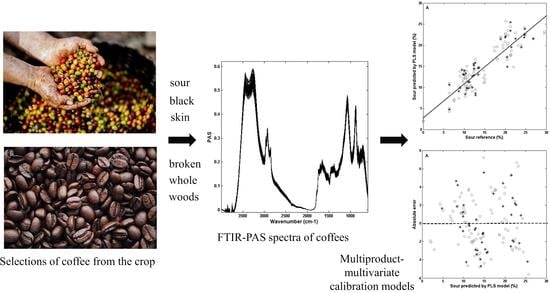
Infrared-Photoacoustic Spectroscopy and Multiproduct Multivariate Calibration to Estimate the Proportion of Coffee Defects in Roasted Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coffee Samples
2.2. FTIR-PAS Spectroscopy Assays
2.3. Multivariate Calibration
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribeiro, J.S.; Ferreira, M.M.C.; Salva, T.J.G. Chemometric models for the quantitative descriptive sensory analysis of Arabica coffee beverages using near infrared spectroscopy. Talanta 2011, 83, 1352–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacalone, D.; Degn, T.K.; Yang, N.; Liu, C.; Fisk, I.; Münchow, M. Common roasting defects in coffee: Aroma composition, sensory characterization and consumer perception. Food Qual. Prefer. 2019, 71, 463–474. [Google Scholar] [CrossRef] [Green Version]
- Franca, A.S.; Mendonça, J.C.F.; Oliveira, S.D. Composition of green and roasted coffees of different cup qualities. LWT Food Sci. Technol. 2005, 38, 709–715. [Google Scholar] [CrossRef]
- Craig, A.P.; Botelho, B.G.; Oliveira, L.S.; Franca, A.S. Mid infrared spectroscopy and chemometrics as tools for the classification of roasted coffees by cup quality. Food Chem. 2018, 245, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Baqueta, M.R.; Coqueiro, A.; Março, P.H.; Valderrama, P. Multivariate classification for the direct determination of cup profile in coffee blends via handheld near-infrared spectroscopy. Talanta 2021, 222, 121526. [Google Scholar] [CrossRef]
- Agresti, P.D.C.M.; Franca, A.S.; Oliveira, L.S.; Augusti, R. Discrimination between defective and non-defective Brazilian coffee beans by their volatile profile. Food Chem. 2008, 106, 787–796. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S. Spectroscopic methods for chemometric identification of defective and nondefective coffees. In Coffee in Health and Disease Prevention, 1st ed.; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 1, pp. 943–952. [Google Scholar]
- Dias, R.C.E.; Valderrama, P.; Dos Santos Scholz, M.B.; Edelmann, M.; Yeretzian, C. Quantitative assessment of specific defects in roasted ground coffee via infrared-photoacoustic spectroscopy. Food Chem. 2018, 255, 132–138. [Google Scholar] [CrossRef]
- Craig, A.P.; Franca, A.S.; Oliveira, L.S. Discrimination between defective and non-defective roasted coffees by diffuse reflectance infrared Fourier transform spectroscopy. LWT Food Sci. Technol. 2012, 47, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Sittipod, S.; Schwartz, E.; Paravisini, L.; Peterson, D.G. Identification of flavor modulating compounds that positively impact coffee quality. Food Chem. 2019, 301, 125250. [Google Scholar] [CrossRef]
- Samoggia, A.; Riedel, B. Coffee consumption and purchasing behavior review: Insights for further research. Appetite 2018, 129, 70–81. [Google Scholar] [CrossRef]
- Boaventura, P.S.M.; Abdalla, C.C.; Araújo, C.L.; Arakelian, J.S. Value co-creation in the specialty coffee value chain: The third-wave coffee movement. Rev. Adm. Empres. 2018, 58, 254–266. [Google Scholar] [CrossRef] [Green Version]
- Craig, A.P.; Franca, A.S.; Oliveira, L.S.; Irudayaraj, J.; Ileleji, K. Fourier transform infrared spectroscopy and near infrared spectroscopy for the quantification of defects in roasted coffees. Talanta 2015, 134, 379–386. [Google Scholar] [CrossRef]
