δ13C-Ethanol as a Potential Exclusionary Criterium for the Authentication of Scotch Whiskies in Taiwan: Normal vs. 3-Parameter Lognormal Distributions of δ13C-Ethanol Found in Single Malt and Blended Scotch Whiskies
Abstract
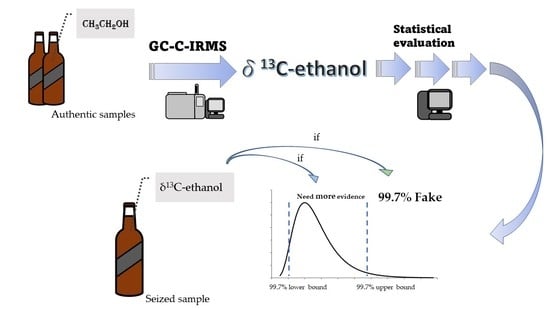
:1. Introduction
2. Materials and Methods
2.1. Preparation of Reference Whiskies and Simulative Samples
2.2. Stable Isotopic Measurement of Ethansol
2.3. Statistical Analysis
3. Results and Discussion
3.1. Measurements and Statistical Analysis of δ13C-ethanol Data in Scotch Whiskies
3.2. Confidence Interval of δ13C-Ethanol Data and Its Implication for Scotch Whiskies
3.3. Exclusionary Criterium for Scotch Whiskies Authentication on the Basis of the Confidence Interval of δ13C-Ethanol Data
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Trade, Scotch Whisky 2021 Exports Report, Scotch Whisky Association. Available online: https://www.scotch-whisky.org.uk/insights/international-trade/ (accessed on 11 February 2021).
- foodNEXT. Rare in the World! Why Does Taiwan Love to Drink Single Malt Whisky so Much? (Chinese Version). Available online: https://reurl.cc/kEXV0r (accessed on 30 October 2020).
- Câmara, J.S.; Marques, J.C.; Perestrelo, R.M.; Rodrigues, F.; Oliveira, L.; Andrade, P.; Caldeira, M. Comparative study of the whisky aroma profile based on headspace solid phase microextraction using different fibre coatings. J. Chromatogr. A 2007, 1150, 198–207. [Google Scholar] [CrossRef] [PubMed]
- González-Arjona, D.; López-Pérez, G.; González-Gallero, V.; González, A.G. Supervised Pattern Recognition Procedures for Discrimination of Whiskeys from Gas Chromatography/Mass Spectrometry Congener Analysis. J. Agric. Food Chem. 2006, 54, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, P.; Dymerski, T.; Wardencki, W.; Namiesnik, J. Chemical composition analysis and authentication of whisky. J. Sci. Food Agric. 2015, 95, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Stupak, M.; Goodall, I.; Tomaniova, M.; Pulkrabova, J.; Hajslova, J. A novel approach to assess the quality and authenticity of Scotch Whisky based on gas chromatography coupled to high resolution mass spectrometry. Anal. Chim. Acta 2018, 1042, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.L.; Hughes, D.M.; Badu-Tawiah, A.K.; Eccles, R.; Goodall, I.; Maher, S. Rapid Scotch Whisky Analysis and Authentication using Desorption Atmospheric Pressure Chemical Ionisation Mass Spectrometry. Sci. Rep. 2019, 9, 7994. [Google Scholar] [CrossRef]
- Kew, W.; Goodall, I.; Clarke, D. Chemical Diversity and Complexity of Scotch Whisky as Revealed by High-Resolution Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2017, 28, 200–213. [Google Scholar] [CrossRef]
- Power, A.C.; Néill, C.N.; Geoghegan, S.; Currivan, S.; Deasy, M.; Cozzolino, D. A Brief History of Whiskey Adulteration and the Role of Spectroscopy Combined with Chemometrics in the Detection of Modern Whiskey Fraud. Beverages 2020, 6, 49. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Boqué, R.; Borràs, E.; Busto, O.; Wardencki, W.; Namieśnik, J.; Dymerski, T. Authentication of whisky due to its botanical origin and way of production by instrumental analysis and multivariate classification methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 849–853. [Google Scholar] [CrossRef]
- MacKenzie, W.M.; Aylott, R.I. Analytical strategies to confirm Scotch whisky authenticity. Part II;: Mobile brand authentication. Analyst 2004, 129, 607–612. [Google Scholar] [CrossRef]
- Ashok, P.C.; Praveen, B.B.; Dholakia, K. Near infrared spectroscopic analysis of single malt Scotch whisky on an optofluidic chip. Opt. Express 2011, 19, 22982–22992. [Google Scholar] [CrossRef] [Green Version]
- Sujka, K.; Koczńn, P. The application of FT-IR spectroscopy in discrimination of differently originated and aged whisky. Eur. Food Res. Technol. 2018, 244, 2019–2025. [Google Scholar] [CrossRef]
