A.; Tate, M.E.; Able, A.J. Proteinaceous Metabolites from Pyrenophora teres Contribute to Symptom Development of Barley Net Blotch. Phytopathology 2007, 97, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Jalli, M. The Virulence of Finnish Pyrenophora Teres f.Teres Isolates and Its Implications for Resistance Breeding. Ph.D. Thesis, Faculty of Agriculture and Forestry of the University of Helsinki, Helsinki, Finland, 2010. Available online: http://www.mtt.fi/mtttiede/pdf/mtttiede9.pdf (accessed on 15 December 2021).
- Rau, D.; Maier, F.J.; Papa, R.; Brown, A.H.; Balmas, V.; Saba, E.; Schaefer, W.; Attene, G. Isolation and characterization of the mating-type locus of the barley pathogen Pyrenophora teres and frequencies of mating-type idiomorphs within and among fungal populations collected from barley landraces. Genome 2005, 48, 855–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clare, S.J.; Wyatt, N.A.; Brueggeman, R.S.; Friesen, T.L. Research advances in the Pyrenophora teres–barley interaction. Mol. Plant Pathol. 2019, 21, 272–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muria-Gonzalez, M.J.; Zulak, K.G.; Allegaert, E.; Oliver, R.P.; Ellwood, S.R. Profile of the in vitro secretome of the barley net blotch fungus, Pyrenophora teres f. teres. Physiol. Mol. Plant Pathol. 2019, 109, 101451. [Google Scholar] [CrossRef]
- Haidukowski, M.; Pascale, M.; Perrone, G.; Pancaldi, D.; Campagna, C.; Visconti, A. Effect of fungicides on the development ofFusarium head blight, yield and deoxynivalenol accumulation in wheat inoculated under field conditions with Fusarium graminearum and Fusarium culmorum. J. Sci. Food Agric. 2004, 85, 191–198. [Google Scholar] [CrossRef]
- Mejri, S. Efficacités et Modes D’action de Nouveaux Simulateurs de Défenses des Plantes sur le Pathosystème Blé—Zymoseptoria tritici; Sciences Agricoles; Université du Littoral Côte d’Opale: Dunkerque, France, 2018. [Google Scholar]
- Bendahmane, B.; Barrault, G.; Albertini, L.; Toubia-Rahme, H. Etude de l’action in Vitro de Divers Fongicides Sur Le Développement de Drechslera Teres f. Teres et f. Maculata. Phytopathol. Mediterr. 1992, 31, 77–84. [Google Scholar]
- Jørgensen, L.; Olsen, L. Control of tan spot (Drechslera tritici-repentis) using cultivar resistance, tillage methods and fungicides. Crop Prot. 2007, 26, 1606–1616. [Google Scholar] [CrossRef]
- Bayer les Méthodes Agronomiques Pour Limiter la Pression Maladies: Bayer-Agri, Conseils Phyto. Available online: https://www.bayer-agri.fr/cultures/les-methodes-agronomiques-pour-limiter-la-pression-maladies_1498/ (accessed on 5 December 2021).
- Pfender, W.F.; Wootke, S.L. Microbial communities ofPyrenophora-infested wheat straw as examined by multivariate analysis. Microb. Ecol. 1988, 15, 95–113. [Google Scholar] [CrossRef]
- Bankina, B.; Bimšteine, G.; Arhipova, I.; Kaņeps, J.; Stanka, T. Importance of Agronomic Practice on the Control of Wheat Leaf Diseases. Agriculture 2018, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Dill-Macky, R.; Jones, R.K. The Effect of Previous Crop Residues and Tillage on Fusarium Head Blight of Wheat. Plant Dis. 2000, 84, 71–76. [Google Scholar] [CrossRef] [Green Version]
- McDonald, B.A.; Mundt, C.C. How Knowledge of Pathogen Population Biology Informs Management of Septoria Tritici Blotch. Phytopathology 2016, 106, 948–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, E.M.; Boareto, C.; Danelli, A.L.D.; Zoldan, S.M. Anthesis, the infectious process and disease progress curves for fusarium head blight in wheat. Summa Phytopathol. 2016, 42, 134–139. [Google Scholar] [CrossRef]
- Liu, X.; Herbert, S.; Hashemi, A.; Zhang, X.; Ding, G. Effects of agricultural management on soil organic matter and carbon transformation—A review. Plant Soil Environ. 2011, 52, 531–543. [Google Scholar] [CrossRef] [Green Version]
- Soonvald, L.; Loit, K.; Runno-Paurson, E.; Astover, A.; Tedersoo, L. The role of long-term mineral and organic fertilisation treatment in changing pathogen and symbiont community composition in soil. Appl. Soil Ecol. 2019, 141, 45–53. [Google Scholar] [CrossRef]
- Flor, H.H. Current Status of the Gene-For-Gene Concept. Annu. Rev. Phytopathol. 1971, 9, 275–296. [Google Scholar] [CrossRef]
- Zhong, Z.; Marcel, T.C.; Hartmann, F.E.; Ma, X.; Plissonneau, C.; Zala, M.; Ducasse, A.; Confais, J.; Compain, J.; Lapalu, N.; et al. A small secreted protein in Zymoseptoria tritici is responsible for avirulence on wheat cultivars carrying the Stb6 resistance gene. New Phytol. 2017, 214, 619–631. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.K.; Chartrain, L.; Lasserre-Zuber, P.; Saintenac, C. Genetics of resistance to Zymoseptoria tritici and applications to wheat breeding. Fungal Genet. Biol. 2015, 79, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Chartrain, L.; Joaquim, P.; Berry, S.T.; Arraiano, L.S.; Azanza, F.; Brown, J.K.M. Genetics of resistance to septoria tritici blotch in the Portuguese wheat breeding line TE 9111. Theor. Appl. Genet. 2005, 110, 1138–1144. [Google Scholar] [CrossRef]
- Williams, K.J.; Lichon, A.; Gianquitto, P.; Kretschmer, J.M.; Karakousis, A.; Manning, S.; Langridge, P.; Wallwork, H. Identification and mapping of a gene conferring resistance to the spot form of net blotch (Pyrenophora teres f maculata) in barley. Theor. Appl. Genet. 1999, 99, 323–327. [Google Scholar] [CrossRef]
- Grewal, T.S.; Rossnagel, B.G.; Pozniak, C.J.; Scoles, G.J. Mapping quantitative trait loci associated with barley net blotch resistance. Theor. Appl. Genet. 2007, 116, 529–539. [Google Scholar] [CrossRef]
- Manninen, O.; Jalli, M.; Kalendar, R.; Schulman, A.; Afanasenko, O.; Robinson, J. Mapping of major spot-type and net-type net-blotch resistance genes in the Ethiopian barley line CI 9819. Genome 2006, 49, 1564–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupinska, K.; Haussühl, K.; Schäfer, A.; van der Kooij, T.A.; Leckband, G.; Lorz, H.; Falk, J. A Novel Nucleus-Targeted Protein Is Expressed in Barley Leaves during Senescence and Pathogen Infection. Plant Physiol. 2002, 130, 1172–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glick, B.R. Beneficial Plant-Bacterial Interactions; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-13920-3. [Google Scholar]
- Agence Française de Sécurité Sanitaire des Aliments. Avis de l’Agence Française de Sécurité Sanitaire des Aliments Relatif à une Demande D’autorisation de Mise sur le Marché de la Préparation CERALL à Base de Pseudomonas chlororaphis souche MA 342. In Produite par la Société BELCHIM CROP PROTECTION NV/SA; AFSSA: Maisons-Alfort, France, 2008; pp. 1–9. [Google Scholar]
- Nascimento, F.X.; Rossi, M.J.; Glick, B.R.; Nascimento, F.X.; Rossi, M.J.; Glick, B.R. Ethylene and 1-Aminocyclopropane-1-carboxylate (ACC) in Plant–Bacterial Interactions. Front. Plant Sci. 2018, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Corteva Agriscience Inatreq Active. Technical Bulletin. 2018, pp. 4–15. Available online: https://www.corteva.com/content/dam/dpagco/corteva/global/corporate/general/files/active-ingredients/INA.045.Corteva.Inatreq.Global.Technical.Bulletin.cereals.pdf (accessed on 5 December 2021).
