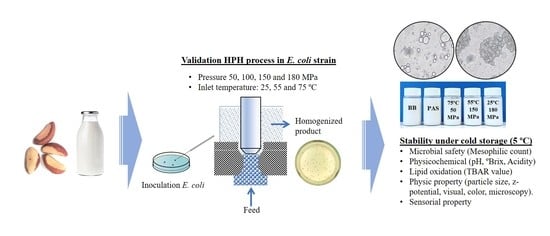
Validation of High-Pressure Homogenization Process to Pasteurize Brazil Nut (Bertholletia excelsa) Beverages: Sensorial and Quality Characteristics during Cold Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Solvents and Reagents
2.3. Production of Brazil Nut Beverages
2.4. Bacterial Strains and Inoculation
2.5. HPH Treatment and Thermal Pasteurization of Brazil Nut Beverage
2.6. Physicochemical Analysis
2.7. Microbiological Analysis
2.8. Color Measurement
2.9. Lipid Oxidation Analysis
2.10. Microstructure
2.11. Analysis of Particle Size and Electrical Charge
2.12. Sensory Evaluation
2.13. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical and Microbiological Characteristics of the Brazil Nut Beverage
3.2. Effect by HPH on Inoculated E. coli in Brazil Nut Beverage
3.3. Stability of the HPH-Treated Beverage in Cold Storage
3.3.1. Microbiological Stability
3.3.2. Physicochemical and Oxidative Stability
3.3.3. Physical Properties of Processed Brazil Nut Beverage
Color
Particle Size and Electrical Charge
Visual Stability and Microstructure
3.3.4. Sensorial Quality
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vasquez-Rojas, W.V.; Martín, D.; Miralles, B.; Recio, I.; Fornari, T.; Cano, M.P. Composition of Brazil Nut (Bertholletia excels HBK), Its Beverage and By-Products: A Healthy Food and Potential Source of Ingredients. Foods 2021, 10, 3007. [Google Scholar] [CrossRef]
- Mesa, J.; Barrera, C.; Betoret, E.; Betoret, N. High Homogenization Pressures to Improve Food. Molecules 2020, 25, 3305. [Google Scholar] [CrossRef]
- Patrignani, F.; Lanciotti, R. Applications of high and ultra high pressure homogenization for food safety. Front. Microbiol. 2016, 7, 1132. [Google Scholar] [CrossRef]
- European Community. Commission Regulation (EC) No 2073/2005: Microbiological Criteria for Foodstuffs; Agencia Estatal Boloetín Oficial del Estado: Madrid, Spain, 2005. [Google Scholar]
- BOE. Real Decreto 1338/1988: Reglamentación Técnico-Sanitaria para la Elaboración y Venta de Horchata de Chufa; Agencia Estatal Boloetín Oficial del Estado: Madrid, Spain, 1988. [Google Scholar]
- Codina-Torrella, I.; Guamis, B.; Zamora, A.; Quevedo, J.M.; Trujillo, A.J. Microbiological stabilization of tiger nuts’ milk beverage using ultra-high pressure homogenization. A preliminary study on microbial shelf-life extension. Food Microbiol. 2018, 69, 143–150. [Google Scholar] [CrossRef]
- Poliseli-Scopel, F.H.; Hernández-Herrero, M.; Guamis, B.; Ferragut, V. Comparison of ultra high pressure homogenization and conventional thermal treatments on the microbiological, physical and chemical quality of soymilk. LWT-Food Sci. Technol. 2012, 46, 42–48. [Google Scholar] [CrossRef]
- Ferragut, V.; Valencia-Flores, D.C.; Pérez-González, M.; Gallardo, J.; Hernández-Herrero, M. Quality characteristics and shelf-life of ultra-high pressure homogenized (Uhph) almond beverage. Foods 2015, 4, 159–172. [Google Scholar] [CrossRef]
- Valencia-Flores, D.C.; Hernández-Herrero, M.; Guamis, B.; Ferragut, V. Comparing the Effects of Ultra-High-Pressure Homogenization and Conventional Thermal Treatments on the Microbiological, Physical, and Chemical Quality of Almond Beverages. J. Food Sci. 2013, 78, E199–E205. [Google Scholar] [CrossRef]
- Cruz, N.; Capellas, M.; Hernández, M.; Trujillo, A.J.; Guamis, B.; Ferragut, V. Ultra high pressure homogenization of soymilk: Microbiological, physicochemical and microstructural characteristics. Food Res. Int. 2007, 40, 725–732. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration (FDA). Guidance for Industry: Juice Hazard Analysis Critical Control Point Hazards and Controls Guidance, 1st ed.; U.S. Food & Drug Administration: Silver Spring, MD, USA, 2004.
