3.1. Characteristics of the Cereal Mixtures
This work aimed to highlight any differences in the final product resulting from the use of three different species of
Triticum, also considering the hulled emmer (
Triticum dicoccum). The intention of the raw material analysis shown in
Table 1;
Table 2 is to describe the composition of the starting cereal materials and to give an insight into some of the components responsible for the colloidal stability, such as proteins, polyphenols, and β-glucans [
26], with the last two classes of compounds also known for their bioactive and antioxidant effects and nutritional value [
27].
Em had the lowest moisture and protein content and the highest total phenolic content, while
Da showed the highest ash and the lowest β–glucan content, and intermediate total phenolic content. The interest towards these compounds is related to their role in haze formation, as proteins conjoined with polyphenols (mainly proanthocyanidins and polymeric forms) are the major colloidal haze components in Belgian white beers with a certain contribution of glucose polymers (mainly starch or degraded starch and only to a minor extent β-glucans) [
28]. Although the polymeric phenols have not been quantified in raw materials, the highest total phenolic content retrieved in the durum and common wheat can be considered as indicators of higher haze formation in the corresponding future beers with respect to those produced with unmalted emmer. The known role of high-molecular-weight (HMW) proteins as precursors of haze-active proteins [
26] and the higher HMW glutenin subunits retrieved in durum and common wheat in respect to emmer [
29] reinforce this prediction, with the same brewing process applied.
The interest towards phenolic content and profile of the raw materials is influenced by their contribution to polyphenols of the final beer considering that about 70% of beer phenolics come from the mashed cereal mixtures because of their significantly higher starting amount [
30]. In terms of total phenols,
Em had the highest concentrations followed by, in a decreasing order,
Da and
Ri(
Table 2). Since phenolic compounds are the most important group of antioxidants [
31], it was expected that the antioxidant activity of cereal mixtures was related to their total phenolic concentration. Instead, the remaining concentration of the DPPH radical (expected to be inversely proportional to the phenolic concentration) was, in a decreasing order,
Da,
Ri, and
Em, and further quite different results were obtained by expressing the antioxidant activity in terms of Trolox equivalents, with
Da highlighting the statistically significantly lowest values. This only apparent inconsistency depends on three factors: the different antioxidant assays applied; the different qualitative phenolic composition of the three cereal mixtures; the different phenolic distribution among free, conjugated, and bond forms of the three cereal mixtures. It is well known that different phenolic acids have different antioxidant activity because substituents on their aromatic rings affect the stabilization of structure and consequently their radical-quenching ability [
32]. Rice-Evans et al. established a certain antioxidant activity hierarchy of phenolics measured as Trolox Equivalent with, citing some of those found in our cereal mixtures: rutin and catechin > p-coumaric acid > ferulic acid > chlorogenic and caffeic acids [
33]. Although the phenolics retrieved in the highest concentrations were generally the same in the three considered mixtures (p-hydroxybenzoic, caffeic, and ferulic acids),
Em differed from the others for the high concentration of 3,4 hydroxybenzoic acid (
Table 2). Moreover, significant differences in the percentage of the free phenolic forms were highlighted among the three mixtures, with the following decreasing concentration order:
Em (78%) >
Ri (74%) >
Da (71%).
3.2. Physicochemical Characteristics of the Finished Beers
The results of the physicochemical analyses of the 12 finished beers are reported in
Table 3. As can be inferred from the data, the interactive effects of cereal mixtures, hops, and yeast strains were statistically significant on all parameters.
Table 3 also includes the single effects of the independent variables, which will be discussed from time to time for those effects that are significant not only in statistical point terms. The average pH values were in the 3.76–4.26 range and these results are consistent with what is found in commercial Belgian white beers [
28]. Types of cereal mixtures also exerted significant single effects on pH with the lowest values found for
Em, as a consequence of its lower buffering potential, which in turn is due to its lower protein content. The beer soluble solid content ranged from 2.72 to 5.83 °Bx and significant single effects were exerted by cereal mixture and yeasts, with the lowest concentrations found for
Da and
Ci, respectively. The effect of
Ci yeast was related to its higher attenuation ability with respect to
Wi that leaves residual sugars. Dry matter varied from 1.83 (
RiCoWi) and 7.56 (
EmCaWi) % but this variability cannot be attributed to any of the independent variables. The colour of beers showed a great variability (2.62–8.45 EBC), with significant single effects exerted by cereal mixtures (respectively higher and lower when
Da and
Em are used), hop (higher when Cascade was used), and yeast (higher when the cider yeast was used). It is known that the beer colour depends mainly on the cereals used in the wort production process, in particular on their pigment content. The cereal mixtures used in this work had the following concentrations of carotenoids: 3.3, 3.0, and 2.6 μg/g for
Da,
Ri, and
Em, respectively). It is also known that hopping with bitter hops led to a significant darkening of wort but in the present work, the bitter
Co was added in lower amount that
Ca in order to obtain the same final IBU [
34]. Concerning the effect of yeast, it has been reported that high fermentation yeasts (as
Wi is) produce beers with higher values of absorbance due to the browning and oxidation of the melanoidins [
35].
