3.1. FAN and Amino Acid Profiles of GF Worts
Nitrogen metabolism by yeast during primary fermentation is complex and dependent on the other constituents in the wort (e.g., FS), the type of yeast utilized, and fermentation conditions, but in terms of amino acids, it is dependent on both the total concentration of assimilable nitrogen (FAN) and the concentrations of individual amino acids [
4,
8,
9]. The uptake of amino acids from the wort by brewing yeasts tends to follow a specific order, and the amino acids are classified into 4 groups based on the uptake preference Group A (most rapidly assimilated) to Group D, solely proline (little to no utilization). The amino acids were segmented into their respective groups using the initial classifications proposed by Jones and Pierce in 1964, except that methionine was included in Group A, as proposed by Lekkas, Hill, and Stewart in 2014 [
1,
25]. The sums of these amino acid groups and the relative abundances of each amino acid within its group are shown for both barley and each GF wort in
Figure 1. An unidentified peak in the chromatograms varied significantly across the malts, suggesting the presence of an amino-containing compound not included in the amino acid standards was of a similar concentration to the α-amino acids in the worts. Injection and derivatization of a γ-aminobutyric acid (GABA) standard indicated that this unknown peak was indeed GABA, and therefore was identified and quantified as such in each of the worts. GABA is a non-proteogenic γ-amino acid found in the wort that can react with the ninhydrin reagent [
30], thus included in the FAN measurement, and has been shown to be utilized by yeasts [
11,
35,
36,
37], suggesting it can also be a source of nitrogen for yeast in beer. However, investigations into its role in beer fermentations are limited, and it is not classified into one of the utilization groups. We then defined total amino acid content as the sum of all quantified amino acids, and total usable amino acids as the sum of all the α-amino acids and GABA concentrations in the wort, excluding proline, which requires an aerobic environment for utilization and thus is not utilized in a typical beer (i.e., anaerobic) fermentation [
1].
As wort fermentations require both sufficient concentrations of yeast assimilable sugar and nitrogen sources, the FAN and amino acid profiles of GF worts made by the ExGM mashing procedure [
20] were investigated. While several of these worts from GF malts (millet, sorghum, ivory teff, brown teff, and GFB1) contained similar or even higher concentrations of FS compared to a barley-derived wort prepared using a common isothermal infusion mash, deficiencies in FAN would require supplementation, and changes in the relative abundance of the amino acid profiles could lead to differences in fermentation characteristics. As shown in
Figure 1A, there were large differences in the FAN content across the tested worts (the individual amino acid concentrations are provided in the
supplementary data). Using the typical goal of producing a wort with 150–200 mg/L FAN, the GFB1 and sorghum malt worts could be considered FAN deficient (143 mg/L and 129 mg/L, respectively), indicating a need for further optimization. The corn, buckwheat, and rice malt worts were all deficient in FAN and deficient in FS using this mashing process, suggesting they lacked the required level of enzyme activity and would not currently be suitable for brewing using this approach. In contrast, the ivory and brown teff malt samples were high in FAN (445 mg/L and 490 mg/L) and produced high concentrations of FS (~90 g/L [
20]), so while these malts were highly successful in producing FAN and FS, excess FAN could have some negative impacts on beer flavor or microbial stability [
4,
38]. The barley malt wort (201 mg/L FAN) and the millet malt wort (169 mg/L FAN) both had FAN contents within the suggested range. Overall, these results indicate that GF malts can be utilized successfully to produce worts with sufficient concentrations of FS and FAN. While some of the worts shown in
Figure 1A were either FAN-sufficient or -abundant, differences in the amino acid profiles could lead to differences in the fermentation and flavor profiles.