- Bubici, G.N. Streptomyces spp. as biocontrol agents against Fusarium species. CAB Rev. Perspect. Agric. Veter. Sci. Nutr. Nat. Resour. 2018, 13, 50. [Google Scholar] [CrossRef]
- Jemmali, L. Stimulateurs des Défenses Naturelles du blé dur en Tunisie et du blé Tendre en France Contre la Septoriose Causée par Zymoseptoria tritici. Ph.D. Thesis, Université du Littoral Côte d’Opale, Dunkerque, France, 2015. Available online: https://tel.archives-ouvertes.fr/tel-01626008 (accessed on 3 December 2021).
- Knogge, W.; Lee, J.; Rosahl, S.; Scheel, D. Signal Perception and Transduction in Plants. In The Mycota; Deising, H.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 5, pp. 337–361. ISBN 978-3-540-87406-5. [Google Scholar]
- Villena, J.; Kitazawa, H.; Van Wees, S.; Pieterse, C.M.J.; Takahashi, H. Receptors and Signaling Pathways for Recognition of Bacteria in Livestock and Crops: Prospects for Beneficial Microbes in Healthy Growth Strategies. Front. Immunol. 2018, 9, 2223. [Google Scholar] [CrossRef]
- Tyagi, S.; Mulla, S.I.; Lee, K.-J.; Chae, J.-C.; Shukla, P. VOCs-mediated hormonal signaling and crosstalk with plant growth promoting microbes. Crit. Rev. Biotechnol. 2018, 38, 1277–1296. [Google Scholar] [CrossRef]
- Van Oosten, V.R.; Bodenhausen, N.; Reymond, P.; Van Pelt, J.A.; Van Loon, L.C.; Dicke, M.; Pieterse, C.M.J. Differential Effectiveness of Microbially Induced Resistance Against Herbivorous Insects in Arabidopsis. Mol. Plant Microbe Interact. 2008, 21, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Champigny, M.J.; Shearer, H.; Mohammad, A.; Haines, K.; Neumann, M.; Thilmony, R.; He, S.Y.; Fobert, P.; Dengler, N.; Cameron, R.K. Localization of DIR1 at the tissue, cellular and subcellular levels during Systemic Acquired Resistance in Arabidopsisusing DIR1:GUS and DIR1:EGFP reporters. BMC Plant Biol. 2011, 11, 125. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [Green Version]
- Bakker, P.A.; Doornbos, R.F.; Zamioudis, C.; Berendsen, R.; Pieterse, C.M. Induced Systemic Resistance and the Rhizosphere Microbiome. Plant Pathol. J. 2013, 29, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankiewicz, U.; Kołtonowicz, M. The involvement of Pseudomonas bacteria in induced systemic resistance in plants (Review). Appl. Biochem. Microbiol. 2012, 48, 244–249. [Google Scholar] [CrossRef]
- Backer, R.; Naidoo, S.; van den Berg, N. The nonexpressor of pathogenesis-related genes 1 (NPR1) and related family: Mechanistic insights in plant disease resistance. Front. Plant Sci. 2019, 10, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orozco-Mosqueda, M.D.C.; Santoyo, G. Plant-microbial endophytes interactions: Scrutinizing their beneficial mechanisms from genomic explorations. Curr. Plant Biol. 2020, 25, 100189. [Google Scholar] [CrossRef]
- Bren, A.; Eisenbach, M. How Signals Are Heard during Bacterial Chemotaxis: Protein-Protein Interactions in Sensory Signal Propagation. J. Bacteriol. 2000, 182, 6865–6873. [Google Scholar] [CrossRef] [Green Version]
- Porter, S.L.; Wadhams, G.H.; Armitage, J.P. Signal processing in complex chemotaxis pathways. Nat. Rev. Microbiol. 2011, 9, 153–165. [Google Scholar] [CrossRef]
- Mark, G.L.; Dow, J.M.; Kiely, P.D.; Higgins, H.; Haynes, J.; Baysse, C.; Abbas, A.; Foley, T.; Franks, A.; Morrissey, J.; et al. Transcriptome profiling of bacterial responses to root exudates identifies genes involved in microbe-plant interactions. Proc. Natl. Acad. Sci. USA 2005, 102, 17454–17459. [Google Scholar] [CrossRef] [Green Version]
- Elasri, M.; Delorme, S.; Lemanceau, P.; Stewart, G.; Laue, B.; Glickmann, E.; Oger, P.M.; Dessaux, Y. Acyl-Homoserine Lactone Production Is More Common among Plant-Associated Pseudomonas spp. than among Soilborne Pseudomonas spp. Appl. Environ. Microbiol. 2001, 67, 1198–1209. [Google Scholar] [CrossRef] [Green Version]
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and promise of bacterial quorum sensing research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef]
- Bassler, B.L. Small Talk: Cell-to-Cell Communication in Bacteria. Cell 2002, 109, 421–424. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Shaaban, M.I.; Bar, F.A.; El-Mahdy, A.; Shokralla, S. Quorum Sensing Inhibiting Activity of Streptomyces coelicoflavus Isolated from Soil. Front. Microbiol. 2016, 7, 659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaeel, Q.; Sanchez, L.; Robineau, M.; Dorey, S.; Clément, C.; Jacquard, C.; Ait Barka, E. Paraburkholderia phytofirmans PsJN-Plants Interaction: From Perception to the Induced Mechanisms. Front. Microbiol. 2018, 9, 2093. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ayesha; Hameed, S.; Imran, A.; Iqbal, M.; Iqbal, J.; Oresnik, I.J. Functional characterization of a soybean growth stimulator Bradyrhizobium sp. strain SR-6 showing acylhomoserine lactone production. FEMS Microbiol. Ecol. 2016, 92, fiw115. [Google Scholar] [CrossRef] [Green Version]
- Jung, B.K.; Khan, A.R.; Hong, S.-J.; Park, G.-S.; Park, Y.-J.; Kim, H.-J.; Jeon, H.-J.; Khan, M.A.; Waqas, M.; Lee, I.-J.; et al. Quorum sensing activity of the plant growth-promoting rhizobacterium Serratia glossinae GS2 isolated from the sesame (Sesamum indicum L.) rhizosphere. Ann. Microbiol. 2017, 67, 623–632. [Google Scholar] [CrossRef]
- Hori, K.; Matsumoto, S. Bacterial adhesion: From mechanism to control. Biochem. Eng. J. 2010, 48, 424–434. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Stein, T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef]
- Böhm, M.; Hurek, T.; Reinhold-Hurek, B. Twitching Motility Is Essential for Endophytic Rice Colonization by the N2-Fixing Endophyte Azoarcus sp. Strain BH72. Mol. Plant Microbe Interact. 2007, 20, 526–533. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, N.; Sathishkumar, R.; Selvakumar, G.; Shyamkumar, R.; Arjunekumar, K. Phyllospheric Microbiomes: Diversity, Ecological Significance, and Biotechnological Applications. Plant Microbiomes Sustain. Agric. 2020, 25, 113–172. [Google Scholar] [CrossRef]
- Saranraj, P. Screening of Pectinase Producing Bacteria and Fungi for Its Pectinolytic Activity Using Fruit Wastes. Int. J. Biochem. Biotechnol. Sci. 2012, 1, 30–40. [Google Scholar]
- Beldman, G.; Leeuwen, M.F.S.-V.; Rombouts, F.M.; Voragen, F.G.J. The cellulase of Trichoderma viride. JBIC J. Biol. Inorg. Chem. 1985, 146, 301–308. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Zhang, Y.-H.P. Cellulases: Characteristics, Sources, Production, and Applications. In Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals, and Polymers; Yang, S.-T., El-Enshasy, H.A., Thongchul, N., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; Volume 8, pp. 131–146. ISBN 978-1-118-64204-7. [Google Scholar]
- Reinhold-Hurek, B.; Maes, T.; Gemmer, S.; Van Montagu, M.; Hurek, T. An Endoglucanase Is Involved in Infection of Rice Roots by the Not-Cellulose-Metabolizing Endophyte Azoarcus sp. Strain BH72. Mol. Plant Microbe Interact. 2006, 19, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Environ. Microbiol. Rep. 2014, 7, 40–50. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Duan, J.; Charles, T.; Glick, B.R. A bioinformatics approach to the determination of genes involved in endophytic behavior in Burkholderia spp. J. Theor. Biol. 2014, 343, 193–198. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted Interactions Between Endophytes and Plant: Developments and Prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Zeidler, D.; Zähringer, U.; Gerber, I.; Dubery, I.; Hartung, T.; Bors, W.; Hutzler, P.; Durner, J. Innate immunity in Arabidopsis thaliana: Lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc. Natl. Acad. Sci. USA 2004, 101, 15811–15816. [Google Scholar] [CrossRef] [Green Version]