- Pathanibul, P.; Taylor, T.M.; Davidson, P.M.; Harte, F. Inactivation of Escherichia coli and Listeria innocua in apple and carrot juices using high pressure homogenization and nisin. Int. J. Food Microbiol. 2009, 129, 316–320. [Google Scholar] [CrossRef]
- Kumar, S.; Thippareddi, H.; Subbiah, J.; Zivanovic, S.; Davidson, P.M.; Harte, F. Inactivation of Escherichia coli K-12 in apple juice using combination of high-pressure homogenization and chitosan. J. Food Sci. 2009, 74, M8–M14. [Google Scholar] [CrossRef]
- Briñez, W.J.; Roig-Sagués, A.X.; Herrero, M.M.H.; López, B.G. Inactivation by ultrahigh-pressure homogenization of Escherichia coli strains inoculated into orange juice. J. Food Prot. 2006, 69, 984–989. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Method of Analysis, 18th ed.; AOAC International: Washington, DC, USA, 2005; ISBN 9780935584424. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple technique to rule out occlusion of right coronary artery after aortic valve surgery. J. Biol. Chem. 1956, 226, 497–509. [Google Scholar] [CrossRef]
- Frampton, E.W.; Restaino, L.; Blaszko, N. Evaluation of the β-Glucuronidase Substrate 5-Bromo-4-Chloro-3-Indolyl-β-D-Glucuronide (X-GLUC) in a 24-Hour Direct Plating Method for Escherichia coli. J. Food Prot. 1988, 51, 402–404. [Google Scholar] [CrossRef]
- Pakzadeh, R.; Goli, S.A.H.; Abdollahi, M.; Varshosaz, J. Formulation optimization and impact of environmental and storage conditions on physicochemical stability of pistachio milk. J. Food Meas. Charact. 2021, 15, 4037–4050. [Google Scholar] [CrossRef]
- Poliseli-Scopel, F.H.; Gallardo-Chacón, J.-J.; Juan, B.; Guamis, B.; Ferragut, V. Characterisation of volatile profile in soymilk treated by ultra high pressure homogenisation. Food Chem. 2013, 141, 2541–2548. [Google Scholar] [CrossRef]
- Souza, M.L.d.; Holanda, L.F.F.d.; Maia, G.A.; Gaspar, J.C., Jr.; Figueiredo, R.W.d. Processamento e estabilidade do leite de amêndoa de castanha-do-brasil (Bertholletia excelsa H.B.K.). Ciência Agronômica 1987, 18, 137–146. [Google Scholar]
- Felberg, I.; Antoniassi, R.; Deliza, R.; de Freitas, S.C.; Modesta, R.C. Della Bebida de soja e castanha do Brasil: Processamento, composição, avaliação sensorial e de cor. Cienc. Tecnol. Aliment. 2009, 29, 609–617. [Google Scholar] [CrossRef]
- Angelino, D.; Rosi, A.; Vici, G.; Russo, M.D.; Pellegrini, N.; Martini, D. Nutritional quality of plant-based drinks sold in Italy: The Food Labelling of Italian Products (FLIP) study. Foods 2020, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Chalupa-Krebzdak, S.; Long, C.J.; Bohrer, B.M. Nutrient density and nutritional value of milk and plant-based milk alternatives. Int. Dairy J. 2018, 87, 84–92. [Google Scholar] [CrossRef]
- Smelt, J.P.P.M.; Brul, S. Thermal Inactivation of Microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 1371–1385. [Google Scholar] [CrossRef]