As shown in
Figure 1B,C, there were significant differences in the total amino acid contents for the various worts, with usable amino acids generally following the same trend as FAN measurements. On average, the measured FAN content is ~1/8 the sum of the usable amino acid concentrations because the ninhydrin reaction measures the nitrogen component of the amino group in each amino acid (nitrogen accounts for ~1/8 of the average mass of the amino acids). As is also shown in
Figure 1C, there were significant differences in the GABA and proline contents of the worts. Proline was the most abundant free amino acid in the barley malt wort, as reported previously [
1], and the barley malt contained more proline than any GF wort tested. Although proline is not typically utilized by yeast, it is an osmolyte that can serve as a cryoprotectant for yeast [
39], and the proline-specific yeast permease can also uptake GABA [
40], so differences between barley and GF malts in proline and GABA could lead to differences in fermentation and fermentation metabolites. Additionally, the proteins in each malt are likely different; for instance, barley is gluten-containing, and the GF malts are not. One of the hallmarks of immunogenic gluten peptides is the presence of proline-rich regions [
41], and so the high proline content in a barley wort may be due to some gluten proteolysis during malting and mashing, but gluten peptides survive the brewing and fermentation processes. In contrast, GF malts may contain less proline overall compared to barley [
41,
42], so the lower proline content observed in these GF worts may be due in part to the lack of gluten proteins. The proline content alone does not make a gluten protein, as they are a complex suite of proteins found in barley, wheat, and rye, and differing individual sequences of the gluten proteins from each grain can produce differing effects in the immunogenicity, but the proline differences observed here between barley and GF malts could be some initial rationale for further investigation as to why the proteins of GF malts tend not to trigger gluten-sensitive individuals.
The underlying distribution of amino acids in the wort is important, as many amino acids serve as metabolic precursors to flavor-active compounds such as fusel alcohols and acetate esters via the Ehrlich pathway, diacetyl or other vicinal diketones, or sulfur-containing compounds via yeast sulfur assimilation and sulfur amino acid pathways [
4,
5,
8,
9,
43,
44]. As shown in
Figure 1D–F, there are differences in the relative abundances of most amino acids within each utilization group between the produced worts. Millet, ivory teff, and brown teff malt worts all had higher relative abundances of methionine and lower phenylalanine than the barley wort, but overall, the millet malt wort had a similar profile to the barley malt wort (except proline). While the differences in relative abundances across these worts could lead to differences in the generation of flavor-active compounds, the relative profiles still need to be considered in the context of the absolute amino acid content as well. The effects of high amino acid contents on the fermentation of a teff wort are of particular interest as the FAN content was more than double that of barley, and it is unknown what impact this would have on the fermentation and flavor profiles. As the millet was close in FAN content to barley, could produce adequate FS, and was commercially available, we decided to utilize millet as a model GF malt to investigate if the FAN and amino acid content of a wort could be improved by modifying the mashing procedures. The remainder of this study now focuses on various manipulations to mashing with millet malt to investigate the role of mashing in the generation of amino acids to increase the wort amino acid content.
3.2. Amino Acid Content in the Mash from Millet Malt during the Enzyme Extraction Phase
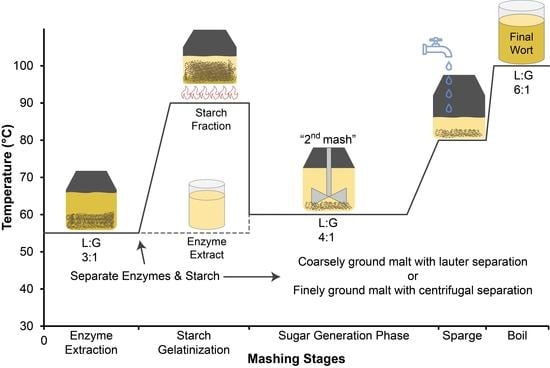
To investigate the effect of processing conditions such as grind size or exogenous enzyme supplementation on the generation of amino acids during the enzyme extraction from a millet malt mash, samples were taken from the enzyme extract at the conclusion of the enzyme extraction phase. In all these experiments, the enzyme extraction phase was held constant at 55 °C for 30 min at an L:G of 3:1, and the malt grind size (fine or coarse), extract separation technique (centrifuge or lauter), or enzyme system (endogenous or exogenous) was varied. As this step at 55 °C is comparable to the time and temperature regimes utilized for a protein rest in conventional barley brewing, it was expected that the different mashing modifications might lead to different amino acid contents in these extracts. In our previous study that analyzed the fermentable sugar concentrations of these samples, freely extracted sugar concentrations were higher with more finely ground samples, and the second batch had higher freely extracted fermentable sugars [
