A Comprehensive Comparison of Gluten-Free Brewing Techniques: Differences in Gluten Reduction Ability, Analytical Attributes, and Hedonic Perception
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pilot-Scale Brewing
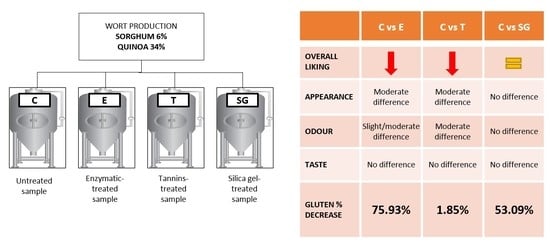
2.1.1. Wort Production
2.1.2. Fermentation, Maturation, and Bottling
- Enzymatic-treated beer (E): Brewers Clarex® (DSM, Delft, The Netherlands) was added at the beginning of fermentation, without dilution, directly to the top of the tank and apart from the yeast, using a recommended dosage of 6.5 g/hL;
- Tannins-treated beer (T): 2 g/hL of tannic acid (Brewtan® B, ≥98%, S.A. Ajinomoto, OmniChem N.V., Wetteren, Belgium) were added on the last day of cold maturation;
- Silica gel-treated beer (SG): 100 g/hL of silica xerogel (Daraclar® 7500, Grace GmbH & Co.KG, Ind. Hollerhecke 1, D-67547 Worms, Germany) were added on the last day of cold maturation;
- Cold maturation beer: after 15 days of cold maturation, 35 L of untreated beer (C) was bottle-conditioned. The remaining 35 L was kept in the fermentation tank for an additional 15 days at 0 °C for a total maturation period of 30 days (C30).

2.2. Physicochemical Characterization
2.3. Sensory Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Gluten Content and Percentage Reduction
3.2. Effect of Gluten Reduction Treatments on Physicochemical Parameters
3.3. Sensory Evaluation: Difference from Control Test Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-Gil, M.; Simon, E.; Gibert, A.; Miranda, J.; Alcoba, E.R.; Martínez, O.; Cerezo, E.V.; Bustamante, M. Gluten Assessment in Beers: Comparison by Different Commercial ELISA Kits and Evaluation of NIR Analysis as a Complementary Technique. Foods 2021, 10, 1170. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.; Decloedt, A.; Vanderputten, D.; Van Landschoot, A. Variation in gluten protein and peptide concentrations in Belgian barley malt beers. J. Inst. Brew. 2018, 124, 148–157. [Google Scholar] [CrossRef]
- European Commission, Commission Implementing Regulation (EU) No. 828/2014 of 30 July 2014 on the Requirements for the Provision of Information to Consumers on the Absence or Reduced Presence of Gluten in Food. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014R0828&from=EN (accessed on 19 January 2023).
- Vriesekoop, F. Beer and Allergens. Beverages 2021, 7, 79. [Google Scholar] [CrossRef]
- Colgrave, M.L.; Goswami, H.; Howitt, C.; Tanner, G.J. What is in a Beer? Proteomic Characterization and Relative Quantification of Hordein (Gluten) in Beer. J. Proteome Res. 2011, 11, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Fanari, M.; Porcu, M.C.; Zinellu, M.; Farina, D.; Scognamillo, S.; Forteschi, M.; Pretti, L. A Preliminary Study about Gluten Levels in Sardinian Craft Beers. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1195–1198. [Google Scholar] [CrossRef]
- Guerdrum, L.J.; Bamforth, C.W. Levels of gliadin in commercial beers. Food Chem. 2011, 129, 1783–1784. [Google Scholar] [CrossRef]
- Kerpes, R.; Göller, F.; Kollmannsberger, H.; Becker, T. Aroma profile of a gluten-free barley malt beer crafted to remove gluten using a barley malt extract with high peptidase activity. Eur. Food Res. Technol. 2022, 249, 23–32. [Google Scholar] [CrossRef]
- Pernica, M.; Boško, R.; Svoboda, Z.; Benešová, K.; Běláková, S. Monitoring of gluten in Czech commercial beers. Czech J. Food Sci. 2020, 38, 255–258. [Google Scholar] [CrossRef]