- Martins, R.; Talhavini, M.; Vieira, M.L.; Zacca, J.J.; Braga, J.W.B. Discrimination of whisky brands and counterfeit identification by UV–Vis spectroscopy and multivariate data analysis. Food Chem. 2017, 229, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.M.; Zeren, C.; Aydin, Z.; Akcan, R.; Dokuyucu, R.; Keten, A.; Cekin, N. Analysis of methanol and its derivatives in illegally produced alcoholic beverages. J. Forensic Leg. Med. 2015, 33, 56–60. [Google Scholar] [CrossRef]
- Aylott, R.I.; Clyne, A.H.; Fox, A.P.; Walker, D.A. Analytical strategies to confirm Scotch whisky authenticity. Analyst 1994, 119, 1741–1746. [Google Scholar] [CrossRef]
- Aylott, R.I.; MacKenzie, W.M. Analytical Strategies to Confirm the Generic Authenticity of Scotch Whisky. J. Inst. Brew. 2010, 116, 215–229. [Google Scholar] [CrossRef]
- Singer, D.D. The proportion of 2-methylbutanol and 3-methylbutanol in some brandies and whiskies as determined by direct gas chromatography. Analyst 1966, 91, 790–794. [Google Scholar] [CrossRef]
- Huang, H.-W.; Chang, W.-T. Methanol Concentration as a Preceding Eliminative Marker for the Authentication of Scotch Whiskies in Taiwan. Forensic Sci. Int. 2022, 339, 111413. [Google Scholar] [CrossRef]
- Parker, G.; Kelly, S.D.; Sharman, M.; Dennis, M.J.; Howie, D. Investigation into the use of carbon isotope ratios (13C/12C) of Scotch whisky congeners to establish brand authenticity using gas chromatography-combustion-isotope ratio mass spectrometry. Food Chem. 1998, 63, 423–428. [Google Scholar] [CrossRef]
- Rhodes, C.N.; Heaton, K.; Goodall, I.; Brereton, P.A. Gas chromatography carbon isotope ratio mass spectrometry applied to the detection of neutral alcohol in Scotch whisky: An internal reference approach. Food Chem. 2009, 114, 697–701. [Google Scholar] [CrossRef]
- Meier-Augenstein, W. Applied gas chromatography coupled to isotope ratio mass spectrometry. J. Chromatogr. A 1999, 842, 351–371. [Google Scholar] [CrossRef]
- Meier-Augenstein, W.; Kemp, H.F.; Hardie, S. Detection of counterfeit scotch whisky by 2H and 18O stable isotope analysis. Food Chem. 2012, 133, 1070–1074. [Google Scholar] [CrossRef]
- Cook, G.; Dunbar, E.; Tripney, B.; Fabel, D. Using Carbon Isotopes to Fight the Rise in Fraudulent Whisky. Radiocarbon 2020, 62, 51–62. [Google Scholar] [CrossRef]
- Bender, M.M. Variations in the 13C/12C ratios of plants in relation to the pathway of photosynthetic carbon dioxide fixation. Phytochemistry 1971, 10, 1239–1244. [Google Scholar] [CrossRef]
- Gregoricka, L.A. Stable isotopes. In The International Encyclopedia of Biological Anthropology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; ISBN 9781118584422. [Google Scholar] [CrossRef]
- Guy, R.D.; Fogel, M.; Berry, J.A. Photosynthetic Fractionation of the Stable Isotopes of Oxygen and Carbon. Plant Physiol. 1993, 101, 37–47. [Google Scholar] [CrossRef]
- Meier-Augenstein, W. Stable Isotope Forensics: An Introduction to the Forensic Application of Stable Isotope Analysis; Academic Press: Cambridge, MA, USA, 2010; ISBN 978-0-470-51705-5. [Google Scholar]
- The Scotch Whisky Regulations 2009 No. 2890. 2009. Available online: https://www.legislation.gov.uk/uksi/2009/2890/pdfs/uksi_20092890_en.pdf (accessed on 23 November 2009).
- Stewart, G.; Russell, I. Whisky: Technology, Production and Marketing, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; ISBN 978-0124017351. [Google Scholar]
- Simpkins, W.A.; Rigby, D. Detection of the illicit extension of potable spirituous liquors using13C:12C ratios. J. Sci. Food Agric. 1982, 33, 898–903. [Google Scholar] [CrossRef]
- Bauer-Christoph, C.; Wachter, H.; Christoph, N.; Rossmann, A.; Adam, L. Assignment of raw material and authentication of spirits by gas chromatography, hydrogen- and carbon-isotope ratio measurements I. Analytical methods and results of a study of commercial products. Z. Lebensm. Unters. Forsch. 1997, 204, 445–452. [Google Scholar] [CrossRef]
- Calderone, G.; Holland, M.V.; Reneiro, F.; Guillou, C. An Overview of Isotopic Analysis for the Control of Alcoholic Drinks and Spirits. European Commission Joint Research Centre. 2005, pp. 1–31. Available online: https://www.researchgate.net/publication/285365882 (accessed on 1 January 2005).