- Taghavi, S.; van der Lelie, D.; Hoffman, A.; Zhang, Y.-B.; Walla, M.D.; Vangronsveld, J.; Newman, L.; Monchy, S. Genome Sequence of the Plant Growth Promoting Endophytic Bacterium Enterobacter sp. 638. PLoS Genet. 2010, 6, e1000943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallet, B. Playing Dr Jekyll and Mr Hyde: Combined mechanisms of phase variation in bacteria. Curr. Opin. Microbiol. 2001, 4, 570–581. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Yu, K.; Pieterse, C.M.; Pieterse, C.M.; Bakker, P.A.; Bakker, P.A.; Berendsen, R.L.; Berendsen, R.L.; Yu, K.; Yu, K.; et al. Beneficial microbes going underground of root immunity. Plant Cell Environ. 2019, 42, 2860–2870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clay, N.K.; Adio, A.M.; Denoux, C.; Jander, G.; Ausubel, F.M. Glucosinolate Metabolites Required for an Arabidopsis Innate Immune Response. Science 2009, 323, 95–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millet, Y.A.; Danna, C.H.; Clay, N.K.; Songnuan, W.; Simon, M.D.; Werck-Reichhart, D.; Ausubel, F.M. Innate Immune Responses Activated in Arabidopsis Roots by Microbe-Associated Molecular Patterns. Plant Cell 2010, 22, 973–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akum, F.N.; Esteinbrenner, J.; Ebiedenkopf, D.; Eimani, J.; Kogel, K.-H. The Piriformospora indica effector PIIN_08944 promotes the mutualistic Sebacinalean symbiosis. Front. Plant Sci. 2015, 6, 906. [Google Scholar] [CrossRef] [Green Version]
- Davidescu, D.; Davidescu, V. Evaluation of Fertility by Plant and Soil Analysis; Taylor & Francis: Abingdon-on-Thames, UK; Abacus Press: London, UK, 1982; Volume 1, ISBN 978-0-85626-123-7. [Google Scholar]
- Alippi, A.; Perelló, A.; Sisterna; Greco, N.; Cordo, C. Potential of Spore-Forming Bacteria as Biocontrol Agents of Wheat Foliar Diseases under Laboratory and Greenhouse Conditions. J. Plant Dis. Prot. 2000, 107, 155–169. [Google Scholar]
- Mejri, S.; Siah, A.; Coutte, F.; Magnin-Robert, M.; Randoux, B.; Tisserant, B.; Krier, F.; Jacques, P.; Reignault, P.; Halama, P. Biocontrol of the wheat pathogen Zymoseptoria tritici using cyclic lipopeptides from Bacillus subtilis. Environ. Sci. Pollut. Res. 2017, 25, 29822–29833. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Cantoro, R.; Palazzini, J.M.; Yerkovich, N.; Miralles, D.J.; Chulze, S.N. Bacillus velezensis RC 218 as a biocontrol agent against Fusarium graminearum: Effect on penetration, growth and TRI5 expression in wheat spikes. BioControl 2020, 66, 259–270. [Google Scholar] [CrossRef]
- Palazzini, J.M.; Ramirez, M.; Alberione, E.; Torres, A.; Chulze, S. Osmotic stress adaptation, compatible solutes accumulation and biocontrol efficacy of two potential biocontrol agents on Fusarium head blight in wheat. Biol. Control 2009, 51, 370–376. [Google Scholar] [CrossRef]
- Palazzini, J.; Roncallo, P.; Cantoro, R.; Chiotta, M.; Yerkovich, N.; Palacios, S.; Echenique, V.; Torres, A.; Ramirez, M.; Karlovsky, P.; et al. Biocontrol of Fusarium graminearum sensu stricto, Reduction of Deoxynivalenol Accumulation and Phytohormone Induction by Two Selected Antagonists. Toxins 2018, 10, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Li, R.; Qin, S.; Huang, L.; Wei, L.; Brian, K. Screening of Antagonistic Strain against Fusarium Head Blight and Its Inhibition Efficiency. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-ZBJS201705002.htm (accessed on 6 June 2021).
- Wang, J.; Liu, J.; Chen, H.; Yao, J. Characterization of Fusarium graminearum inhibitory lipopeptide from Bacillus subtilis IB. Appl. Microbiol. Biotechnol. 2007, 76, 889–894. [Google Scholar] [CrossRef]
- Pan, D.; Mionetto, A.; Tiscornia, S.; Bettucci, L. Endophytic bacteria from wheat grain as biocontrol agents of Fusarium graminearum and deoxynivalenol production in wheat. Mycotoxin Res. 2015, 31, 137–143. [Google Scholar] [CrossRef]
- Crane, J.M.; Gibson, D.M.; Vaughan, R.H.; Bergstrom, G.C. Iturin Levels on Wheat Spikes Linked to Biological Control of Fusarium Head Blight by Bacillus amyloliquefaciens. Phytopathology 2013, 103, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Gong, A.-D.; Li, H.-P.; Yuan, Q.-S.; Song, X.-S.; Yao, W.; He, W.-J.; Zhang, J.-B.; Liao, Y.-C. Antagonistic Mechanism of Iturin A and Plipastatin A from Bacillus amyloliquefaciens S76-3 from Wheat Spikes against Fusarium graminearum. PLoS ONE 2015, 10, e0116871. [Google Scholar] [CrossRef] [Green Version]
- Jamal, Q.; Cho, J.-Y.; Moon, J.-H.; Kim, K.Y. Purification and antifungal characterization of Cyclo (D-Pro-L- Val) from Bacillus amyloliquefaciens Y1 against Fusarium graminearum to control head blight in wheat. Biocatal. Agric. Biotechnol. 2017, 10, 141–147. [Google Scholar] [CrossRef]
- Grosu, A.I.; Sicuia, O.-A.; Dobre, A.; Voaideş, C.; Cornea, C.P. Evaluation of Some Bacillus spp. Strains for the Biocontrol of Fusarium graminearum and F. culmorum in Wheat. Agric. Agric. Sci. Procedia 2015, 6, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Zalila-Kolsi, I.; Ben Mahmoud, A.; Ali, H.; Sellami, S.; Nasfi, Z.; Tounsi, S.; Jamoussi, K. Antagonist effects of Bacillus spp. strains against Fusarium graminearum for protection of durum wheat (Triticum turgidum L. subsp. durum). Microbiol. Res. 2016, 192, 148–158. [Google Scholar] [CrossRef]
- Kildea, S.; Ransbotyn, V.; Khan, M.R.; Fagan, B.; Leonard, G.; Mullins, E.; Doohan, F.M. Bacillus megaterium shows potential for the biocontrol of septoria tritici blotch of wheat. Biol. Control 2008, 47, 37–45. [Google Scholar] [CrossRef]
- Nourozian, J.; Etebarian, H.R.; Khodakaramian, G. Biological Control of Fusarium graminearum on Wheat by Antagonistic Bacteria. Songklanakarin J. Sci. Technol. 2006, 28, 29–38. [Google Scholar]
- Palazzini, J.M.; Ramirez, M.L.; Torres, A.M.; Chulze, S.N. Potential biocontrol agents for Fusarium head blight and deoxynivalenol production in wheat. Crop Prot. 2007, 26, 1702–1710. [Google Scholar] [CrossRef]
- Palazzini, J.M.; Alberione, E.; Torres, A.; Donat, C.; Köhl, J.; Chulze, S. Biological control of Fusarium graminearum sensu stricto, causal agent of Fusarium head blight of wheat, using formulated antagonists under field conditions in Argentina. Biol. Control 2016, 94, 56–61. [Google Scholar] [CrossRef]
- Palazzini, J.M.; Dunlap, C.; Bowman, M.J.; Chulze, S.N. Bacillus velezensis RC 218 as a biocontrol agent to reduce Fusarium head blight and deoxynivalenol accumulation: Genome sequencing and secondary metabolite cluster profiles. Microbiol. Res. 2016, 192, 30–36. [Google Scholar] [CrossRef]
- Palazzini, J.; Torres, A.; Chulze, S. Tolerance of triazole-based fungicides by biocontrol agents used to control Fusarium head blight in wheat in Argentina. Lett. Appl. Microbiol. 2018, 66, 434–438. [Google Scholar] [CrossRef]