- Diels, A.M.J.; Callewaert, L.; Wuytack, E.Y.; Masschalck, B.; Michiels, C.W. Inactivation of Escherichia coli by high-pressure homogenisation is influenced by fluid viscosity but not by water activity and product composition. Int. J. Food Microbiol. 2005, 101, 281–291. [Google Scholar] [CrossRef]
- Taylor, T.M.; Roach, A.; Black, D.G.; Davidson, P.M.; Harte, F. Inactivation of Escherichia coli K-12 exposed to pressures in excess of 300 MPa in a high-pressure homogenizer. J. Food Prot. 2007, 70, 1007–1010. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G.; Lenza, E.; Maresca, P. Main factors regulating microbial inactivation by high-pressure homogenization: Operating parameters and scale of operation. Chem. Eng. Sci. 2009, 64, 520–532. [Google Scholar] [CrossRef]
- Dong, P.; Zhou, B.; Zou, H.; Wang, Y.; Liao, X.; Hu, X.; Zhang, Y. High pressure homogenization inactivation of Escherichia coli and Staphylococcus aureus in phosphate buffered saline, milk and apple juice. Lett. Appl. Microbiol. 2021, 73, 159–167. [Google Scholar] [CrossRef]
- Diels, A.M.J.; Callewaert, L.; Wuytack, E.Y.; Masschalck, B.; Michiels, C.W. Moderate Temperatures Affect Escherichia coli Inactivation by High-Pressure Homogenization Only through Fluid Viscosity. Biotechnol. Prog. 2004, 20, 1512–1517. [Google Scholar] [CrossRef]
- Ferragut, V.; Hernández-Herrero, M.; Veciana-Nogués, M.T.; Borras-Suarez, M.; González-Linares, J.; Vidal-Carou, M.C.; Guamis, B. Ultra-high-pressure homogenization (UHPH) system for producing high-quality vegetable-based beverages: Physicochemical, microbiological, nutritional and toxicological characteristics. J. Sci. Food Agric. 2014, 95, 953–961. [Google Scholar] [CrossRef]
- Poliseli-Scopel, F.H.; Hernández-Herrero, M.; Guamis, B.; Ferragut, V. Characteristics of soymilk pasteurized by ultra high pressure homogenization (UHPH). Innov. Food Sci. Emerg. Technol. 2013, 20, 73–80. [Google Scholar] [CrossRef]
- Gul, O.; Saricaoglu, F.T.; Mortas, M.; Atalar, I.; Yazici, F. Effect of high pressure homogenization (HPH) on microstructure and rheological properties of hazelnut milk. Innov. Food Sci. Emerg. Technol. 2017, 41, 411–420. [Google Scholar] [CrossRef]
- Bernat, N.; Cháfer, M.; Rodríguez-García, J.; Chiralt, A.; González-Martínez, C. Effect of high pressure homogenisation and heat treatment on physical properties and stability of almond and hazelnut milks. LWT 2015, 62, 488–496. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Soto-Reyes, N.; Dávila-Rodríguez, M.; Lorenzo-Leal, A.C.; Jiménez-Munguía, M.T.; Mani-López, E.; López-Malo, A. Plant-Based Milk Alternatives: Types, Processes, Benefits, and Characteristics. Food Rev. Int. 2021, 00, 1–32. [Google Scholar] [CrossRef]
- Cortés, C.; Esteve, M.J.; Frígola, A.; Torregrosa, F. Quality characteristics of horchata (a Spanish vegetable beverage) treated with pulsed electric fields during shelf-life. Food Chem. 2005, 91, 319–325. [Google Scholar] [CrossRef]