- Wermelinger, T.; D’Ambrosio, L.; Klopprogge, B.; Yeretzian, C. Quantification of the Robusta fraction in a coffee blend via Raman spectroscopy: Proof of principle. J. Agric. Food Chem. 2011, 59, 9074–9079. [Google Scholar] [CrossRef]
- Alves, S.T.; Dias, R.C.E.; Benassi, M.T.; Dos Santos Scholz, M.B. Metodologia para análise simultânea de ácido nicotínico, trigonelina, ácido clorogênico e cafeína em café torrado por cromatografia líquida de alta eficiência. Quím. Nova 2006, 29, 1164–1168. [Google Scholar] [CrossRef]
- Dias, R.C.E.; Campanha, F.G.; Vieira, L.G.E.; Ferreira, L.P.; Pot, D.; Marraccini, P.; Benassi, M.T. Evaluation of Kahweol and Cafestol in coffee tissues and roasted coffee by a new high-performance liquid chromatography methodology. J. Agric. Food Chem. 2010, 58, 88–93. [Google Scholar] [CrossRef]
- Dias, R.C.E.; Benassi, M.T. Discrimination between arabica and robusta coffees using hydrosoluble compounds: Is the efficiency of the parameters dependent on the roast degree? Beverages 2015, 1, 127–139. [Google Scholar] [CrossRef]
- Ongo, E.A.; Montevecchi, G.; Antonelli, A.; Sberveglieri, V.; Sevilla III, F. Metabolomics fingerprint of Philippine coffee by SPME-GC-MS for geographical and varietal classification. Food Res. Int. 2020, 134, 109227. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Cui, C.; Fisk, I.D. Variability of single bean coffee volatile compounds of Arabica and robusta roasted coffees analysed by SPME-GC-MS. Food Res. Int. 2018, 108, 628–640. [Google Scholar] [CrossRef]
- Dong, W.; Hu, R.; Long, Y.; Li, H.; Zhang, Y.; Zhu, K.; Chu, Z. Comparative evaluation of the volatile profiles and taste properties of roasted coffee beans as affected by drying method and detected by electronic nose, electronic tongue, and HS-SPME-GC-MS. Food Chem. 2019, 272, 723–731. [Google Scholar] [CrossRef]
- Casas, M.I.; Vaughan, M.J.; Bonello, P.; Gardener, B.M.; Grotewold, E.; Alonso, A.P. Identification of biochemical features of defective Coffea arabica L. beans. Food Res. Int. 2017, 95, 59–67. [Google Scholar] [CrossRef]
- Mendonça, J.C.F.; Franca, A.S.; Oliveira, L.S.; Nunes, M. Chemical characterisation of non-defective and defective green arabica and robusta coffees by electrospray ionization-mass spectrometry (ESI-MS). Food Chem. 2008, 111, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.C.E.; Alves, S.T.; Benassi, M.T. Spectrophotometric method for quantification of kahweol in coffee. J. Food Compos. Anal. 2013, 31, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Pires, F.C.; Pereira, R.G.F.A.; Baqueta, M.R.; Valderrama, P.; Rocha, R.A. Near-infrared spectroscopy and multivariate calibration as an alternative to the Agtron to predict roasting degrees in coffee beans and ground coffees. Food Chem. 2021, 365, 130471. [Google Scholar] [CrossRef] [PubMed]
- Baqueta, M.R.; Coqueiro, A.; Março, P.H.; Valderrama, P. Quality control parameters in the roasted coffee industry: A proposal by using MicroNIR spectroscopy and multivariate calibration. Food Anal. Methods 2020, 13, 50–60. [Google Scholar] [CrossRef]
- Baqueta, M.R.; Coqueiro, A.; Valderrama, P. Brazilian coffee blends: A simple and fast method by Near-Infrared Spectroscopy for the determination of the sensory attributes elicited in professional coffee cupping. J. Food Sci. 2019, 84, 1247–1255. [Google Scholar] [CrossRef]