27], so we would have expected a similar effect here. However, as shown in
Figure 2A, there was largely no effect of our treatments on the overall amino acid content of this first wort (an analysis of all the worts is provided in
Supplementary Figure S1). A fine or coarse grind yielded similar amino acid concentrations and profiles, and the addition of exogenous enzymes containing a neutral protease did not produce a large effect on the amino acid content. Rather, as shown in
Figure 2B, the differences in the worts were largely due to which batch of millet was utilized, as CL-SR and CL-Exo were generated from the same batch of millet, and this batch tended to have higher GABA concentrations compared to the one utilized for FF-55 °C. The lack of effect of the grind size on amino acid extraction is interesting as it suggests that proteolysis at this stage may only be on small, easily extractable peptides that were likely generated during malting, and not larger protein bodies that might be rendered more accessible due to milling. The addition of an exogenous protease also had no significant effect on amino acid generation, suggesting that either the activity of the endogenous proteases was not limiting or, we suspect more likely, the added protease was not active under these conditions. To maintain consistency across these experiments, conditions deviated slightly from the protease manufacturer’s recommendations in terms of pH (acidification not recommended) and temperature (recommended maximum of 53 °C). Overall, these modifications to the initial phase of mashing had a minimal impact on amino acid production, leading us to further investigate whether there were any differences in amino acid generation between these protocols throughout the subsequent second mash.
3.3. Amino Acid Generation across a Mash
There is no consensus on the percentage of amino acids in a wort attributed to the result of mashing versus what is already present in the malt [
21,
23], but many barley malt step mashes utilize a protein rest of ~30 min at 55 °C or lower, and some commercial brewers will skip a protein rest all together, instead subjecting the mash to temperatures ≥ 62 °C. Given that good FS production was shown at 55 °C [
20,
27], we hypothesized that extended mashes at this temperature near that of a protein rest may be a simple avenue for improving the FAN content of a GF wort. The results shown in
Figure 3 demonstrate the time-dependent concentration of 2 amino acids from each utilization group across the FF, CL-SR, and CL-Exo experiments, and graphs for all amino acids across time are provided as
Supplemental Figures S2–S5, and values for the individual amino acid concentrations are provided in the
supplementary data. As shown in all the panels of
Figure 3, the concentration of amino acids that can be generated during the second mash was minimal. While
Figure 3A,D,E show some change in those amino acids over time, arginine increased the most of any amino acid, and yet only increased 13% over the 2 h mash. As seen with the extracts, the batch of millet malt affected the amino acid content, while the grind size and addition of the exogenous enzymes had a minimal effect on amino acid generation. However, looking more closely at the FF samples across the three mashing temperatures (55–65 °C), there was some change in arginine, isoleucine, methionine, alanine, and tyrosine between 55 and 60 °C (all
p ≤ 0.024), but no change at 65 °C (all
p ≥ 0.162). This suggests that although the overall effects are minimal, some amino acid generation is possible within the relatively lower temperature range of 55–60 °C. Interestingly, the above-mentioned amino acids have hydrophobic side chains (except arginine), suggesting that exopeptidase(s) generating these amino acids may prefer substrates with terminal hydrophobic residues. Carboxypeptidases with similar product specificities (carboxypeptidase III) have been reported in triticale, wheat, and barley [
45], and a similar enzyme in millet could be responsible for the formation of these amino acids. As some limited amino acid generation is possible during the mash, what might be limiting more significant amino acid generation in this system was the next investigation.
3.4. FAN and Amino Acid Profiles Produced from Millet under Different Mashing Conditions
As there were little observed differences in the amino acid concentrations in the extracts over the course of the mash, we decided to analyze two additional millet malt mashing treatments: the XT (55 °C for 2 h 45 min, no gelatinization step) and IE (temperature abused to denature enzyme activity) from our previous study [
20] to determine if the gelatinization step in our experiments might be inhibiting protein degradation, or if the millet malt used could only produce small concentrations of amino acids overall during mashing.