- Colombo, E.M.; Kunova, A.; Pizzatti, C.; Saracchi, M.; Cortesi, P.; Pasquali, M. Selection of an Endophytic Streptomyces sp. Strain DEF09 from Wheat Roots as a Biocontrol Agent Against Fusarium graminearum. Front. Microbiol. 2019, 10, 2356. [Google Scholar] [CrossRef] [Green Version]
- Jungkwan, L.; Park, S.-Y.; Lee, Y.-W.; Lee, J. Biological Efficacy of Streptomyces sp. Strain BN1 against the Cereal Head Blight Pathogen Fusarium graminearum. Plant Pathol. J. 2013, 29, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Bello, G.D.; Mónaco, C.; Simon, M.R. Biological control of seedling blight of wheat caused by Fusarium graminearum with beneficial rhizosphere microorganisms. World J. Microbiol. Biotechnol. 2002, 18, 627–636. [Google Scholar] [CrossRef]
- Khan, N.I.; Schisler, D.A.; Boehm, M.J.; Slininger, P.J.; Bothast, R.J. Selection and Evaluation of Microorganisms for Biocontrol of Fusarium Head Blight of Wheat Incited by Gibberella zeae. Plant Dis. 2001, 85, 1253–1258. [Google Scholar] [CrossRef] [Green Version]
- Schisler, D.A.; Khan, N.I.; Boehm, M.J.; Slininger, P.J. Greenhouse and Field Evaluation of Biological Control of Fusarium Head Blight on Durum Wheat. Plant Dis. 2002, 86, 1350–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunlap, C.A.; Schisler, D.A.; Bowman, M.J.; Rooney, A.P. Genomic analysis of Bacillus subtilis OH 131.1 and co-culturing with Cryptococcus flavescens for control of Fusarium head blight. Plant Gene 2015, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lynch, K.; Zannini, E.; Guo, J.; Axel, C.; Arendt, E.K.; Kildea, S.; Coffey, A. Control of Zymoseptoria tritici cause of septoria tritici blotch of wheat using antifungal Lactobacillus strains. J. Appl. Microbiol. 2016, 121, 485–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavermicocca, P.; Valerio, F.; Evidente, A.; Lazzaroni, S.; Corsetti, A.; Gobbetti, M. Purification and Characterization of Novel Antifungal Compounds from the Sourdough Lactobacillus plantarum Strain 21B. Appl. Environ. Microbiol. 2000, 66, 4084–4090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lounaci, L.; Guemouri-Athmani, S.; Boureghda, H.; Achouak, W.; Heulin, T. Suppression of crown and root rot of wheat by the rhizobacterium Paenibacillus polymyxa. Phytopathol. Mediterr. 2017, 55, 355–365. [Google Scholar]
- He, J.; Boland, G.; Zhou, T. Concurrent Selection for Microbial Suppression of Fusarium graminearum, Fusarium Head Blight and Deoxynivalenol in Wheat. J. Appl. Microbiol. 2009, 106, 1805–1817. [Google Scholar] [CrossRef]
- Samain, E.; Aussenac, T.; Selim, S. The Effect of Plant Genotype, Growth Stage, and Mycosphaerella graminicola Strains on the Efficiency and Durability of Wheat-Induced Resistance by Paenibacillus sp. Strain B2. Front. Plant Sci. 2019, 10, 587. [Google Scholar] [CrossRef] [Green Version]
- Levy, E.; Eyal, Z.; Carmely, S.; Kashman, Y.; Chet, I. Suppression of Septoria tritici and Puccinia recondita of wheat by an antibiotic-producing fluorescent pseudomonad. Plant Pathol. 1989, 38, 564–570. [Google Scholar] [CrossRef]
- Levy, E.; Gough, F.J.; Berlin, K.D.; Guiana, P.W.; Smith, J.T. Inhibition of Septoria tritici and other phytopathogenic fungi and bacteria by Pseudomonas fluorescens and its antibiotics. Plant Pathol. 1992, 41, 335–341. [Google Scholar] [CrossRef]
- Schisler, D.; Khan, N.; Boehm, M.; Lipps, P.; Slininger, P.; Zhang, S. Selection and evaluation of the potential of choline-metabolizing microbial strains to reduce Fusarium head blight. Biol. Control 2006, 39, 497–506. [Google Scholar] [CrossRef]
- Flaishman, M.A.; Eyal, Z.; Voisard, C.; Haas, D. Suppression of Septoria Tritici Blotch and Leaf Rust of Wheat by Recombiant Cyanide-Producing Strains of Pseudomonas putida. Mol. Plant-Microbe Interact. 1996, 9, 642. [Google Scholar] [CrossRef]
- Flaishman, M.; Eyal, Z.; Voisard, C.; Haas, D. Suppression of Septoria tritici by Phenazine- or Siderophore-deficient mutants ofPseudomonas. Curr. Microbiol. 1990, 20, 121–124. [Google Scholar] [CrossRef]
- Johnsson, L.; Hökeberg, M.; Gerhardson, B. Performance of the Pseudomonas chlororaphis biocontrol agent MA 342 against cereal seed-borne diseases in field experiments. Eur. J. Plant Pathol. 1998, 104, 701–711. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Yang, N.; Wen, Z.; Sun, X.; Chai, Y.; Ma, Z. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat. Commun. 2018, 9, 3429. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-Y.; Xie, Y.-S.; Cui, Y.-Y.; Xu, J.; He, W.; Chen, H.-G.; Guo, J.-H. Conjunctively screening of biocontrol agents (BCAs) against fusarium root rot and fusarium head blight caused by Fusarium graminearum. Microbiol. Res. 2015, 177, 34–42. [Google Scholar] [CrossRef]
- Morimura, H.; Ito, M.; Yoshida, S.; Koitabashi, M.; Tsushima, S.; Camagna, M.; Chiba, S.; Takemoto, D.; Kawakita, K.; Sato, I. In Vitro Assessment of Biocontrol Effects on Fusarium Head Blight and Deoxynivalenol (DON) Accumulation by DON-Degrading Bacteria. Toxins 2020, 12, 399. [Google Scholar] [CrossRef]
- Khan, M.R.; Brien, E.O.; Carney, B.F.; Doohan, F.M. A fluorescent pseudomonad shows potential for the control of net blotch disease of barley. Biol. Control 2010, 54, 41–45. [Google Scholar] [CrossRef]
- Hökeberg, M.; Gerhardson, B.; Johnsson, L. Biological control of cereal seed-borne diseases by seed bacterization with greenhouse-selected bacteria. Eur. J. Plant Pathol. 1997, 103, 25–33. [Google Scholar] [CrossRef]
- Tombolini, R.; van der Gaag, D.J.; Gerhardson, B.; Jansson, J.K. Colonization Pattern of the Biocontrol Strain Pseudomonas chlororaphis MA 342 on Barley Seeds Visualized by Using Green Fluorescent Protein. Appl. Environ. Microbiol. 1999, 65, 3674–3680. [Google Scholar] [CrossRef] [Green Version]
- Chemitei, K.; Amendi, M.B.; Mwamburi, L.A.; Ochuodho, J.O. Bio-Control of Net-Blotch and Scald Pathogens of Barley Using Paenibacillus Polymyxa KAI245 Isolated from Sorghum Rhizosphere in Western Kenya. Am. J. Microbiol. Res. 2019, 7, 28–36. [Google Scholar] [CrossRef]
- Backes, A.; Vaillant-Gaveau, N.; Esmaeel, Q.; Barka, E.A.; Jacquard, C. A biological agent modulates the physiology of barley infected with Drechslera teres. Sci. Rep. 2021, 11, 8330. [Google Scholar] [CrossRef]
- Backes, A.; Charton, S.; Planchon, S.; Esmaeel, Q.; Sergeant, K.; Hausman, J.-F.; Renaut, J.; Barka, E.A.; Jacquard, C.; Guerriero, G. Gene expression and metabolite analysis in barley inoculated with net blotch fungus and plant growth-promoting rhizobacteria. Plant Physiol. Biochem. 2021, 168, 488–500. [Google Scholar] [CrossRef]
- Romera, F.J.; García, M.J.; Lucena, C.; Martinez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef]
- Ahn, I.-P.; Lee, S.-W.; Suh, S.-C. Rhizobacteria-Induced Priming in Arabidopsis Is Dependent on Ethylene, Jasmonic Acid, and NPR1. Mol. Plant Microbe Interact. 2007, 20, 759–768. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.; Sultana, F.; Kubota, M.; Hyakumachi, M. Differential inducible defense mechanisms against bacterial speck pathogen in Arabidopsis thaliana by plant-growth-promoting-fungus Penicillium sp. GP16-2 and its cell free filtrate. Plant Soil 2008, 304, 227–239. [Google Scholar] [CrossRef]