- Craig, A.P.; Franca, A.S.; Oliveira, L.S.; Irudayaraj, J. Application of elastic net and infrared spectroscopy in the discrimination between defective and non-defective roasted coffees. Talanta 2014, 128, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Shan, J.; Suzuki, T.; Suhandy, D.; Ogawa, Y.; Kondo, N. Chlorogenic acid (CGA) determination in roasted coffee beans by Near Infrared (NIR) spectroscopy. Eng. Agric. Environ. Food 2014, 7, 139–142. [Google Scholar] [CrossRef]
- Dias, R.C.E.; Madivadua, D.A.; Marco, P.H.; Valderrama, P. Commercial coffee evaluation based on Vis-Nir spectroscopy and principal component analysis. In Innovations in Coffee Quality, 1st ed.; Kalschne, D.L., Corso, M.P., Dias, R.C.E., Eds.; Nova Science Publishers: New York, NY, USA, 2020; Volume 1, pp. 219–224. [Google Scholar]
- Dias, R.C.E.; Yeretzian, C. Investigating coffee samples by Raman spectroscopy for quality control–preliminary study. Int. J. Spectrosc. Tech. 2016, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Stuart, B. Infrared spectroscopy. In Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; Kirk, R.E., Othmer, D.F., Mann, C.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; Volume 1, pp. 1–18. [Google Scholar]
- Spragg, R.A. IR Spectroscopy Sample Preparation Methods. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Linden, J., Ed.; Academic Press: New York, NY, USA, 2017; pp. 437–447. [Google Scholar]
- Barbin, D.F.; Felicio, A.L.D.S.M.; Sun, D.W.; Nixdorf, S.L.; Hirooka, E.Y. Application of infrared spectral techniques on quality and compositional attributes of coffees: An over-view. Food Res. Int. 2014, 61, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Araújo, T.K.L.; Nóbrega, R.O.; Sousa Fernandes, D.D.; Araújo, M.C.U.; Diniz, P.H.G.D.; Silva, E.C. Non-destructive authentication of Gourmet ground roasted coffees using NIR spectroscopy and digital images. Food Chem. 2021, 364, 130452. [Google Scholar] [CrossRef]
- Hernández-Aguilar, C.; Domínguez-Pacheco, A.; Cruz-Orea, A.; Ivanov, R. Photoacoustic spectroscopy in the optical characterization of foodstuff: A review. J. Spectrosc. 2019, 2019, 5920948. [Google Scholar] [CrossRef]
- Huang, J.; Bekiaris, G.; Fitamo, T.; Scheutz, C.; Bruun, S. Prediction of biochemical methane potential of urban organic waste using Fourier transform mid-infrared photoacoustic spectroscopy and multivariate analysis. Sci. Total Environ. 2021, 790, 147959. [Google Scholar] [CrossRef]
- Grzegorczyk, M.; Pogorzelski, S.; Rochowski, P. Towards a novel class of photoacoustics-based water contamination sensors. J. Environ. Chem. Eng. 2022, 10, 107983. [Google Scholar] [CrossRef]
- Riech, I.; Zambrano, M.; Abelenda, A.; Maldonado, F.; Rojas-Marroquín, A.; Jaime, J.; Calderón, A.; Marín, E. Evaluation of thin films intermixing by photoacoustic spectroscopy. Thin Solid Films 2021, 735, 138871. [Google Scholar] [CrossRef]
- Schmid, T.; Helmbrecht, C.; Panne, U.; Haisch, C.; Niessner, R. Process analysis of biofilms by photoacoustic spectroscopy. Anal. Bioanal. Chem. 2003, 375, 1124–1129. [Google Scholar] [CrossRef]
- Sgobba, F.; Sampaolo, A.; Patimisco, P.; Giglio, M.; Menduni, G.; Ranieri, A.C.; Hoelzl, C.; Rossmadl, H.; Brehm, C.; Mackowiak, V.; et al. Compact and portable quartz-enhanced photoacoustic spectroscopy sensor for carbon monoxide environmental monitoring in urban areas. Photoacoustics 2022, 25, 100318. [Google Scholar] [CrossRef]