- Rubio-Flores, M.; Serna-Saldivar, S.O. Technological and Engineering Trends for Production of Gluten-Free Beers. Food Eng. Rev. 2016, 8, 468–482. [Google Scholar] [CrossRef]
- Van Landschoot, A. Gluten-free barley malt beers. Cerevisia 2011, 36, 93–97. [Google Scholar] [CrossRef]
- Cela, N.; Condelli, N.; Caruso, M.C.; Perretti, G.; Di Cairano, M.; Tolve, R.; Galgano, F. Gluten-Free Brewing: Issues and Perspectives. Fermentation 2020, 6, 53. [Google Scholar] [CrossRef]
- Zdaniewicz, M.; Pater, A.; Hrabia, O.; Duliński, R.; Cioch-Skoneczny, M. Tritordeum malt: An innovative raw material for beer production. J. Cereal Sci. 2020, 96, 103095. [Google Scholar] [CrossRef]
- Yorke, J.; Cook, D.; Ford, R. Brewing with Unmalted Cereal Adjuncts: Sensory and Analytical Impacts on Beer Quality. Beverages 2021, 7, 4. [Google Scholar] [CrossRef]
- Demeester, A.; Laureys, D.; Baillière, J.; Huys, J.; Vermeir, P.; De Leyn, I.; Vanderputten, D.; De Clippeleer, J. Comparison of Congress Mash with Final 65 °C Mash for Wort Production with Unmalted Barley, Tritordeum, and Quinoa, with or without Pregelatinization and/or Enzyme Addition. J. Am. Soc. Brew. Chem. 2021, 81, 66–75. [Google Scholar] [CrossRef]
- Myncke, E.; Laureys, D.; Baillière, J.; Vanderputten, D.; De Clippeleer, J. Mashing with Unmalted Buckwheat: Influence of Proportion, Grind Size, and Exogenous Enzymes on Small-Scale Lautering. MBAA TQ 2022, 59, 55–62. [Google Scholar]
- Cela, N.; Galgano, F.; Perretti, G.; Di Cairano, M.; Tolve, R.; Condelli, N. Assessment of brewing attitude of unmalted cereals and pseudocereals for gluten free beer production. Food Chem. 2022, 384, 132621. [Google Scholar] [CrossRef]
- Baillière, J.; Laureys, D.; Vermeir, P.; Van Opstaele, F.; De Rouck, G.; De Cooman, L.; Vanderputten, D.; De Clippeleer, J. 10 unmalted alternative cereals and pseudocereals: A comparative analysis of their characteristics relevant to the brewing process. J. Cereal Sci. 2022, 106, 103482. [Google Scholar] [CrossRef]
- Kordialik-Bogacka, E.; Bogdan, P.; Pielech-Przybylska, K.; Michałowska, D. Suitability of unmalted quinoa for beer production. J. Sci. Food Agric. 2018, 98, 5027–5036. [Google Scholar] [CrossRef] [PubMed]
- Di Ghionno, L.; Marconi, O.; Sileoni, V.; De Francesco, G.; Perretti, G. Brewing with prolyl endopeptidase from Aspergillus niger: The impact of enzymatic treatment on gluten levels, quality attributes and sensory profile. Int. J. Food Sci. Technol. 2017, 52, 1367–1374. [Google Scholar] [CrossRef]
- Guerdrum, L.J.; Bamforth, C.W. Prolamin Levels through Brewing and the Impact of Prolyl Endoproteinase. J. Am. Soc. Brew. Chem. 2012, 70, 35–38. [Google Scholar] [CrossRef]
- Knorr, V.; Wieser, H.; Koehler, P. Production of gluten-free beer by peptidase treatment. Eur. Food Res. Technol. 2016, 242, 1129–1140. [Google Scholar] [CrossRef]
- Watson, H.; Vanderputten, D.; Van Landschoot, A.; Decloedt, A. Applicability of different brewhouse technologies and gluten-minimization treatments for the production of gluten-free (barley) malt beers: Pilot- to industrial-scale. J. Food Eng. 2019, 245, 33–42. [Google Scholar] [CrossRef]
- Fiedler, K.L.; Cao, W.; Zhang, L.; Naziemiec, M.; Bedford, B.; Yin, L.; Smith, N.; Arbuckle, M.; Lopez-Hernandez, A.; Jackson, L.S. Detection of gluten in a pilot-scale barley-based beer produced with and without a prolyl endopeptidase enzyme. Food Addit. Contam. Part A 2019, 36, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.G.; Decloedt, A.I.; Hemeryck, L.Y.; Van Landschoot, A.; Prenni, J. Peptidomics of an industrial gluten-free barley malt beer and its non-gluten-free counterpart: Characterisation and immunogenicity. Food Chem. 2021, 355, 129597. [Google Scholar] [CrossRef]