- Iavicoli, A.; Boutet, E.; Buchala, A.; Métraux, J.-P. Induced Systemic Resistance in Arabidopsis thaliana in Response to Root Inoculation with Pseudomonas fluorescens CHA0. Mol. Plant Microbe Interact. 2003, 16, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Ryu, C.-M.; Murphy, J.F.; Mysore, K.S.; Kloepper, J.W. Plant growth-promoting rhizobacteria systemically protect Arabidopsis thaliana against Cucumber mosaic virus by a salicylic acid and NPR1-independent and jasmonic acid-dependent signaling pathway. Plant J. 2004, 39, 381–392. [Google Scholar] [CrossRef]
- Weller, D.M.; Mavrodi, D.V.; van Pelt, J.A.; Pieterse, C.M.J.; van Loon, L.C.; Bakker, P.A.H.M. Induced Systemic Resistance in Arabidopsis thaliana Against Pseudomonas syringae pv. tomato by 2,4-Diacetylphloroglucinol-Producing Pseudomonas fluorescens. Phytopathology 2012, 102, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Katsir, L.; Chung, H.S.; Koo, A.J.; Howe, G.A. Jasmonate signaling: A conserved mechanism of hormone sensing. Curr. Opin. Plant Biol. 2008, 11, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Pozo, M.J.; Van Der Ent, S.; Van Loon, L.C.; Pieterse, C.M.J. Transcription factor MYC2 is involved in priming for enhanced defense during rhizobacteria-induced systemic resistance in Arabidopsis thaliana. New Phytol. 2008, 180, 511–523. [Google Scholar] [CrossRef]
- Galletti, R.; Ferrari, S.; De Lorenzo, G. Arabidopsis MPK3 and MPK6 Play Different Roles in Basal and Oligogalacturonide- or Flagellin-Induced Resistance against Botrytis cinerea. Plant Physiol. 2011, 157, 804–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stringlis, I.A.; Yu, K.; Feussner, K.; de Jonge, R.; Van Bentum, S.; Van Verk, M.C.; Berendsen, R.L.; Bakker, P.A.H.M.; Feussner, I.; Pieterse, C.M.J. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl. Acad. Sci. USA 2018, 115, E5213–E5222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef] [PubMed]
- Petti, C.; Khan, M.; Doohan, F. Lipid transfer proteins and protease inhibitors as key factors in the priming of barley responses to Fusarium head blight disease by a biocontrol strain of Pseudomonas fluorescens. Funct. Integr. Genom. 2010, 10, 619–627. [Google Scholar] [CrossRef]
- Mur, L.A.; Kenton, P.; Atzorn, R.; Miersch, O.; Wasternack, C. The Outcomes of Concentration-Specific Interactions between Salicylate and Jasmonate Signaling Include Synergy, Antagonism, and Oxidative Stress Leading to Cell Death. Plant Physiol. 2005, 140, 249–262. [Google Scholar] [CrossRef] [Green Version]
- Nie, P.; Li, X.; Wang, S.; Guo, J.; Zhao, H.; Niu, D. Induced Systemic Resistance against Botrytis cinerea by Bacillus cereus AR156 through a JA/ET- and NPR1-Dependent Signaling Pathway and Activates PAMP-Triggered Immunity in Arabidopsis. Front. Plant Sci. 2017, 8, 238. [Google Scholar] [CrossRef]
- Pastor, V.; Luna, E.; Mauch-Mani, B.; Ton, J.; Flors, V. Primed plants do not forget. Environ. Exp. Bot. 2013, 94, 46–56. [Google Scholar] [CrossRef]
- Iriti, M. Plant Innate Immunity; Editorial Office; MDPI: Basel, Switzerland, 2019; Volume 2, ISBN 978-3-03897-580-9. [Google Scholar]
- Bhattacharyya, D.; Garladinne, M.; Lee, Y.H. Volatile Indole Produced by Rhizobacterium Proteus vulgaris JBLS202 Stimulates Growth of Arabidopsis thaliana Through Auxin, Cytokinin, and Brassinosteroid Pathways. J. Plant Growth Regul. 2014, 34, 158–168. [Google Scholar] [CrossRef]
- Beckers, G.J.; Jaskiewicz, M.; Liu, Y.; Underwood, W.R.; He, S.Y.; Zhang, S.; Conrath, U. Mitogen-Activated Protein Kinases 3 and 6 Are Required for Full Priming of Stress Responses in Arabidopsis thaliana. Plant Cell 2009, 21, 944–953. [Google Scholar] [CrossRef] [Green Version]
- Tateda, C.; Zhang, Z.; Shrestha, J.; Jelenska, J.; Chinchilla, D.; Greenberg, J.T. Salicylic Acid Regulates Arabidopsis Microbial Pattern Receptor Kinase Levels and Signaling. Plant Cell 2014, 26, 4171–4187. [Google Scholar] [CrossRef] [Green Version]
- Bonanomi, G.; D’Ascoli, R.; Scotti, R.; Gaglione, S.; Caceres, M.G.; Sultana, S.; Scelza, R.; Rao, M.A.; Zoina, A. Soil quality recovery and crop yield enhancement by combined application of compost and wood to vegetables grown under plastic tunnels. Agric. Ecosyst. Environ. 2014, 192, 1–7. [Google Scholar] [CrossRef]
- Haas, D.; Défago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Genet. 2005, 3, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Legrand, F.; Picot, A.; Díaz, J.F.C.; Chen, W.; Le Floch, G. Challenges facing the biological control strategies for the management of Fusarium Head Blight of cereals caused by F. graminearum. Biol. Control 2017, 113, 26–38. [Google Scholar] [CrossRef]
- Ullah, H.; Yasmin, H.; Mumtaz, S.; Jabeen, Z.; Naz, R.; Nosheen, A.; Hassan, M.N. Multitrait Pseudomonas spp. Isolated from Monocropped Wheat (Triticum aestivum) Suppress Fusarium Root and Crown Rot. Phytopathology 2020, 110, 582–592. [Google Scholar] [CrossRef]
- Sharma, A.; Johri, B. Growth promoting influence of siderophore-producing Pseudomonas strains GRP3A and PRS9 in maize (Zea mays L.) under iron limiting conditions. Microbiol. Res. 2003, 158, 243–248. [Google Scholar] [CrossRef]
- Lambrese, Y.; Guiñez, M.; Calvente, V.; Sansone, G.; Cerutti, S.; Raba, J.; Sanz, M.I. Production of siderophores by the bacterium Kosakonia radicincitans and its application to control of phytopathogenic fungi. Bioresour. Technol. Rep. 2018, 3, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Thakur, S.; Dhingra, G.; Singh, A.; Pal, M.K.; Harshvardhan, K.; Dubey, R.; Maheshwari, D. Inoculation of siderophore producing rhizobacteria and their consortium for growth enhancement of wheat plant. Biocatal. Agric. Biotechnol. 2018, 15, 264–269. [Google Scholar] [CrossRef]
- Sayyed, R.Z.; Chincholkar, S.B.; Reddy, M.S.; Gangurde, N.S.; Patel, P.R. Siderophore Producing PGPR for Crop Nutrition and Phytopathogen Suppression. In Bacteria in Agrobiology: Disease Management; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 449–471. ISBN 978-3-642-33638-6. [Google Scholar]
- Trapet, P.; Avoscan, L.; Klinguer, A.; Pateyron, S.; Citerne, S.; Chervin, C.; Mazurier, S.; Lemanceau, P.; Wendehenne, D.; Besson-Bard, A. The Pseudomonas fluorescens Siderophore Pyoverdine Weakens Arabidopsis thaliana Defense in Favor of Growth in Iron-Deficient Conditions. Plant Physiol. 2016, 171, 675–693. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.J.; Silva, H.; Cunha, A. Siderophore-Producing Rhizobacteria as a Promising Tool for Empowering Plants to Cope with Iron Limitation in Saline Soils: A Review. Pedosphere 2019, 29, 409–420. [Google Scholar] [CrossRef]
- Morot-Gaudry, J.F. Nitrogen Assimilation by Plants: Physiological, Biochemical, and Molecular Aspects; CRC Press: Boca Raton, FL, USA, 2001; ISBN 978-1-4822-7984-9. [Google Scholar]
- Söğüt, S.; Çiğ, F. Determination of the effect of plant growth promoting bacteria on wheat (Triticum aestivum L.) Development under salinity stress conditions. Appl. Ecol. Environ. Res. 2019, 17, 1129–1141. [Google Scholar] [CrossRef]
- Barh, D.; Azevedo, V. Omics Technologies and Bio-Engineering: Towards Improving Quality of Life; Barh, D., Azevedo, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, ISBN 978-0-12-815899-9. [Google Scholar]