The carbon dioxide in the investigated beers was in the range 2.55 (RiCoCi)-7.80 (EmCaCi) g/L. Carbonation contributes to the refreshing effect of a traditional wheat beer, which is why beers with a low level of carbonation are not accepted by consumers. Em and Wi positively affected CO2 content, the latter because it is specifically selected to produce carbonated beer (a rapid CO2 production in moderate sugar medium is an important phenotype in strains suitable for producing sparkling beverages).
Alcohol content was comprised between 2.81 (
EmCoCi) and 4.86 (
RiCoCi) % but 10 of the 12 types of beers produced had values lower than 4.5%. Alcohol content of traditional Belgian-type white beer is generally in the range 4.5–5.5%. Concerning the single effect of cereal mixture, the highest alcohol content of
Ri and
Da beers is explained by the higher carbohydrate contents in common (75%) and durum wheat (71%) compared to those of ancient wheats such as emmer [
36].
Acidity is an important trait of white beers: they should have a moderate-to-high titratable acidity, but low-to-moderate volatile acidity since it is often an indicator of presence of acetic acid and other volatile components, such as acetaldehyde, responsible for off flavours. Very broad ranges of titratable (0.61–3.05 g/L) and volatile (0.30–2.59 g/L) acidity were observed, with the highest and the lowest values of both types of acidity found in
RiCaCi and
DaCaCi beers, respectively. Concerning the single effects of cereal mixture, the lowest values were found when
Da was used, as a consequence of the strong buffer potential of its protein. Regarding yeast, the highest values were found when
Wi strain was used, as expected by a yeast specifically selected as Belgian wit yeast. The discussion on the acidity of the finished beers can be deepened by considering their composition in organic acids (
Table 4). First, the following assumptions are necessary: among the six compounds detected, only fumaric acid showed the same concentration in all the samples; during mashing, the same amount of lactic acid was added to worts to bring their pH to a value close to 5.4. Some organic acids related to the Krebs cycle were quantified and were found to vary widely from a beer to another: citric (0.67–1.02), malic (0.59–1.54), succinic (0.86–2.97), lactic (0.68–1.36), and acetic (5.67–1.52). Concerning the single effect of cereal mixtures, the highest concentration of citric and malic acids and the lowest concentrations of succinic, lactic, and acetic acids were detected in
Da beers. Regarding the effect of yeast, the lowest concentration of citric acid and the highest concentrations of malic, succinic, and lactic and acetic acids were found in beers fermented by
Wi. This data could be explained with the ability of yeast metabolism to influence levels of beer organic acids, which has been documented for decades [
37], as well as the application of selection and crossing techniques to obtain yeasts with an overproduction of organic acids through a permanently altered carbon flow in the two branches of the tricarboxylic acid cycle.
The remaining carbohydrates from starch hydrolysis in the final beer mainly include non-fermentable dextrins (90%), which cannot be metabolized by yeast strains, and only low levels of fermentable sugars (maltotriose and maltose). They have been quantified in the produced beers (
Table 4). Maltodextrin has a moderately sweet taste and enhances the body and palate fullness of beer. The maltodextrin concentrations in the final beers ranged from 26.39 and 40.73 g/L and mainly depended on cereal mixtures (
Em >
Ri >
Da). The content of the fermentable sugar maltotriose ranged from 8.01 to 29.92 g/L and showed a behaviour similar to that of soluble solids. Maltose content was below the detection limit for all the beers, thus indicating that it was completely fermented by yeasts.
Finally,
Table 3 shows the glycerol concentrations of the beers, which ranged from 2.05 to 4.06 g/L and the most significant effect was exerted by yeast, with higher glycerol production highlighted by
Ci.