- Martin, G.J.; Martin, M.L.; Mabon, F.; Michon, M.J. A new method for the identification of the origin of ethanols in grain and fruit spirits: High-field quantitative deuterium nuclear magnetic resonance at the natural abundance level. J. Agric. Food Chem. 1983, 31, 311–315. [Google Scholar] [CrossRef]
- Gajek, M.; Pawlaczyk, A.; Maćkiewicz, E.; Albińska, J.; Wysocki, P.; Jóźwik, K.; Szynkowska-Jóźwik, M.I. Assessment of the Authenticity of Whisky Samples Based on the Multi-Elemental and Multivariate Analysis. Foods 2022, 11, 2810. [Google Scholar] [CrossRef]
- Limpert, E.; Stahel, W.A.; Abbt, M. Log-normal Distributions across the Sciences: Keys and Clues. BioScience 2001, 51, 341–352. [Google Scholar] [CrossRef]
- Motulsky, H.J. Common misconceptions about data analysis and statistics. Br. J. Pharmacol. 2015, 172, 2126–2132. [Google Scholar] [CrossRef]
- Razali, N.M. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson–Darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- Cousineau, D.; Engmann, S. Comparing distributions: The two-sample Anderson–Darling test as an alternative to the Kolmogorov–Smirnov test. J. Appl. Quant. Methods 2011, 6, 1–17. [Google Scholar]
- Siano, D.B. The log-normal distribution function. J. Chem. Educ. 1972, 49, 755–757. [Google Scholar] [CrossRef]
- Annila, A.; Grönholm, T. Natural Distribution. Math. Biosci. 2007, 210, 659–667. [Google Scholar]
- Graddum, J.H. Lognormal distribution. Nature 1945, 156, 463–466. [Google Scholar] [CrossRef]
- Koch, L. The logarithm in biology 1. Mechanisms generating the log-normal distribution exactly. J. Theor. Biol. 1966, 23, 276–290. [Google Scholar] [CrossRef] [PubMed]
- The Tobacco and Alcohol Administration Act, Ministry of Finance (R.O.C). Available online: https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=G0330011 (accessed on 27 December 2017).
- Spitzke, M.E.; Fauhl-Hassek, C. Determination of the 13C/12C ratios of ethanol and higher alcohols in wine by GC-C-IRMS analysis. Eur. Food Res. Technol. 2010, 231, 247–257. [Google Scholar] [CrossRef]




| Anderson–Darling Method | Kolmogorov–Smirnov Method | |||||
|---|---|---|---|---|---|---|
| Statistic | df | Sig. | Statistic | df | Sig. | |
| Single malt Scotch whisky | 0.152 | 51 | 0.958 | 0.479 | 51 | 0.976 |
| Blended Scotch whisky | 0.526 | 34 | 0.167 | 0.762 | 34 | 0.607 |
| No. of Samples | Min | Max | Threshold | Mean | Standard Deviation * | Standard Deviation ** | |
|---|---|---|---|---|---|---|---|
| Single malt Scotch whisky | 51 | −29.19‰ | −24.21‰ | −49.97 ‰ | −26.89‰ | 1.050 | 0.021 |
| Blended Scotch whisky | 34 | −28.04‰ | −17.14‰ | −30.35 ‰ | −25.13‰ | 1.542 | 0.188 |
| 68.3% | 95.5% | 99.7% | ||||
|---|---|---|---|---|---|---|
| Upper Bound | Lower Bound | Upper Bound | Lower Bound | Upper Bound | Lower Bound | |
| Unit: (‰) | ||||||
| Single malt Scotch whisky | −25.73 | −28.01 | −24.50 | −29.06 | −23.21 | −30.07 |
| Blended Scotch whisky | −22.29 | −26.96 | −17.93 | −28.15 | −11.19 | −28.93 |
| Supplier | Taiwan Sugar Corp. | Taiwan Tobacco & Liquor Corp. | Echo Chemical Co., Ltd. |
|---|---|---|---|
| δ13C-ethanol | −13.50 (‰) | −14.63 (‰) | −24.76 (‰) |
| Type | C4-derived spirit | C4-derived spirit | C3-derived spirit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-W.; Chang, W.-T. δ13C-Ethanol as a Potential Exclusionary Criterium for the Authentication of Scotch Whiskies in Taiwan: Normal vs. 3-Parameter Lognormal Distributions of δ13C-Ethanol Found in Single Malt and Blended Scotch Whiskies. Beverages 2023, 9, 13. https://doi.org/10.3390/beverages9010013
Huang H-W, Chang W-T. δ13C-Ethanol as a Potential Exclusionary Criterium for the Authentication of Scotch Whiskies in Taiwan: Normal vs. 3-Parameter Lognormal Distributions of δ13C-Ethanol Found in Single Malt and Blended Scotch Whiskies. Beverages. 2023; 9(1):13. https://doi.org/10.3390/beverages9010013
Chicago/Turabian StyleHuang, Hsiao-Wen, and Wei-Tun Chang. 2023. "δ13C-Ethanol as a Potential Exclusionary Criterium for the Authentication of Scotch Whiskies in Taiwan: Normal vs. 3-Parameter Lognormal Distributions of δ13C-Ethanol Found in Single Malt and Blended Scotch Whiskies" Beverages 9, no. 1: 13. https://doi.org/10.3390/beverages9010013