- Takenaka, S.; Nishio, Z.; Nakamura, Y. Induction of Defense Reactions in Sugar Beet and Wheat by Treatment with Cell Wall Protein Fractions from the Mycoparasite Pythium oligandrum. Phytopathology 2003, 93, 1228–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaeel, Q.; Pupin, M.; Jacques, P.; Leclère, V. Nonribosomal peptides and polyketides of Burkholderia: New compounds potentially implicated in biocontrol and pharmaceuticals. Environ. Sci. Pollut. Res. 2017, 25, 29794–29807. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.; Camele, I. An Overview of Metabolic Activity, Beneficial and Pathogenic Aspects of Burkholderia spp. Metabolites 2021, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Saad, M. Destruction of Rhizoctonia Solani and Phytophthora Capsici Causing Tomato Root-Rot by Pseudomonas fluorescences Lytic Enzymes. Res. J. Agric. Biol. Sci. 2006, 2, 274–281. [Google Scholar]
- Zhao, Y.; Selvaraj, J.N.; Xing, F.; Zhou, L.; Wang, Y.; Song, H.; Tan, X.; Sun, L.; Sangare, L.; Folly, Y.M.E.; et al. Antagonistic Action of Bacillus subtilis Strain SG6 on Fusarium graminearum. PLoS ONE 2014, 9, e92486. [Google Scholar] [CrossRef]
- Beauregard, P.B.; Chai, Y.; Vlamakis, H.; Losick, R.; Kolter, R. Bacillus subtilis biofilm induction by plant polysaccharides. Proc. Natl. Acad. Sci. USA 2013, 110, E1621–E1630. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.-H.; Xu, J.-L.; Li, X.-Z.; Zhang, L.-H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 2000, 97, 3526–3531. [Google Scholar] [CrossRef]
- Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial Volatiles: Small Molecules with an Important Role in Intra- and Inter-Kingdom Interactions. Front. Microbiol. 2017, 8, 2484. [Google Scholar] [CrossRef]
- Farag, M.A.; Ryu, C.-M.; Sumner, L.W.; Paré, P.W. GC–MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry 2006, 67, 2262–2268. [Google Scholar] [CrossRef]
- Li, X.-Y.; Mao, Z.-C.; Wu, Y.-X.; Ho, H.-H.; He, Y.-Q. Comprehensive volatile organic compounds profiling of Bacillus species with biocontrol properties by head space solid phase microextraction with gas chromatography-mass spectrometry. Biocontrol Sci. Technol. 2014, 25, 132–143. [Google Scholar] [CrossRef]
- Boukaew, S.; Cheirsilp, B.; Prasertsan, P.; Yossan, S. Antifungal effect of volatile organic compounds produced by Streptomyces salmonis PSRDC-09 against anthracnose pathogen Colletotrichum gloeosporioides PSU-03 in postharvest chili fruit. J. Appl. Microbiol. 2021, 131, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yuan, J.; E, Y.; Raza, W.; Shen, Q.; Huang, Q. Effects of volatile organic compounds from Streptomyces albulus NJZJSA2 on growth of two fungal pathogens. J. Basic Microbiol. 2015, 55, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
- Esmaeel, Q.; Pupin, M.; Kieu, N.P.; Chataigné, G.; Béchet, M.; Deravel, J.; Krier, F.; Höfte, M.; Jacques, P.; Leclère, V. Burkholderia genome mining for nonribosomal peptide synthetases reveals a great potential for novel siderophores and lipopeptides synthesis. MicrobiologyOpen 2016, 5, 512–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimkić, I.; Janakiev, T.; Petrović, M.; Degrassi, G.; Fira, D. Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms—A review. Physiol. Mol. Plant Pathol. 2021, 117, 101754. [Google Scholar] [CrossRef]
- Hoskisson, P.A.; van Wezel, G.P. Streptomyces coelicolor. Trends Microbiol. 2019, 27, 468–469. [Google Scholar] [CrossRef]
- Andrić, S.; Meyer, T.; Ongena, M. Bacillus Responses to Plant-Associated Fungal and Bacterial Communities. Front. Microbiol. 2020, 11, 1350. [Google Scholar] [CrossRef]
- Oni, F.E.; Esmaeel, Q.; Onyeka, J.T.; Adeleke, R.; Jacquard, C.; Clement, C.; Gross, H.; Barka, E.A.; Höfte, M. Pseudomonas Lipopeptide-Mediated Biocontrol: Chemotaxonomy and Biological Activity. Molecules 2022, 27, 372. [Google Scholar] [CrossRef]
- Höfte, M. The Use of Pseudomonas spp. as Bacterial Biocontrol Agents to Control Plant Diseases. In Burleigh Dodds Series in Agricultural Science; Köhl, J., Ed.; Wageningen University & Research: Wageningen, The Netherlands; Burleigh Dodds Science Publishing: Cambridge, UK, 2021; pp. 301–374. ISBN 978-1-78676-813-1. [Google Scholar]
- Pierson, I.L.S.; Pierson, E.A. Phenazine antibiotic production in Pseudomonas aureofaciens: Role in rhizosphere ecology and pathogen suppression. FEMS Microbiol. Lett. 1996, 136, 101–108. [Google Scholar] [CrossRef]
- Thomashow, L.S.; Weller, D.M. Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J. Bacteriol. 1988, 170, 3499–3508. [Google Scholar] [CrossRef] [Green Version]
- Keel, C.; Schnider, U.; Maurhofer, M.; Voisard, C.; Laville, J.; Burger, U.; Wirthner, P.J.; Haas, D.; Défago, G. Suppression of Root Diseases by Pseudomonas fluorescens CHA0: Importance of the Bacterial Secondary Metabolite 2,4-Diacetylphloroglucinol. Mol. Plant Microbe Interact. 1992, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Keel, C.; Oberhansli, T.; Haas, D.; Defag, G.; Wirthner, P. Pseudomonads as Antagonists of Plant Pathogens in the Rhizosphere: Role of the Antibiotic 2,4-Diacetylphloroglucinol in the Suppression of Black Root Rot of Tobacco. Symbiosis 1990, 9, 327–341. [Google Scholar]
- Baron, S.S.; Terranova, G.; Rowe, J.J. Molecular mechanism of the antimicrobial action of pyocyanin. Curr. Microbiol. 1989, 18, 223–230. [Google Scholar] [CrossRef]
- Baron, S.S.; Rowe, J.J. Antibiotic action of pyocyanin. Antimicrob. Agents Chemother. 1981, 20, 814–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLoughlin, A.J. Plasmid stability and ecological competence in recombinant cultures. Biotechnol. Adv. 1994, 12, 279–324. [Google Scholar] [CrossRef]
- Chin-A-Woeng, T.F.C.; Bloemberg, G.V.; Lugtenberg, B.J.J. Phenazines and their role in biocontrol by Pseudomonas bacteria. New Phytol. 2003, 157, 503–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, T.H.; Sørensen, D.; Tobiasen, C.; Andersen, J.B.; Christophersen, C.; Givskov, M.; Sørensen, J. Antibiotic and Biosurfactant Properties of Cyclic Lipopeptides Produced by Fluorescent Pseudomonas spp. from the Sugar Beet Rhizosphere. Appl. Environ. Microbiol. 2002, 68, 3416–3423. [Google Scholar] [CrossRef] [Green Version]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef]
- Anckaert, A.; Arias, A.A.; Hoff, G.; Calonne-Salmon, M.; Declerck, S.; Ongena, M. The Use of Bacillus spp. as Bacterial Biocontrol Agents to Control Plant Diseases. In Burleigh Dodds Series in Agricultural Science; Köhl, J., Ed.; Wageningen University & Research: Wageningen, The Netherlands; Burleigh Dodds Science Publishing: Cambridge, UK, 2021; pp. 247–300. ISBN 978-1-78676-813-1. [Google Scholar]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef] [Green Version]
- Falardeau, J.; Wise, C.; Novitsky, L.; Avis, T.J. Ecological and Mechanistic Insights Into the Direct and Indirect Antimicrobial Properties of Bacillus subtilis Lipopeptides on Plant Pathogens. J. Chem. Ecol. 2013, 39, 869–878. [Google Scholar] [CrossRef]
- Parent, Z.K. Potentiel de Bacillus Amyloliquefaciens Pour Lutter Contre les Maladies Fongiques Endémiques du Maïs au Sud Kivu: Efficacité et Mode d’Action. Ph.D. Thesis, Liège University Gembloux Agro-Bio Tech, Gembloux, Belgium, 2017. Available online: https://hdl.handle.net/2268/213753 (accessed on 4 January 2022).