3.3. Phenolic Concentration and Antioxidant Activity of the Finished Beers
Together with its low alcohol content, the phenolic concentration and antioxidant activity of beer are relevant factors in terms of nutritional quality of beer [
30]. The total phenolic content of the beers was between 173 (
EmCoCi) and 364 mg/L, with the highest values found in
DaCoCi,
RiCoCi, and
EmCoWi (
Table 5). These are the results of interactions among cereal mixtures, hops, and yeasts, while the single effects of the same variables were not statistically significant. The TPCs were higher than those (76.8–177 mg/L) retrieved in the literature for both malted and unmalted wheat beers [
4,
38]. The remaining DPPH was in the range 51.29–73.23% while the millimoles of Trolox per L varied from 0.678 to 1.255. TPCs were not correlated with antioxidant activity while better correlations were found between the two ways in which antioxidant activity was measured, with the highest values detected in
DaCoCi and
RiCoWi and the lowest values observed in
DaCaCi beers. It is only an apparent discrepancy since the antioxidant power of a beer does not depend only on the contribution of phenolic compounds provided by the ingredients but is further influenced by the complex transformations that take place during brewing, which are well documented by the scientific literature. It has been observed that mashing-in temperature affects the release of polyphenols: temperature around 40–45 °C allows the activity of arabinoxylan- and protein-degrading enzymes and thus the release of phenolic acids bound to cell walls, polysaccharides, or proteins [
39]. Sparging, i.e., the step when the spent grain is washed through with hot water, is able to further recover phenolics. During boiling, the antioxidant activity increases thanks to the dissolution of hop polyphenols in the wort but, on the other hand, loss of polyphenols is experienced since they react with proteins and the resulting complexes precipitate and are separated from wort by whirlpooling [
40,
41]. However, always during boiling, Maillard reactions occur bringing to the formation of melanoidins that, together with phenolics, are the most important beer antioxidants [
42]. During fermentation, the antioxidant activity can remain unchanged or suffers a decrease. As a result, the overall brewing process reduces the initial content of total phenols by 50%.
All these changes strongly affected the phenolic profiles of the finished beers (
Table 5). Twelve phenolic compounds were detected in all the beers but in different concentrations depending on the starting cereal mixture, type of hop, and yeast strain: seven phenolic acids (gallic, 4-hydroxybenzoic, vanillic, caffeic, syringic, ferulic,
p-coumaric); two flavanols (catechin and epicatechin); two flavonols (quercetin and rutin); one hydroxystilbene (resveratrol). Eight of these compounds were also retrieved in the cereal mixtures used in brewing: catechin, rutin, and the phenolic acids gallic, vanillic, caffeic, syringic, ferulic, and
p-coumaric. Among these compounds, catechin and the phenolic acids, namely, gallic, vanillic, caffeic, ferulic, and
p-coumaric were also contributed by hops. Epicatechin, quercetin, 4-hydroxybenzoic, and resveratrol were hop-derived beer polyphenols. The contribution of the yeasts is limited from a quantitative point of view but can have an influence on the organoleptic characteristics of the beers: part of xanthumol and higher oligomeric proanthocyanidins are adsorbed to yeast cells during fermentation [
43,
44]; ferulic acid increases during fermentation, due to possible feruloyl esterase activity in yeast [
45].
The phenolics retrieved in the highest concentrations were, in a decreasing order, gallic acid (5.844–36.162 g/L), epicatechin (8.341–12.497 mg/L), rutin (2.574–10.126 mg/L), and 4-hydroxybenzoic acid (1.924–8.296mg/L). The other compounds were found in concentrations lower than 2.5 mg/L). Regarding the single effects exerted by the cereal mixtures on the beer phenolic profiles, Da allowed to obtain the highest concentrations of gallic acid, 4-hydroxybenzoic acid, syringic acid, rutin, resveratrol, and p-coumaric acid (together with Em), while Em showed the highest concentrations of catechin (together with Ri), caffeic acid, and quercetin. The single effects of the type of cereal mixtures on amounts of vanillic acid, epicatechin, and ferulic acid were not statistically significant. Concerning the single effect of hops, Ca beers showed the highest concentrations of gallic, 4-hydroxybenzoic, vanillic, syringic, and ferulic acids, rutin, and resveratrol while Co beers had the highest amounts of catechin. The effects of the type of hops on caffeic acid, p-coumaric acid, epicatechin, and quercetin were not significant. No differences were highlighted between the two yeast strains in terms of concentrations of caffeic acid, syringic acid, epicatechin, p-coumaric acid, and resveratrol. The highest concentrations of catechin were observed in beers fermented with Ci, while the highest concentrations of rutin, quercetin, and the phenolic acids, namely, gallic, 4-hydroxybenzoic, vanillic, ferulic were retrieved in beers fermented with Wi.