- MacKenzie, H.A.; Christison, G.B.; Hodgson, P.; Blanc, D. A laser photoacoustic sensor for analyte detection in aqueous systems. Sens. Actuators B Chem. 1993, 11, 213–220. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, W.; Zhang, B.; Du, C.; Wei, N.; Liang, D.; Sun, K.; Tu, K.; Peng, J.; Pan, L. Determination of total protein and wet gluten in wheat flour by Fourier transform infrared photoacoustic spectroscopy with multivariate analysis. J. Food Compos. Anal. 2022, 106, 104349. [Google Scholar] [CrossRef]
- Hernandez-Aguilar, C.; Dominguez-Pacheco, A.; Valderrama-Bravo, C.; Cruz-Orea, A.; Martínez Ortiz, E.; Ivanov, R.; Ordonez-Miranda, J. Photoacoustic characterization of wheat bread mixed with Moringa oleifera. Curr. Res. Food Sci. 2021, 4, 521–531. [Google Scholar] [CrossRef]
- Cesar, C.L.; Vargas, H.; Lima, C.A.S.; Mendes Filho, J.; Miranda, L.C.M. On the use of photoacoustic spectroscopy for investigating adulterated or altered powdered coffee samples. J. Agric. Food Chem. 1984, 32, 1355–1358. [Google Scholar] [CrossRef]
- Gordillo-Delgado, F.; Marín, E.; Cortés-Hernández, D.M.; Mejía-Morales, C.; García- Salcedo, A.J. Discrimination of organic coffee via Fourier transform infrared–photoacoustic spectroscopy. J. Sci. Food Agric. 2012, 92, 2316–2319. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.C.E.; Valderrama, P.; Março, P.H.; Dos Santos Scholz, M.B.; Edelmann, M.; Yeretzian, C. Data on roasted coffee with specific defects analyzed by infrared-photoacoustic spectroscopy and chemometrics. Data Brief 2018, 20, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Geladi, P.; Kowalski, B.R. Partial least-squares regression: A tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Brereton, R.G. Introduction to multivariate calibration in analytical chemistry. Analyst 2000, 125, 2125–2154. [Google Scholar] [CrossRef]
- Dos Santos, D.A.; De Lima, K.P.; Cavalcante, V.; Coqueiro, A.; Consolin, M.F.B.; Consolin Filho, N.; Março, P.H.; Valderrama, P. Multiproduct, multicomponent and multivariate calibration: A case study by using Vis-NIR spectroscopy. Food Anal. Methods 2017, 11, 1915–1919. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Kennard, R.W.; Stone, L.A. Computer aided designing of experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- ASTM E1655-00; Standard Practices for Infrared Multivariate Quantitative Analysis. ASTM—American Society for Testing and Materials: West Conshohocken, PA, USA. Available online: https://www.astm.org/e1655-00.html (accessed on 28 October 2022).
- Valderrama, P.; Braga, J.W.B.; Poppi, R.J. Estado da arte de figuras de mérito em calibração multivariada. Quím. Nova 2009, 32, 1278–1287. [Google Scholar] [CrossRef] [Green Version]
- Oliveri, P.; Malegori, C.; Simonetti, R.; Casale, M. The impact of signal pre-processing on the final interpretation of analytical outcomes–A tutorial. Anal. Chim. Acta 2019, 1058, 9–17. [Google Scholar] [CrossRef]
- Ferreira, M.M.C. Quimiometria: Conceitos, Métodos e Aplicações, 1st ed.; Editora da UNICAMP: Campinas, Brazil, 2015; pp. 394–398. [Google Scholar]
- ASTM E1655-17; Standard Practices for Infrared Multivariate Quantitative Analysis. ASTM—American Society for Testing and Materials: West Conshohocken, PA, USA. Available online: https://www.astm.org/e1655-17.html (accessed on 28 October 2022).