- Chouliara, E.; Georgogianni, K.G.; Kanellopoulou, N.; Kontominas, M.G. Effect of ultrasonication on microbiological, chemical and sensory properties of raw, thermized and pasteurized milk. Int. Dairy J. 2010, 20, 307–313. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, J.; Xiong, Y.L. High pressure homogenization combined with pH shift treatment: A process to produce physically and oxidatively stable hemp milk. Food Res. Int. 2018, 106, 487–494. [Google Scholar] [CrossRef]
- Gul, O.; Atalar, I.; Mortas, M.; Saricaoglu, F.T.; Yazıcı, F. Application of TOPSIS methodology to determine optimum hazelnut cake concentration and high pressure homogenization condition for hazelnut milk production based on physicochemical, structural and sensory properties. J. Food Meas. Charact. 2018, 12, 2404–2415. [Google Scholar] [CrossRef]
- Mukherjee, D.; Chang, S.K.C.; Zhang, Y.; Mukherjee, S. Effects of Ultra-High Pressure Homogenization and Hydrocolloids on Physicochemical and Storage Properties of Soymilk. J. Food Sci. 2017, 82, 2313–2320. [Google Scholar] [CrossRef]
- McClements, D.J.; Newman, E.; McClements, I.F. Plant-based Milks: A Review of the Science Underpinning Their Design, Fabrication, and Performance. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2047–2067. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Jiang, S.-W.; Cao, X.-M.; Jiang, S.-T.; Pan, L.J. Effect of high pressure homogenization (HPH) on the physical properties of taro (Colocasia esculenta (L). Schott) pulp. J. Food Eng. 2016, 177, 1–8. [Google Scholar] [CrossRef]
- Codina-Torrella, I.; Guamis, B.; Ferragut, V.; Trujillo, A.J. Potential application of ultra-high pressure homogenization in the physico-chemical stabilization of tiger nuts’ milk beverage. Innov. Food Sci. Emerg. Technol. 2017, 40, 42–51. [Google Scholar] [CrossRef]
- Patra, T.; Rinnan, Å.; Olsen, K. The physical stability of plant-based drinks and the analysis methods thereof. Food Hydrocoll. 2021, 118, 106770. [Google Scholar] [CrossRef]
- Pinto Ramos, C.M.; Bora, P.S. Extraction and Functional Characteristics of Brazil Nut (Bertholletia excelsa HBK) Globulin. Food Sci. Technol. Int. 2003, 9, 265–269. [Google Scholar] [CrossRef]
- Zaaboul, F.; Raza, H.; Cao, C.; Yuanfa, L. The impact of roasting, high pressure homogenization and sterilization on peanut milk and its oil bodies. Food Chem. 2019, 280, 270–277. [Google Scholar] [CrossRef]
- Poliseli-Scopel, F.H.; Hernández-Herrero, M.; Guamis, B.; Ferragut, V. Sterilization and aseptic packaging of soymilk treated by ultra high pressure homogenization. Innov. Food Sci. Emerg. Technol. 2014, 22, 81–88. [Google Scholar] [CrossRef]






| Procedure | Description |
|---|---|
| Preliminary step | Brazil nuts were ground and then homogenized at 10,000 rpm with water at 75 °C, in a 7:1 (water: raw material, v/w) ratio for 5 min. They were then filtered to obtain the hot aqueous extract which was immediately cooled to 5 °C in an ice bath. |