Figure 4A,B show the FAN and usable amino acid concentrations at the endpoints of the various mashing experiments. For FAN, no significant differences were observed between barley or any of the millet mashes, except for the IE samples. These results show that a millet wort can match the FAN content of a barley wort (~200 mg/L). Furthermore, given that the IE mash was intentionally temperature-abused to assess the endogenous background content of FS and amino acids in the malt, these results suggest that some proteolysis occurred during the 30 min enzyme extraction at 55 °C leading to the production of the additional observed amino acids. However, the XT samples held at a constant temperature of 55 °C did not differ in usable amino acid content from any other millet mash (except the IE samples), suggesting that the gelatinization step following enzyme extraction is not limiting with respect to additional proteolytic reactions, but that nearly all the additional amino acids observed occurred within 30 min at 55 °C. These results demonstrate that amino acids can be generated during the initial enzyme extraction phase of the mash, but that some mechanism is limiting further protein degradation in this system.
The two main classes of protein-degrading enzymes (endopeptidases and exopeptidases) are both required to efficiently produce small peptides and free amino acids in malts. Despite the different enzymatic properties of the various peptidase classes (pH, temperature, substrate, etc.) [
22,
23,
24,
45], in mashing, the major variable that a brewer can change to modify proteolytic activity is temperature (mash pH can be changed, but modification to <5 where many peptidases have high activity is probably not reasonable), so a brewer should utilize temperatures where the peptidases are thermostable if a goal is to maximize proteolysis. As our results show, some proteolysis occurs during the enzyme extraction at 55 °C, which indicates that some endo- and exoproteolytic activities remain in the malt; however, as this proteolysis seems limited to a short timeframe at 55 °C, they likely are not thermostable at that temperature.
To ascertain whether endo- or exopeptidases are limiting amino acid generation beyond the enzyme extraction, the soluble nitrogen (SN) contents of the worts were evaluated. As each wort had similar amino acid concentrations regardless of modifying the mashing temperature or adding exogenous enzymes, differences in soluble nitrogen content could suggest endopeptidases may still be active in the wort and capable of producing soluble peptides. As the combustion analysis measures all nitrogenous species in the wort, and FAN measures the nitrogen primarily from amino acids and small peptides, assuming the total nitrogen content of a wort is dominated by proteins (rather than other nitrogenous compounds) one can estimate the percentages of SN contributed by FAN and soluble proteins. The SN analysis in
Figure 4C shows only that the IE sample was significantly different from the other millet worts in terms of total soluble nitrogen due to thermally induced enzyme denaturation, and that in the remaining millet worts, FAN accounted for between 27 and 35% of the SN. Since FAN does not account for most of the measured soluble nitrogen, there are likely soluble peptides remaining in the wort that were not measured by the FAN analysis, suggesting that incomplete peptide degradation by exopeptidases (possibly due to inactivation) occurred. Furthermore, that the SN values are not different between millet samples mashed at different temperatures suggests that endopeptidases do not remain active to any significant degree beyond the enzyme extraction step, and both enzyme classes may be limiting. Furthermore, all the millet malt worts had significantly less soluble nitrogen content than the barley malt wort, despite having similar amino acid contents (total amino acids in barley wort: 2133 mg/L vs. average of all millet worts: 1938 mg/L). This suggests there may be more soluble peptides present in the barley malt wort, as FAN only accounted for 17% of the SN in the barley wort, and differences in soluble peptides could lead to attributes such as good foam formation and stability that millet-based worts may be lacking. This may be due to the less peptidase activity in a millet malt that survives kilning and could be utilized in the enzyme extraction or less overall proteolytic degradation during malting. Although previous studies have demonstrated that barley’s endopeptidases can survive kilning and can affect the SN and FAN content of a wort, the proteolytic activities of malts remain relatively understudied [
22,
24].