- Dunlap, C.; Bowman, M.J.; Schisler, D.A. Genomic analysis and secondary metabolite production in Bacillus amyloliquefaciens AS 43.3: A biocontrol antagonist of Fusarium head blight. Biol. Control 2013, 64, 166–175. [Google Scholar] [CrossRef]
- Deleu, M.; Paquot, M.; Nylander, T. Fengycin interaction with lipid monolayers at the air–aqueous interface—Implications for the effect of fengycin on biological membranes. J. Colloid Interface Sci. 2004, 283, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Peypoux, F.; Bonmatin, J.M.; Wallach, J. Recent trends in the biochemistry of surfactin. Appl. Microbiol. Biotechnol. 1999, 51, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Buchoux, S.; Him, J.L.K.; Garnier, M.; Tsan, P.; Besson, F.; Brisson, A.; Dufourc, E.J. Surfactin-Triggered Small Vesicle Formation of Negatively Charged Membranes: A Novel Membrane-Lysis Mechanism. Biophys. J. 2008, 95, 3840–3849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maget-Dana, R.; Thimon, L.; Peypoux, F.; Ptak, M. Surfactin/iturin A interactions may explain the synergistic effect of surfactin on the biological properties of iturin A. Biochimie 1992, 74, 1047–1051. [Google Scholar] [CrossRef]
- Cawoy, H.; Mariutto, M.; Henry, G.; Fisher, C.; Vasilyeva, N.; Thonart, P.; Dommes, J.; Ongena, M. Plant Defense Stimulation by Natural Isolates of Bacillus Depends on Efficient Surfactin Production. Mol. Plant Microbe Interact. 2014, 27, 87–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bais, H.P.; Fall, R.; Vivanco, J.M. Biocontrol of Bacillus subtilis against Infection of Arabidopsis Roots by Pseudomonas syringae Is Facilitated by Biofilm Formation and Surfactin Production. Plant Physiol. 2004, 134, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Kinsinger, R.F.; Shirk, M.C.; Fall, R. Rapid Surface Motility in Bacillus subtilis Is Dependent on Extracellular Surfactin and Potassium Ion. J. Bacteriol. 2003, 185, 5627–5631. [Google Scholar] [CrossRef] [Green Version]
- Leclère, V.; Marti, R.; Béchet, M.; Fickers, P.; Jacques, P. The lipopeptides mycosubtilin and surfactin enhance spreading of Bacillus subtilis strains by their surface-active properties. Arch. Microbiol. 2006, 186, 475–483. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [Green Version]
- Hathout, A.S.; Abel-Fattah, S.M.; Abou-Sree, Y.H.; Fouzy, A.S. Incidence and exposure assessment of aflatoxins and ochratoxin A in Egyptian wheat. Toxicol. Rep. 2020, 7, 867–873. [Google Scholar] [CrossRef]
- Audenaert, K.; Vanheule, A.; Höfte, M.; Haesaert, G. Deoxynivalenol: A Major Player in the Multifaceted Response of Fusarium to Its Environment. Toxins 2014, 6, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoev, S.D. Food Safety and Increasing Hazard of Mycotoxin Occurrence in Foods and Feeds. Crit. Rev. Food Sci. Nutr. 2013, 53, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Fan, Y.; Zhao, L. Review on biological degradation of mycotoxins. Anim. Nutr. 2016, 2, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Altalhi, A.D. Plasmid-mediated Detoxification of Mycotoxin Zearalenone in Pseudomonas sp. ZEA-1. Am. J. Biochem. Biotechnol. 2007, 3, 150–158. [Google Scholar] [CrossRef]
- Yi, P.-J.; Pai, C.-K.; Liu, J.-R. Isolation and characterization of a Bacillus licheniformis strain capable of degrading zearalenone. World J. Microbiol. Biotechnol. 2010, 27, 1035–1043. [Google Scholar] [CrossRef]
- Cho, K.J.; Kang, J.S.; Cho, W.T.; Lee, C.H.; Ha, J.K.; Bin Song, K. In vitro degradation of zearalenone by Bacillus subtilis. Biotechnol. Lett. 2010, 32, 1921–1924. [Google Scholar] [CrossRef]
- Sato, I.; Ito, M.; Ishizaka, M.; Ikunaga, Y.; Sato, Y.; Yoshida, S.; Koitabashi, M.; Tsushima, S. Thirteen novel deoxynivalenol-degrading bacteria are classified within two genera with distinct degradation mechanisms. FEMS Microbiol. Lett. 2011, 327, 110–117. [Google Scholar] [CrossRef] [Green Version]
- He, J.W.; Bondy, G.S.; Zhou, T.; Caldwell, D.; Boland, G.J.; Scott, P.M. Toxicology of 3-epi-deoxynivalenol, a deoxynivalenol-transformation product by Devosia mutans 17-2-E-8. Food Chem. Toxicol. 2015, 84, 250–259. [Google Scholar] [CrossRef]
- Shima, J.; Takase, S.; Takahashi, Y.; Iwai, Y.; Fujimoto, H.; Yamazaki, M.; Ochi, K. Novel detoxification of the trichothecene mycotoxin deoxynivalenol by a soil bacterium isolated by enrichment culture. Appl. Environ. Microbiol. 1997, 63, 3825–3830. [Google Scholar] [CrossRef] [Green Version]
- Platel, R.; Lucau-Danila, A.; Baltenweck, R.; Maia-Grondard, A.; Chaveriat, L.; Magnin-Robert, M.; Randoux, B.; Trapet, P.; Halama, P.; Martin, P.; et al. Bioinspired Rhamnolipid Protects Wheat Against Zymoseptoria tritici Through Mainly Direct Antifungal Activity and without Major Impact on Leaf Physiology. Front. Plant Sci. 2022, 13, 1530. [Google Scholar] [CrossRef]
- Riseh, R.S.; Skorik, Y.A.; Thakur, V.K.; Pour, M.M.; Tamanadar, E.; Noghabi, S.S. Encapsulation of Plant Biocontrol Bacteria with Alginate as a Main Polymer Material. Int. J. Mol. Sci. 2021, 22, 11165. [Google Scholar] [CrossRef] [PubMed]



| Products Name | Company | Beneficial Microbes | Pathogen | Targeted Crop | Mode of Action for Biocontrol |
|---|---|---|---|---|---|
| Polyversum® | De Sangosse | Pythium oligandrum M1 | Fusarium and Sclerotina | Wheat, barley, and colza |
|
| Mycostop® | Lallemend | Streptomyces sp. K61 | Fusarium | Wheat, corn, barley, sugar-beet, and tomato |
|
| Inatreq™ Active® | Corteva | Fenpicoxamid from fermentation broths of the Streptomyces sp. 517–02 | Septoria | Wheat |
|
| Cerall, Cedomon® | Koppert | Pseudomonas chlororaphis MA342 | Fusarium spp., Septoria, wheat bunt and Drechslera teres | Wheat, barley, triticale, and rye |
|
| Strains | Origin | Pathogen | Biostimulation | Mode of Action for Biocontrol | Methodology | References |
|---|---|---|---|---|---|---|
| Bacillus subtilis ATCC 10783; B. cereus ATCC 11778; B. licheniformis NRRLB-510; B. pumilus ATCC 7061; Brevibacillus laterosporus BLA170; Paenibacillus polymyxa NA | Soil | Zymoseptoria tritici, Pyrenophora tritici-repentis, Cochliobolus sativus, Alternaria triticimaculans | n/a | n/a | In vitro and greenhouse (leaves) | [121] |
| B. subtilis BBG125, BBG131, Bs2504 | ProBioGEM, Centre Wallon de Biologie Industrielle | Z. tritici | n/a | Lipopeptides (LPs): mycosubtilin, surfactin, fengycin | In vitro and greenhouse (leaves) | [122] |
| B. velezensis RC 218 | Wheat anthers | Fusarium graminearum | n/a | Ericin lantiobiotic Plant phytohormone modulation (jasmonic and salicylic acid) ISR cell wall thickening preventing cell plasmolysis and collapse | Field and greenhouse (spikes) | [123,124,125,126] |
| B. velezensis LM2303 | Wild yak | F. graminearum | n/a | n/a | In silico and field (spikes) | [127] |