| Partial Defatting Method | |
| BNB 1 | The extract was centrifuged (7000× g, 10 min, 5 °C), obtaining three phases (sediment, aqueous phase, and cream). The cream phase (upper) was separated manually with a spatula. The aqueous and sediment phases were then separated by transferring. Finally, a 30% amount of the cream was mixed (homogenizer) with the aqueous phase. |
| BNB 2 | The extract was kept for 5 h in a refrigerator at 5 °C. The cream was separated manually and the sediment via the transfer of the supernatant. The aqueous phase (supernatant) was used for the assay. |
| BNB 3 | The extract was kept for 15 h in a refrigerator at 5 °C. The cream was separated manually and the sediment via the transfer of the supernatant. The aqueous phase (supernatant) was used for the assay. |
| Samples/Treatment | Log CFU/Ml * | |||||
|---|---|---|---|---|---|---|
| Storage at 5 °C | ||||||
| 0 Day | 2 Day | 5 Day | 9 Day | 15 Day | 21 Day | |
| BNB (untreated) | 3.5 ± 0.0 D | ND | ND | ND | ND | ND |
| PAS (Pasteurized) | 2.3 ± 0.0 aB | 2.4 ± 0.0 a | 2.4 ± 0.1 a | 2.2 ± 0.1 a | 2.4 ± 0.3 a | 2.3 ± 0.1 a |
| T1 (50 MPa/75 °C) | 1.6 ± 0.1 dA | 1.5 ± 0.0 bcd | 1.6 ± 0.1 cd | 1.0 ± 0.0 a | 1.2 ± 0.2 abc | 1.2 ± 0.3 ab |
| T2 (150 MPa/55 °C) | 2.7 ± 0.1 aC | 2.6 ± 0.0 a | 2.7 ± 0.0 a | 2.7 ± 0.1 a | 2.6 ± 0.0 a | 4.0 ± 0.1 b |
| T3 (180 MPa/25 °C) | 3.5 ± 0.0 aD | 3.2 ± 0.0 a | 3.7 ± 0.0 a | 3.9 ± 0.0 a | 4.1 ± 0.1 a | 5.1 ± 0.1 b |
| Parameter | Day | BNB | PAS | T1 | T2 | T3 |
|---|---|---|---|---|---|---|
| L* | 0 | 80.7 ± 0.3 A | 81.4 ± 0.3 bB | 89.2 ± 0.3 aC | 89.8 ± 0.2 aCD | 90.0 ± 0.2 aD |
| 2 | 68.9 ± 0.7 a | 88.9 ± 0.4 a | 90.0 ± 0.2 a | 90.3 ± 0.2 a | ||
| 5 | 69.2 ± 0.2 a | 89.2 ± 0.1 a | 90.9 ± 0.3 a | 91.0 ± 0.1 b | ||
| 9 | 69.7 ± 1.1 a | 88.4 ± 0.7 a | 90.2 ± 0.7 a | 90.1 ± 0.1 a | ||
| 15 | 69.2 ± 1.4 a | 88.2 ± 0.2 a | 90.5 ± 0.0 a | 90.3 ± 0.3 a | ||
| 21 | 69.0 ± 0.7 a | 88.9 ± 0.3 a | 89.8 ± 0.4 a | 90.2 ± 0.4 a | ||
| a* | 0 | 0.5 ± 0.1 A | 1.24 ± 0.3 bAB | 2.43 ± 0.2 bD | 2.30 ± 0.3 cCD | 1.64 ± 0.4 aBC |
| 2 | 0.53 ± 0.0 a | 2.12 ± 0.2 a | 1.92 ± 0.2 ab | 1.81 ± 0.1 a | ||
| 5 | 0.63 ± 0.1 a | 2.27 ± 0.5 ab | 1.99 ± 0.3 a | 1.82 ± 0.3 a | ||
| 9 | 0.45 ± 0.1 a | 2.13 ± 0.2 ab | 1.87 ± 0.4 ab | 1.76 ± 0.1 a | ||
| 15 | 0.47 ± 0.1 a | 2.36 ± 0.3 ab | 2.18 ± 0.1 bc | 1.75 ± 0.1 a | ||
| 21 | 0.59 ± 0.2 a | 2.42 ± 0.7 ab | 2.10 ± 0.3 ab | 1.85 ± 0.4 a | ||
| b* | 0 | 11.3 ± 0.5 B | 9.9 ± 0.3 bA | 10.2 ± 0.6 aA | 10.2 ± 0.4 aA | 9.6 ± 0.3 aA |
| 2 | 6.4 ± 0.5 a | 10.3 ± 0.4 a | 12.9 ± 1.3 a | 8.6 ± 2.0 a | ||
| 5 | 4.7 ± 0.1 a | 10.8 ± 1.5 a | 11.4 ± 1.1 a | 9.2 ± 1.5 a | ||
| 9 | 6.7 ± 1.2 a | 11.0 ± 0.4 a | 11.0 ± 0.6 a | 9.7 ± 2.3 a | ||
| 15 | 6.4 ± 0.4 a | 9.9 ± 0.5 a | 8.5 ± 1.4 a | 9.4 ± 0.2 a | ||
| 21 | 6.6 ± 1.6 a | 10.2 ± 1.6 a | 8.9 ± 0.9 a | 8.4 ± 1.1 a | ||