3.4. Sensory Characteristics of the Finished Beers
Table 6 shows the results of the sensory analysis. The evaluation of two visual (colour of foam and liquid) and eight gustatory (sweetness, bitterness, saltiness, hoppy, floral, fruity, spicy, and alcoholic) parameters showed high variability and thus any significant difference was highlighted between the 12 types of beer with reference to those characteristics. Regarding description, all the beers were characterized by a white foam and a straw yellow colour and were evaluated as moderately sweet, bitter, salty, and alcoholic. Moreover, moderate hoppy, floral, fruity, and spicy flavours were perceived.
Significant differences were highlighted for the other sensory characteristics. The amount of foam ranged between 1 and 5, while its persistence was in the range 1–4. Concerning the single effects, the highest amount of foam was observed in beers produced with
Da,
Ca, and
Wi. On the other hand, little foam was observed in beers produced with
Em. Among the cereal mixtures,
Da and
Ri highlighted the highest foam persistence, as well as Ca and Ci for the hops and yeast strain, respectively. Foaming in beer is mainly due to the interactions between proteins deriving from cereal mixtures and hop acids, while yeast proteins are mainly associated with foam stabilization [
46]. Modification of the barley occurring during germination causes protein hydrolysis, which negatively correlates with foam persistence, due to the degradation of foam-positive protein factors [
46]. The non-modified proteins of wheat added to over-modified malt ameliorates beer foaming characteristics also reducing the size of foam bubbles but the overall effect of wheat on foam stability depends on both the foaming potential of the barley malt and wheat variety used for brewing [
3]. Concerning β-glucans deriving from the starting cereal mixtures, neither foam enhancement nor foam stabilization has been documented [
47]. In the present work, foam persistence and amount correlated well with the protein content of the starting cereal mixtures. Concerning the effects of hop acids,
Ca had lower iso-α-acids than
Co (7.6% vs. 14.9%) but a higher amount of
Ca was used to obtain the same IBU values, thus the final α-acid concentration was similar.
Turbidity, with scores included between 1 and 5, was higher in
Da and
Ri beers, i.e., in beers produced from the starting cereal mixtures having the highest protein content and this result matches well with the finding that haze intensity in wheat beers is mainly governed by the wheat gluten content of the beer, which in turn depends on the protein content of the starting cereals [
48]. The single effect of yeasts was also significant, with the highest turbidity values observed in
Ci-fermented beers. A recent study of Huismann et al. successfully applied fractionation for purifying and isolating proteins from high and low haze beers [
49]. They observed two protein peaks in the high haze samples and one protein peak in the low haze ones, also discovering the presence of yeast cell wall mannoproteins.
The trained sensory panel also found remarkable differences in sourness/acidity of beers (scores from 2 to 5), with the highest intensity evaluated in Em beers, which were also the samples with the lowest pH values and the highest acetic acid contents. The highest intensity of the malty taste (whose scores ranged from 1 to 4) was attributed to Da beers, despite the maltose concentrations being under the detection limit for all the samples and the maltodextrins and maltotriose contents were higher in beers produced from Em and Ri cereal mixtures.
A great variability among beers was observed for effervescence (1 ≤ score ≤ 5) with a single significant effect exerted only by the type of hop. Beers produced with Ca were evaluated as the most effervescent, but that judgement was affected by the highest amount and stability of foam observed in those beers. The body ranged from 1 to 4, with the lowest scores obtained by Em beers, although they had the highest content of maltodextrins.
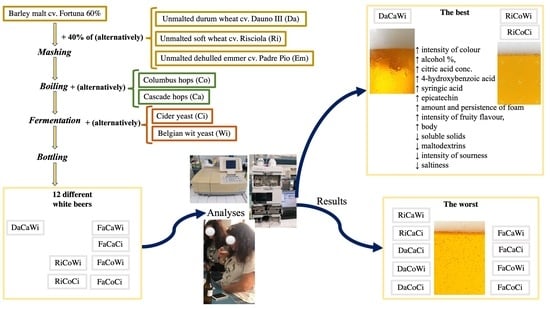
Finally, the best overall ratings were attributed to Ri and Da beers, which were the beers richer in foam, more full-bodied, less acidic, and (Da beers) with a more pronounced malt taste.
3.5. Statistical Data Evaluation
Principal Component Analysis was performed to visualize the relationship between the beers, their chemical composition, and their physical and sensory attributes.