- Taylor, J.P.; Jacob, F.; Arendt, E.K. Fundamental study on the impact of silica gel and tannic acid on hordein levels in beer. Innov. Food Sci. Emerg. Technol. 2015, 31, 177–184. [Google Scholar] [CrossRef]
- Gazzetta Ufficiale della Repubblica Italiana. Disciplina Igienica della Produzione e del Commercio Della Birra, Legge del 16/08/1962, n. 1354; Gazzetta Ufficiale della Repubblica Italiana: Rome, Italy, 1962. [Google Scholar]
- Cela, N.; Condelli, N.; Perretti, G.; Di Cairano, M.; Tolve, R.; Galgano, F. Gluten reduction in beer: Effect of sorghum:quinoa ratio and protein rest time on brewing parameters and consumer acceptability. J. Cereal Sci. 2023, 109, 103607. [Google Scholar] [CrossRef]
- Analytica-EBC. European Brewery Convention, 5th ed.; Fachverlag Hans Carl: Nürnberg, Germany, 2007. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kerpes, R.; Fischer, S.; Becker, T. The production of gluten-free beer: Degradation of hordeins during malting and brewing and the application of modern process technology focusing on endogenous malt peptidases. Trends Food Sci. Technol. 2017, 67, 129–138. [Google Scholar] [CrossRef]
- Benítez, E.I.; Acquisgrana, M.R.; Peruchena, N.M.; Sosa, G.L.; Lozano, J.E. Effects of silica gel on reduction in gluten during several beer brewing stages. Int. J. Food Sci. Technol. 2016, 51, 920–928. [Google Scholar] [CrossRef]
- van Donkelaar, L.H.G.; Hageman, J.A.; Oguz, S.; Noordman, T.R.; Boom, R.M.; van der Goot, A.-J. Combining unmalted barley and pearling gives good quality brewing. J. Inst. Brew. 2016, 122, 228–236. [Google Scholar] [CrossRef]
- Evans, D.E.; Finn, J.E.C.; Robinson, L.H.; Eglinton, J.K.; Sheehy, M.; Stewart, D.C. The Effects of Hop-α-Acids and Proline-Specific Endoprotease (PSEP) Treatments on the Foam Quality of Beer. J. Inst. Brew. 2011, 117, 335–342. [Google Scholar] [CrossRef]
- Kunze, W. Finished Beer. In Technology Brewing and Malting; VLB: Berlin, Germany, 2004; p. 741. [Google Scholar]
- Kunze, W. Wort Production. In Technology Brewing and Malting; VLB: Berlin, Germany, 2004; pp. 283–284. [Google Scholar]
- Habschied, K.; Košir, I.J.; Krstanović, V.; Kumrić, G.; Mastanjević, K. Beer Polyphenols—Bitterness, Astringency, and Off-Flavors. Beverages 2021, 7, 38. [Google Scholar] [CrossRef]
- Gazzetta Ufficiale della Repubblica Italiana. LEGGE 28 Luglio 2016, n. 154 Deleghe Al Governo e Ulteriori Disposizioni in Materia Di Semplificazione, Razionalizzazione e Competitivita’ Dei Settori Agricolo e Agroalimentare, Nonche’ Sanzioni in Materia Di Pesca Illegale. Available online: https://www.gazzettaufficiale.it/eli/id/2016/08/10/16G00169/sg%20 (accessed on 19 January 2023).
- Stewart, G.G. The Chemistry of Beer. J. Chem. Educ. 2004, 81, 963. [Google Scholar] [CrossRef]
- Krekora, M.; Nawrocka, A. Effect of a polyphenol molecular size on the gluten proteins—Polyphenols interactions studied with FT-Raman spectroscopy. Food Biophys. 2022, 17, 535–544. [Google Scholar] [CrossRef]
- Królak, K.; Kobus, K.; Kordialik-Bogacka, E. Effects on beer colloidal stability of full-scale brewing with adjuncts, enzymes, and finings. Eur. Food Res. Technol. 2022, 249, 47–53. [Google Scholar] [CrossRef]
- Fanari, M.; Forteschi, M.; Sanna, M.; Zinellu, M.; Porcu, M.C.; Pretti, L. Comparison of enzymatic and precipitation treatments for gluten-free craft beers production. Innov. Food Sci. Emerg. Technol. 2018, 49, 76–81. [Google Scholar] [CrossRef]