| B. subtilis IB | Soil | F. graminearum | n/a | Fengycin | In vitro | [128] |
| B. megaterium BM1 and B. subtilis BS43, BSM0, BSM2 | Wheat spikes | F. graminearum | n/a | Degradation of DON Metabolites | In vitro and field (spikes) | [129] |
| B. subtilis BaSu1/BaSu3, B. amyloliquefaciens BaAm and Chaetomium globosum CG1/CG2, Phoma glomerata PG1, Aureobasidium proteae AP5 and Sarocladium kiliense SK1/SK2 | Wheat endosphere | F. graminearum and F. culmorum | n/a | Antibiosis | In vitro and greenhouse (detached wheat spikelets) | [22] |
| B. amyloliquefaciens TrigoCor | Wheat rhizosphere | F. graminearum | n/a | Iturin | Field and greenhouse (spikes) | [130] |
| B. amyloliquefaciens S76-3 | Wheat spikes | F. graminearum | n/a | Iturin A and plipastatin | In vitro | [131] |
| B. amyloliquefaciens Y1 | Soil | F. graminearum | n/a | Metabolites Cyclo D-PRO-L- VAL | In vitro | [132] |
| B. amyloliquefaciens B8 and B3 | Soil | F. graminearum and culmorum | Yes | Phytohormones | In vitro and greenhouse | [133] |
| B. amyloliquefaciens BLB369, B. subtilis BLB277, Pae. polymyxa BLB267 | Soil | F. graminearum | Yes | Supernatant (iturin and surfactin, fengycin, fusaricidin and polymyxin) | In vitro and greenhouse | [134] |
| B. megaterium MKB135 Pseudomonas fluorescens MKB21 and MKB91 | Cereal rhizospheres, leaves, grain and weeds | Z. tritici | Yes | Cell free surpernantant and VOC | Field and greenhouse (leaves) | [135] |
| B. subtilis strains 53 and 71, P. fluorescens biov1 strain 32 and Streptomyces sp. strain 3 | Wheat kernels | F. graminearum | Yes | Volatiles metabolites | In vitro and greenhouse (spikes) | [136] |
| Antibiotics tubercidin, phosphlactomycin and candicidin, 2,4-diacetylphloroglucinol, phenasin, fengymcine, bacillomycin | ||||||
| Phytohormone regulation | ||||||
| B. subtilis RC 218, S. sp. BRC87B. and Brevibacillus sp. BRC263 | Wheat anthers | F. graminearum | Yes | Space and nutrients competition | Field, greenhouse and in vitro | [125,126,137,138,139,140] |
| Metabolites | ||||||
| Streptomyces. sp. DEF09 | Wheat root | F. graminearum | Yes | Metabolites | Field, greenhouse (spikes) and in vitro | [141] |
| IAA | ||||||
| Streptomyces. sp. BN1 | Rice kernels | F. graminearum | n/a | n/a | In vitro and greenhouse (seeds and spikes) | [142] |
| B. cereus | Soil from wheat fields | F. graminearum | Yes | Dose and cultivar dependent | In vitro and greenhouse (seeds) | [143] |
| B. subtilis AS43.3/AS43.4, Cryptococcus sp. OH71.4 and Cryptococcus nodaensis OH182.9 | Wheat anthers | F. graminearum | n/a | n/a | Field, greenhouse (spikes) and in vitro | [144,145] |
| Co-cultures of B. subtilis OH 131.1 and Cryptococcus flavescens OH 182.9 | ARS NRRL | F. graminearum | n/a | Plipastatin and subtilomycin | Greenhouse (spikes) | [146] |
| Lactobacillus brevis JJ2P; Lactobacillus reuteri R2 | Porcine gut, cheese | Z. tritici | n/a | Cell free supernatant (phenyllactic acid and hydroxyphenyllactic acid) | In vitro and greenhouse (leaves) | [147] |
| Lactobacillus plantarum strain 21B | Sourdough breads | F. graminearum | n/a | Antifungal phenyllactic acid and 4-hydroxyphenyllactic acid | In vitro | [148] |
| Paenibacillus polymyxa SGK2 | Wheat rhizosphere | F. graminearum, F. culmorum, F. verticillioides | Yes | Competition for nutrients (iron) | In vitro and greenhouse (seeds) | [149] |
| Pae. polymyxa W1-14-3 and C1-8-b | Rhizosphere | F. graminearum | Yes | Inhibition of fungal germination | In vitro and greenhouse (spikes) | [150] |
| glucanolytic enzyme, cellulase, mannanase xylase, chitinase and protease | ||||||
| supernatant activity (enzymatic or antibiotic activities: polymyxins, benzoic acid, fusaricidin A and antibiotic peptides) | ||||||
| Pae. sp. B2 | INRAE Dijon | Z. tritici | n/a | ISR | Field and greenhouse (leaves) | [151] |
| P. fluorescens LEC1 | Wheat phyllosphere | Z. tritici | n/a | Antibiotics 1- hydroxyphenazine and chlororaphin | In vitro and greenhouse (leaves) | [152] |
| P. fluorescens PFM2 | Wheat phyllosphere | M. graminicola | n/a | Antibiotics 2-4-diacetylphoroglucinol and phenazine-l-carboxylic acid | In vitro | [153] |
| P sp. AS 64.4 | Wheat anthers | F. graminearum | n/a | Nutrients competition (choline metabolizing strain) | Field, greenhouse (spikes) and in vitro | [154] |
| P. putida BK8661 | Wheat phyllosphere | Z. tritici | Yes | HCN, siderophore, antibiotics | In vitro and greenhouse (leaves) | [155] |
| P. aeruginosa LEC1 | Soil | Z. tritici | n/a | Antibiotic (Pyocyanine) and Siderophore (pyoverdine) | In vitro and field (leaves) | [156] |
| P. chlororaphis MA 342 | Craw berry rhizosphere | Septoria nodorum | n/a | n/a | Field (seeds) | [157] |
| P. piscium ZJU60 | Wheat anthers | F. graminearum | n/a | Phenazine-1-carboxamide | Field and greenhouse (spikes) | [158] |
| P. fluorescens LY1-8 | Wheat tissues | F. graminearum | n/a | Extracellular hydrolytic enzymes (protease, chitinase, cellulose, glucanase and siderophore) and antagonistic activity | Field and greenhouse (spikes) | [159] |
| Devosia sp. strain NKJ1 and Nocardioides spp. strains SS3 or SS4 | Wheat field soil | F. graminearum | Yes | Degradation of DON | In vitro | [160] |
| Strains | Origin | Pathogen | Biostimulation | Mode of Action of Biocontrol | M&M | Source |
|---|---|---|---|---|---|---|
| Pseudomonas fluorescens MKB100 and MKB156 | Cereal rhizosphere | P. teres | n/a | ISR | Field, greenhouse (leaves and drenching) and in vitro | [161] |
| Production of antifungal compounds (2,4-DAPG and HCN) | ||||||
| Pseudomonas chlororaphis MA 342 | Craw berry rhizosphere | D. teres | n/a | n/a | Field and greenhouse (seeds) | [157,162,163] |
| D. graminea | ||||||
| U. hordei | ||||||
| Paenibacillus polymyxa KaI245 | Sorghum rhizosphere | Drechsclera teres f. sp. teres and Rhynchosporium commune | Yes | Cell free supernatant | In vitro and greenhouse (leaves) | [164] |
| Burkholderia sp. strain BE25 | Maize rhizosphere | P. teres | Yes | Induction plant genes defense | In vitro and greenhouse (leaves) | [165,166] |
| Limitation of the fungus on photosynthetic and respiratory parameters |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutilloy, E.; Oni, F.E.; Esmaeel, Q.; Clément, C.; Barka, E.A. Plant Beneficial Bacteria as Bioprotectants against Wheat and Barley Diseases. J. Fungi 2022, 8, 632. https://doi.org/10.3390/jof8060632
Dutilloy E, Oni FE, Esmaeel Q, Clément C, Barka EA. Plant Beneficial Bacteria as Bioprotectants against Wheat and Barley Diseases. Journal of Fungi. 2022; 8(6):632. https://doi.org/10.3390/jof8060632
Chicago/Turabian StyleDutilloy, Emma, Feyisara Eyiwumi Oni, Qassim Esmaeel, Christophe Clément, and Essaid Ait Barka. 2022. "Plant Beneficial Bacteria as Bioprotectants against Wheat and Barley Diseases" Journal of Fungi 8, no. 6: 632. https://doi.org/10.3390/jof8060632