The amino acid profiles of three different final millet worts are shown in
Figure 4D, and, when compared to the barley malt wort, show that although there are differences in amino acids such as proline, glutamine, serine, GABA, phenylalanine, and leucine, the overall amino acid profile of a millet malt wort can be similar to a barley malt wort. While these differences could still lead to changes in concentrations of higher alcohols in finished beers, there is no obvious amino acid deficiency in the millet malt-based worts, suggesting their promise as a base malt for GF beer production. Returning to our overall hypothesis that the amino acid content of a wort could be modified by maintaining milder temperatures (55–60 °C) across the mash, a small quantity of amino acids can be generated, but the only step where significant protein degradation can likely occur is during the enzyme extraction.
3.5. Effect of Mild Temperature Mashing on Amino Acid Generation
As it appeared that some amino acids could be generated in the enzyme extraction phase, but the window of protein degradation was limited, we hypothesized that the peptidases present in the system may not be thermostable at 55 °C; therefore, additional mashes were performed where only the enzyme extraction phase was performed and was held at 40 °C for 2 h, with aliquots taken every 30 min to assess the change in amino acid content over time. As the exogenous enzymes did not appear to have a major effect at temperatures ≥ 55 °C, the effect of the exogenous enzymes at this lower temperature extraction phase was also tested.
Figure 5 shows that at 40 °C, amino acid concentrations could increase over the course of 2 h (individual amino acid concentrations are provided in the
supplementary data). For the endogenous millet system, 30 min at 40 °C extracted slightly less usable amino acids into the wort than the average of the extracts at 55 °C (2284 mg/L vs. 2577 mg/L), but more will be produced at 40 °C if the time is increased. After 2 h at 40 °C, the endogenous millet system increased usable amino acids (CL-40 °C 3191 mg/L, 40% increase; CL-Exo 40 °C 3033 mg/L, 38% increase, respectively over 2 h) and increased 614 mg/L compared to an extraction for 30 min at 55 °C. As these experiments did not undergo the entire mash process, we did not measure what the amino acid content would be in the final wort, but across all the other experiments where the amino acid concentrations varied little beyond the enzyme extraction step, the total (including proline) amino acid content in the final wort was 68.11 ± 2.64% of the total amino acid content of the extract (despite a change in volume ratio from L:G 3:1 to 6:1, the drop is not by half as the sparge process rinses the grain). Using that modified dilution factor, it was estimated what the total wort total amino acid concentrations could have been for CL-40 °C and CL-Exo 40 °C if the process was continued with a second mash temperature of ≥55 °C (estimated final wort total amino acids: 2364 mg/L and 2260 mg/L, respectively), with an estimated increase of 418 mg/L usable amino acids in the final wort for CL-40 °C. Similar estimations for FAN and soluble nitrogen (2 h: 512 mg/L, 0.14% nitrogen; estimated final wort: 348 mg/L, 0.095% nitrogen) would suggest that an extended mash at a mild temperature will improve proteolytic degradation in the millet malt mash, producing a final wort with higher amino acid content, FAN, and soluble nitrogen.
As in the extractions at 55 °C, it appears the addition of exogenous enzymes did not markedly improve amino acid production at 40 °C (
Figure 5A,B,E), suggesting that the thermostability of the exogenous protease was not limiting. While the possibility that acidifying the mash may have limited the proteolytic activity of the neutral protease in the exogenous enzyme cocktail cannot be ruled out, under the conditions tested here, the addition of the exogenous enzymes appears to have slightly hindered the proteolytic activity of the mash. While an underlying mechanism for this result was not explored, one hypothesis could be that the exogenous protease, if active, may be producing peptides that are not ideal substrates for the millet’s exopeptidases.
In this mild (40 °C) temperature system, it was observed that every proteogenic amino acid increased in concentration (to differing extents) across the 2 h step, except for glutamic acid, which decreased. GABA also increased in these mashes, and although non-proteogenic, it can be enzymatically produced by the decarboxylation of glutamic acid by the enzyme glutamate decarboxylase found in plants, animals, and yeasts [
35,
37,
43]. The increase in GABA and decrease in glutamate suggest that there may be some glutamate decarboxylase activity in these millet malts.
These results provide some evidence to support our initial hypothesis that mashing can be utilized to modify the amino acid content of the wort, but that it must occur during a rest in the mashing scheduling at a temperature where the proteolytic enzymes are thermostable. In our system, this translated to a maximum of 55 °C for some amino acid generation, but a lower temperature for a longer time if a larger amount of amino acid generation was required.