- Botelho, B.G.; Mendes, B.A.P.; Sena, M.S. Development and analytical validation of robust near-infrared multivariate calibration models for the quality control of mozzarella cheese. Food Anal. Methods 2013, 6, 881–891. [Google Scholar] [CrossRef]
- Valderrama, P.; Braga, J.W.B.; Poppi, R.J. Validation of multivariate calibrationmodels in the determination of sugar cane quality parameters by near infrared spectroscopy. J. Braz. Chem. Soc. 2007, 18, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, D.A.; Coqueiro, A.; Gonçalves, T.R.; Carvalho, J.C.; Bezerra, J.S., Jr.; Matsushita, M.; De Oliveira, C.A.L.; Março, P.H.; Valderrama, P.; Ribeiro, R.P. Omega-3 and Omega-6 determination in nile tilapia’s fillet based on MicroNIR spectroscopy and multivariate calibration. J. Braz. Chem. Soc. 2020, 31, 1883–1890. [Google Scholar] [CrossRef]
- Valderrama, P.; Braga, J.W.B.; Poppi, R.J. Variable selection, outlier detection, and figures of merit estimation in a partial least-squares regression multivariate calibration model. A case study for the determination of quality parameters in the alcohol industry by Near-Infrared spectroscopy. J. Agric. Food Chem. 2007, 55, 8331–8338. [Google Scholar] [CrossRef]
- Dos Santos, D.A.; Lima, K.P.; Março, P.H.; Valderrama, P. Vitamin C determination by ultraviolet spectroscopy and multiproduct calibration. J. Braz. Chem. Soc. 2016, 27, 1912–1917. [Google Scholar] [CrossRef]
- Dos Santos, D.A.; Lima, K.P.; Consolin, M.F.B.; Consolin Filho, N.; Março, P.H.; Valderrama, P. Multi product multivariate calibration: Determination of quality parameters in soybean industrialized juices. Acta Sci. Technol. 2019, 41, e37382. [Google Scholar] [CrossRef] [Green Version]
- Cordeiro, L.D.; Valderrama, P. Digital images and multivariate calibration in the determination of rheological and quality parameters of wheat flour. Braz. J. Anal. Chem. 2021, 8, 62–77. [Google Scholar] [CrossRef]
- Dos Santos, D.A.; Março, P.H.; Valderrama, P. Multi-product calibration: Preliminar studies to determine quality parameters in industrialized juices based on ultravioleta spectroscopy. Braz. J. Anal. Chem. 2013, 12, 495–498. [Google Scholar]
- Jarque, C.M. Jarque-Bera Test. Int. Encycl. Stat. Sci. 2011, 1, 701–702. [Google Scholar]



| Parameters of Merit | Equation | Sour (8 LVs a) | Black (9 LVs) | Skin (8 LVs) | Whole (9 LVs) | Broken (8 LVs) | Woods (11 LVs) | |
|---|---|---|---|---|---|---|---|---|
| Modeled range | - | 0–29.49% | 0–17.06% | 0–6.28% | 0–11.43% | 0–11.27% | 0–1.02% | |
| Accuracy (%) | RMSEC b | 2.8074 | 1.9244 | 0.7642 | 1.2318 | 1.4005 | 0.0609 | |
| RMSEP c | 2.7979 | 1.6406 | 0.7364 | 1.2471 | 1.3855 | 0.0574 | ||
| Sensitivity (%) d | 0.0022 | 0.0021 | 0.0079 | 0.0034 | 0.0053 | 0.0503 | ||
| Analytical sensitivity−1 (%) e | 0.4072 | 0.3563 | 0.1170 | 0.2240 | 0.1748 | 0.0128 | ||
| Limit of detection (%) e | 1.3438 | 1.1756 | 0.3862 | 0.7394 | 0.5769 | 0.0421 | ||
| Limit of quantification (%) e | 4.0721 | 3.5626 | 1.1702 | 2.2405 | 1.7482 | 0.1276 | ||
| Fit (R-squared) | 0.8080 | 0.7416 | 0.7675 | 0.7863 | 0.7176 | 0.7430 | ||
| Linearity | Jarque-Bera test f,g | A = 1.874 B = 4.774 | A = 0.866 B = 4.8467 | A = 0.955 B = 4.847 | A = 1.420 B = 4.692 | A = 2.114 B = 4.869 | A = 0.896 B = 4.799 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, R.; Valderrama, P.; Março, P.; Scholz, M.; Edelmann, M.; Yeretzian, C. Infrared-Photoacoustic Spectroscopy and Multiproduct Multivariate Calibration to Estimate the Proportion of Coffee Defects in Roasted Samples. Beverages 2023, 9, 21. https://doi.org/10.3390/beverages9010021
Dias R, Valderrama P, Março P, Scholz M, Edelmann M, Yeretzian C. Infrared-Photoacoustic Spectroscopy and Multiproduct Multivariate Calibration to Estimate the Proportion of Coffee Defects in Roasted Samples. Beverages. 2023; 9(1):21. https://doi.org/10.3390/beverages9010021
Chicago/Turabian StyleDias, Rafael, Patrícia Valderrama, Paulo Março, Maria Scholz, Michael Edelmann, and Chahan Yeretzian. 2023. "Infrared-Photoacoustic Spectroscopy and Multiproduct Multivariate Calibration to Estimate the Proportion of Coffee Defects in Roasted Samples" Beverages 9, no. 1: 21. https://doi.org/10.3390/beverages9010021