- Salek, R.N.; Lorencová, E.; Gál, R.; Kůrová, V.; Opustilová, K.; Buňka, F. Physicochemical and Sensory Properties of Czech Lager Beers with Increasing Original Wort Extract Values during Cold Storage. Foods 2022, 11, 3389. [Google Scholar] [CrossRef]
- Gahr, A.; Forster, A.; Van Opstaele, F. Reproducibility trials in a research brewery and effects on the evaluation of hop sub-stances in beer. Part 1: Reproducibility in fresh beers. Brew. Sci. 2016, 69, 103–111. [Google Scholar]


| Physicochemical Attributes | C | E | T | SG |
|---|---|---|---|---|
| Real Extract (% w/w) | 5.27 ± 0.01 c | 5.30 ± 0.02 b | 5.34 ± 0.01 a | 5.21 ± 0.01 d |
| Real Degree of Fermentation (%) | 62.48 ± 0.38 a | 60.86 ± 0.04 c | 60.30 ± 0.07 d | 62.10 ± 0.03 b |
| Alcohol (% v/v) | 5.37 ± 0.12 a | 5.10 ± 0.04 b | 5.01 ± 0.04 b | 5.28 ± 0.01 a |
| pH | 4.29 ± 0.005 c | 4.38 ± 0.01 a | 4.39 ± 0.01 a | 4.35 ± 0.01 b |
| Color (EBC-units) | 28.04 ± 1.38 a | 24.95 ± 0.07 b | 21.20 ± 0.06 c | 23.94 ± 0.41 b |
| Foam stability (s) | 135 ± 3.67 c | 212 ±0.82 a | 210 ± 2.04 a | 163 ± 2.04 b |
| Bitterness (BU) | 34.52 ± 0.58 c | 36.48 ± 0.67 b | 38.82 ± 0.91 a | 38.26 ± 1.02 a |
| Total Polyphenols Content (mg/L) | 294 ± 2.51 c | 301 ± 2.28 b | 307 ± 0.74 a | 290 ± 4.79 c |
| Total Nitrogen (mg/L) | 988 ± 3.27 a | 784 ± 6.13 d | 973 ± 1.63 b | 931 ± 1.25 c |
| Protein (%) | 0.618 ± 0.002 a | 0.490 ± 0.004 d | 0.608 ± 0.001 b | 0.581 ± 0.001 c |
| Soluble Protein (mg/L) | 417 ± 5.14 a | 325 ± 3.16 d | 408 ± 1.09 b | 371 ± 4.93 c |
| Sensitive Protein (EBC FHU) | 33.87 ± 1.18 a | 7.93 ± 0.24 c | 15.71 ± 1.33 b | 7.85 ± 0.29 c |
| Physicochemical Parameters | p-Values |
|---|---|
| Real Extract (% w/w) | 1.000 |
| Real Degree of Fermentation (%) | <0.0001 |
| Alcohol (% v/v) | 0.048 |
| pH | 0.157 |
| Color | 0.001 |
| Foam stability (s) | 0.402 |
| Bitterness (BU) | 0.312 |
| Total Polyphenol Content (mg/L) | 0.162 |
| Total Nitrogen (mg/L) | 0.517 |
| Protein (%) | 0.472 |
| Soluble Protein (mg/L) | 0.636 |
| Sensitive Protein (EBC FHU) | 0.001 |
| Sample | Overall Liking | Mean DFC | ||
|---|---|---|---|---|
| Appearance | Odor | Taste | ||
| C | 6.93 ± 1.48 a | 3.34 | 3.06 | 3.50 |
| E | 6.61 ± 1.22 ab | 3.75 * | 3.53 * | 3.74 |
| T | 6.41 ± 1.34 b | 3.72 * | 3.65 * | 3.77 |
| SG | 6.90 ± 1.33 a | 3.31 | 3.26 | 3.52 |
| p-value | 0.024 | 0.004 | 0.335 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cela, N.; Condelli, N.; Perretti, G.; Di Cairano, M.; De Clippeleer, J.; Galgano, F.; De Rouck, G. A Comprehensive Comparison of Gluten-Free Brewing Techniques: Differences in Gluten Reduction Ability, Analytical Attributes, and Hedonic Perception. Beverages 2023, 9, 18. https://doi.org/10.3390/beverages9010018
Cela N, Condelli N, Perretti G, Di Cairano M, De Clippeleer J, Galgano F, De Rouck G. A Comprehensive Comparison of Gluten-Free Brewing Techniques: Differences in Gluten Reduction Ability, Analytical Attributes, and Hedonic Perception. Beverages. 2023; 9(1):18. https://doi.org/10.3390/beverages9010018
Chicago/Turabian StyleCela, Nazarena, Nicola Condelli, Giuseppe Perretti, Maria Di Cairano, Jessika De Clippeleer, Fernanda Galgano, and Gert De Rouck. 2023. "A Comprehensive Comparison of Gluten-Free Brewing Techniques: Differences in Gluten Reduction Ability, Analytical Attributes, and Hedonic Perception" Beverages 9, no. 1: 18. https://doi.org/10.3390/beverages9010018