3.7. The Potential Impact of GF Worts on Fermentation
Overall, all the results shown here demonstrate that GF worts can vary both in terms of FAN and amino acid profile, influenced by the malt used (
Section 3.1), or in the millet malt experiments by the times and temperatures of the initial enzyme extraction phase and/or protein rest (
Section 3.5). Questions remain as to what effect differences in the amino acid profiles and FS may have on the subsequent fermentations. Higher amino acid contents would likely lead to additional products of Maillard browning during the boil, and the various flavor-active compounds produced by Strecker degradation of those intermediates [
8]. Differences in leucine, isoleucine, and phenylalanine concentrations could lead to differences in the formation of isoamyl alcohol, amyl alcohol, and phenylethanol, and flavor-active esters derived from the condensation of alcohols with acetyl-CoA or longer fatty acids (isoamyl acetate, phenylethyl acetate, ethyl hexanoate, etc.) directly impacting the beer flavor. Prior research has shown that the fermentation of worts with higher concentrations of monosaccharides tends to produce beers with higher concentrations of higher alcohols and esters [
10,
46]. As one of the results observed from these GF worts is they tended to have higher glucose and fructose concentrations compared to barley [
20], those differences in both sugar distribution and the amino acid results shown here could also lead to differences in the production of those important flavor-active metabolites.
Different GABA concentrations in the GF worts compared to barley were also observed, but studies tracking the utilization of GABA in wort and its effects on beer fermentation are limited. Some studies in wine show that GABA can be utilized to a significant degree, and that its primary metabolic product is succinate [
36,
37]. However, the extent to which GABA from a GF wort will be utilized in relation to the other amino acids is unknown, as is its effect on fermentation or flavor profile. Yeast generally has two primary mechanisms of amino acid uptake, a general amino acid permease system that dominates in nitrogen-poor environments, or more specific amino acid transporters in nitrogen-rich environments [
47], and GABA can been assimilated by yeast through the general system, its own specific permeases, or through proline permeases [
40]. The degree to which GABA can be utilized in a wort would likely be dependent on its concentration, the total amino acid concentration, potentially proline concentration, and any other variable that could affect yeast fermentation. Therefore, further research is needed to elucidate the role of increased GABA concentrations present in these GF worts on finished beer characteristics.
While the simplest method to improve the FAN content of a wort may be for a brewer to supplement with exogenous sources of nitrogen, there has been relatively little research investigating avenues to modify the amino acid content and profile of a wort, even when using more traditional barley malts [
22]. Similarly, while yeast nutrient supplements can provide some benefits to yeast nutrition, the supplements can be variable and are often under characterized, and their utilization (especially at higher gravities) without careful consideration could have unintended consequences on the resulting fermentation [
48]. This means further research on various wort components and manners in which brewers could influence those components could aid in identifying more targeted approaches to supplement potential specific wort nutrient deficiencies, such as amino acids. However, beyond a way to support a healthy fermentation, amino acids will serve as flavor precursors, and recent research has sought to better manipulate the amino acid profile of a wort to drive the specific formation of fusel alcohols and acetates [
49]. In that work, the authors sought to improve the release of specific amino acids (valine, phenylalanine, leucine, isoleucine) through the use of exogenous endo- and exoproteases to drive the increased production of specific fusel alcohols and acetates. While this particular study was performed with exogenous enzymes, the results do suggest that better understanding and manipulating the proteolytic mechanisms in a mash could be a potential avenue to purposefully drive beer flavor differences during brewing. Our results here show that the amino acid content of a millet malt wort can be improved by relying on the endogenous proteolytic system with a simple extension of a mild temperature step in the mashing schedule (
Figure 5), potentially providing new opportunities to drive flavor differences without requiring exogenous ingredients. While improving amino acid generation is not strictly necessary in most cases of traditional barley malt brewing or here with the millet malt samples, this study presents evidence that the endogenous proteolytic system could provide more of a direct role in the formation of amino acids during mashing, and that a better understanding of these systems could give brewers new opportunities to influence beer flavor and quality.