| WI | 0 | 77.6 ± 0.4 A | 78.9 ± 0.3 bA | 84.9 ± 0.4 aB | 85.4 ± 0.9 aB | 86.0 ± 0.3 aB |
| 2 | 68.2 ± 0.8 a | 84.7 ± 0.5 a | 83.6 ± 1.7 a | 86.9 ± 1.2 a | ||
| 5 | 68.9 ± 0.2 a | 84.5 ± 1.0 a | 85.2 ± 1.6 a | 87.0 ± 1.1 a | ||
| 9 | 68.9 ± 1.0 a | 83.9 ± 0.3 a | 85.1 ± 1.4 a | 86.0 ± 1.6 a | ||
| 15 | 68.5 ± 1.3 a | 84.4 ± 0.4 a | 87.0 ± 0.9 a | 86.3 ± 0.3 a | ||
| 21 | 68.3 ± 1.0 a | 84.7 ± 0.9 a | 86.3 ± 0.3 a | 87.0 ± 0.4 a | ||
| ΔE | 0 | ND | 1.8 ± 0.8 aA | 8.8 ± 0.3 aB | 9.5 ± 0.3 aB | 9.5 ± 0.3 aB |
| 2 | 12.8 ± 0.6 b | 8.5 ± 0.4 a | 9.8 ± 0.5 a | 10.3 ± 0.5 a | ||
| 5 | 13.0 ± 0.8 b | 8.8 ± 0.3 a | 10.3 ± 0.6 a | 10.7 ± 0.4 a | ||
| 9 | 11.9 ± 1.3 b | 7.9 ± 0.7 a | 7.9 ± 0.7 a | 9.8 ± 0.3 a | ||
| 15 | 12.5 ± 1.4 b | 7.9 ± 0.4 a | 10.4 ± 0.4 a | 9.9 ± 0.3 a | ||
| 21 | 12.6 ± 0.2 b | 8.6 ± 0.2 a | 9.6 ± 0.1 a | 10.1 ± 0.0 a |
| Sample | Particle Size (nm) | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 2 | Day 5 | Day 9 | Day 15 | Day 21 | |
| BNB | 8227.5 ± 782.5 A | - | - | - | - | - |
| PAS | 1780.3 ± 28.7 Ba | 2119.2 ± 87 b | 2813 ± 69.4 c | 3173.8 ± 81.1 d | 2755 ± 109.3 c | 1697 ± 36.7 a |
| T1 | 261.2 ± 13.1 Ca | 290.8 ± 12 a | 353.6 ± 106.3 a | 300.9 ± 25 a | 248.2 ± 5 a | 270.2 ± 21 a |
| T2 | 355.8 ± 51.5 Ca | 350.4 ± 17.2 a | 603.7 ± 104.7 b | 344.2 ± 10.1 a | 325 ± 33.3 a | 283.9 ± 11.3 a |
| T3 | 345.3 ± 21.3 Ca | 387.4 ± 24.9 a | 620 ± 97.3 b | 531.2 ± 66.6 b | 295.95 ± 2a | 310.3 ± 24.9 a |
| Zeta potential (mV) | ||||||
| BNB | −38.6 ± 6.3 A | - | - | - | - | - |
| PAS | −39.1 ± 0.1 Aa | −47.9 ± 1.8 b | −44.1 ± 1.3 a | −40.5 ± 1.3 a | −38.3 ± 4.2 a | −40.9 ± 0.8 a |
| T1 | −36 ± 0.7 Aa | −34.9 ± 1.1 a | −35.3 ± 0.5 a | −34.6 ± 1.2 a | −39.5 ± 0.3 a | −35.21 ± 0.6 a |
| T2 | −35.8 ± 1.7 Aa | −36.4 ± 0.9 a | −37.1 ± 1.9 a | −36.5 ± 1 a | −37.7 ± 1.3 a | −35.9 ± 1.2 a |
| T3 | −32.3 ± 6 Aa | −35.7 ± 1.7 a | −33.6 ± 1.7 a | −37.8 ± 0.9 a | −34.8 ± 2.1 a | −35.8 ± 1.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasquez-Rojas, W.V.; Parralejo-Sanz, S.; Martin, D.; Fornari, T.; Cano, M.P. Validation of High-Pressure Homogenization Process to Pasteurize Brazil Nut (Bertholletia excelsa) Beverages: Sensorial and Quality Characteristics during Cold Storage. Beverages 2023, 9, 22. https://doi.org/10.3390/beverages9010022
Vasquez-Rojas WV, Parralejo-Sanz S, Martin D, Fornari T, Cano MP. Validation of High-Pressure Homogenization Process to Pasteurize Brazil Nut (Bertholletia excelsa) Beverages: Sensorial and Quality Characteristics during Cold Storage. Beverages. 2023; 9(1):22. https://doi.org/10.3390/beverages9010022
Chicago/Turabian StyleVasquez-Rojas, Wilson V., Sara Parralejo-Sanz, Diana Martin, Tiziana Fornari, and M. Pilar Cano. 2023. "Validation of High-Pressure Homogenization Process to Pasteurize Brazil Nut (Bertholletia excelsa) Beverages: Sensorial and Quality Characteristics during Cold Storage" Beverages 9, no. 1: 22. https://doi.org/10.3390/beverages9010022