Figure 1 shows a biplot of Factors 1 and 2 which accounted for 37.65% of the variance in the whole data set. Most of the samples are concentrated around the centre of the factorial plane and, having similar characteristics from each other, appear overlapped. Two clusters of beers stand out for their positioning in the factorial plan:
RiCo beers, which differ from the others for the negative loading of Factor 2; and
DaCaWi beers, with negative loadings of Factors 1 and 2 (upper left quadrant). The negative loading of Factor 2 is associated with low values of titratable and volatile acidity; intermediate amounts of dry matter, carbon dioxide, organic acids (citric, malic, succinic), maltotriose; intermediate turbidity, sourness, effervescence, and body; high values of pH, soluble solids, EBC colour, alcohol %, lactic acid, maltodextrins, glycerol, and overall quality. The upper left quadrant corresponded to low amounts of soluble solids, dry matter, succinic acid, maltodextrins and maltotriose, and low sourness intensity; intermediate contents of carbon dioxide and lactic acid and intermediate intensity of the malty taste; high values of pH, EBC colour, alcohol %, titratable and volatile acidity, organic acids (citric, malic, acetic), glycerol, foam development, turbidity, effervescence, body, and overall quality.
Pearson correlation coefficients were calculated to evaluate significant (
p < 0.01) correlations between pairs of variables. In the present work, the amount of foam was positively correlated with pH (0.60), alcohol % (0.42), and concentrations of gallic acid (0.61), 4-hydroxybenzoic acid (0.53), syringic acid (0.81), vanillic acid (0.46), epicatechin (0.69), and rutin (0.72), and negatively correlated with soluble solids (−0.58), dry matter (−0.45), and caffeic acid (−0.56). The persistence (stability) of foam was positively correlated with pH (0.57), and concentrations of gallic acid (0.64), 4-hydroxybenzoic acid (0.80), syringic acid (0.76), vanillic acid (0.53), epicatechin (0.68), and rutin (0.77), and negatively correlated with soluble solids (−0.64). A very high positive correlation was found between amount and stability of foam (0.82). The results are in agreement with those of Siebert [
50], who found that intermediate pH and ethanol levels lead to the best foam. Concerning the positive correlation among phenolic content and foam development, it should be considered as an indirect measure of the effect of the protein content exerted by the starting cereal mixtures which, at the same time, are responsible for contributing most of the phenolic component to the beer.
The titratable and volatile acidity were positively correlated with CO2 (0.60 and 0.64, respectively) and acetic acid (0.97 and 0.96, respectively) contents. The acetic acid content was negatively correlated with alcohol % (−0.41) since ethanol is firstly oxidized to acetaldehyde that in turn is oxidized to acetic acid.
The total phenolic content was positively correlated with alcohol % (0.42) since beers lower in alcohol are brewed starting from worts having lower original gravity. Furthermore, the total phenolic content and the antioxidant activity expressed as Trolox were negatively correlated with resveratrol concentrations (−0.42 and −0.56, respectively), a behaviour already observed in wines [
51].
Maltodextrin content was negatively correlated with amount and persistence of foam (−0.65 and −0.64, respectively) as well as maltotriose (−0.65 for the amount of foam; −0.54 for its persistence) in contrast with the use of maltodextrins to increase viscosity and stabilize foam of beer. Glycerol content was positively correlated with alcohol % (0.41) in agreement with the finding that the first is produced in great amount as a by-product of alcoholic fermentation.
Concerning sensory attributes, turbidity was positively correlated with pH (0.68) and alcohol % (0.67), and negatively correlated with acetic acid (−0.69), titratable acidity (−0.64), and volatile acidity (−0.69). These results are in agreement with Siebert and Lynn (2003) [
52], whose results indicate that pH and alcohol content are likely to influence both the size of colloidal particles and light scattering. The malty taste was negatively correlated with soluble solids (−0.53) since it would be emphasized by high amount of low-degraded starch molecules. Bitterness was positively correlated with effervescence (0.45), since it is reinforced by acidity. The fruity taste was positively correlated with alcohol % (0.61) since the synthesis of esters (compounds responsible for this taste) increases with fermentation. Body was negatively correlated with soluble solids (−0.44) and maltodextrins (−0.61) and these results contrasted with the practice to add maltodextrins in alcohol-free beers at the end of the dealcoholization process, just in order to increase body and mouthfeel of the beer.
Finally, the overall quality of beers was positively correlated with concentrations of citric acid (0.52), 4-hydroxybenzoic acid (0.53), syringic acid (0.57), epicatechin (0.43), alcohol % (0.52), colour (0.52), amount and persistence of foam (0.55 and 0.63, respectively), intensity of fruity flavour (0.52), body (0.81), and negatively correlated with soluble solids (−0.47), maltodextrin content (−0.48), and intensity of sourness (−0.41) and saltiness (−0.44).