Development and Characterization of Zein/Ag-Sr Doped Mesoporous Bioactive Glass Nanoparticles Coatings for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
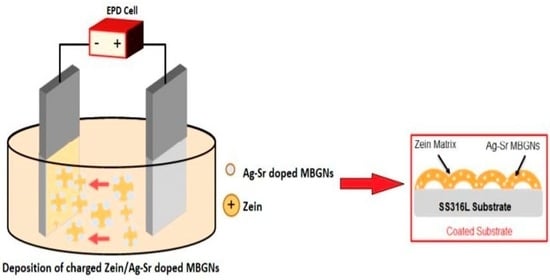
2.2. Suspension Preparation
2.3. EPD Process
2.4. Materials Characterization
2.5. Biological Characterization
3. Results and Discussion
3.1. EPD Kinetics and Suspension Stability
3.2. Morphology of Synthesized Ag-Sr Doped MBGNs and Coatings
3.3. Deposition Yield
3.4. Adhesion Strength
3.5. Wettability Studies
3.6. Wear Studies
3.7. Corrosion Studies
3.8. Biological Characterization
4. Conclusions
- High deposition yield of the coatings was obtained at higher voltages, i.e., 25 V.
- Optical microscopic images showed uniform deposition of coatings on the surface of SS substrates (at optimum deposition parameters). SEM images illustrated the homogenous distribution of Ag-Sr doped MBGNs throughout the zein matrix along with the presence of spherical agglomerates, indicating good mechanical integration of zein/Ag-Sr doped MBGN coatings.
- Pencil scratch test results showed increased hardness of zein/Ag-Sr doped MBGN coatings deposited at 25 V, from which it was inferred that coatings developed at higher voltage showed improved adhesion strength. Furthermore, zein/Ag-Sr doped MBGN coatings exhibited good adhesion strength during bend tests.
- Zein/Ag-Sr doped MBGN coatings deposited at 25 V demonstrated good wettability properties (contact angle of 72 ± 2°), suitable for initial protein and subsequent osteoblast cell attachment.
- Moreover, zein/Ag-Sr doped MBGN coatings showed good wear and corrosion resistance as compared to that of bare SS substrates.
- Coatings exhibited good antibacterial and bioactive potential.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shinde, A.S.S.; Sumant, O. Medical Implant Market by Product Type (Orthopedic Implants, Cardiovascular Implants, Spinal Implant, Neurostimulators, Ophthalmic Implants, Dental Implants, Facial Implants, and Breast Implants) and Biomaterial Type (Metallic Biomaterials, Ceramic Biomaterials, Polymers Biomaterials, and Natural Biomaterials): Global Opportunity Analysis and Industry Forecast, 2020–2027; Allied market Research: Portland, OR, USA, 2020. [Google Scholar]
- Kuhn, L.T. 6—Biomaterials. In Introduction to Biomedical Engineering, 2nd ed.; Enderle, J.D., Blanchard, S.M., Bronzino, J.D., Eds.; Academic Press: Boston, MA, USA, 2005; pp. 255–312. [Google Scholar]
- Kastellorizios, M.; Tipnis, N.; Burgess, D.J. Foreign Body Reaction to Subcutaneous Implants; Springer: Cham, Switzerland, 2015; pp. 93–108. [Google Scholar]
- Godbole, N.; Yadav, S.; Manickam, R.; Belemkar, S. A Review on Surface Treatment of Stainless Steel Orthopedic Implants. Int. J. Pharm. Sci. Rev. Res. 2016, 36, 190–194. [Google Scholar]
- Balestriere, M.A.; Schuhladen, K.; Seitz, K.H.; Boccaccini, A.R.; Cere, S.M.; Ballarre, J. Sol-gel coatings incorporating borosilicate bioactive glass enhance anti corrosive and surface performance of stainless steel implants. J. Electroanal. Chem. 2020, 876, 114735. [Google Scholar] [CrossRef]
- Narmada, I.B.; Baya, R.A.; Hamid, T.J.J.o.I.D.; Research, M. Nickel and Chromium Ions Release from Stainless Steel Bracket Immersed in Fluoridated Mouthwash. J. Int. Dent. Med. Res. 2018, 11, 294–298. [Google Scholar]
- Patnaik, L.; Maity, S.R.; Kumar, S. Status of nickel free stainless steel in biomedical field: A review of last 10 years and what else can be done. Mater. Today Proc. 2020, 26, 638–643. [Google Scholar] [CrossRef]
- Hebert, C.K.; Williams, R.E.; Levy, R.S.; Barrack, R.L. Cost of Treating an Infected Total Knee Replacement. Clin. Orthop. Relat. Res. 1996, 331, 140–145. [Google Scholar] [CrossRef]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical Implants: Corrosion and its Prevention—A Review. Recent Patents Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Nouri, A.; Wen, C. 1—Introduction to surface coating and modification for metallic biomaterials. In Surface Coating and Modification of Metallic Biomaterials; Wen, C., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 3–60. [Google Scholar]
- Felgueiras, H.P.; Antunes, J.C.; Martins, M.C.L.; Barbosa, M.A. 1—Fundamentals of protein and cell interactions in biomaterials. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Barbosa, M.A., Martins, M.C.L., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 1–27. [Google Scholar]
- Gagner, J.E.; Kim, W.; Chaikof, E.L. Designing protein-based biomaterials for medical applications. Acta Biomater. 2013, 10, 1542–1557. [Google Scholar] [CrossRef] [Green Version]
- Paliwal, R.; Palakurthi, S. Zein in controlled drug delivery and tissue engineering. J. Control. Release 2014, 189, 108–122. [Google Scholar] [CrossRef]
- Dong, J.; Sun, Q.; Wang, J.-Y.J.B. Basic study of corn protein, zein, as a biomaterial in tissue engineering, surface morphology and biocompatibility. Biomaterials 2004, 25, 4691–4697. [Google Scholar] [CrossRef]
- Tortorella, S.; Maturi, M.; Vetri Buratti, V.; Vozzolo, G.; Locatelli, E.; Sambri, L.; Franchini, M.C. Zein as a versatile biopolymer: Different shapes for different biomedical applications. RSC Adv. 2021, 11, 39004–39026. [Google Scholar] [CrossRef]
- Ahmed, Y.; Rehman, M.A.U. Improvement in the surface properties of stainless steel via zein/hydroxyapatite composite coatings for biomedical applications. Surf. Interfaces 2020, 20, 100589. [Google Scholar] [CrossRef]
- El-Ghannam, A.; Ducheyne, P. 1.9 Bioactive Ceramics. In Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2017; pp. 204–234. [Google Scholar]
- Van Vugt, T.A.; Geurts, J.A.P.; Arts, J.J.; Lindfors, N.C. 3—Biomaterials in treatment of orthopedic infections. In Management of Periprosthetic Joint Infections (PJIs); Arts, J.J.C., Geurts, J., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 41–68. [Google Scholar]
- Zahid, S.; Shah, A.T.; Jamal, A.; Chaudhry, A.A.; Khan, A.S.; Khan, A.F.; Muhammad, N.; Rehman, I.U. Biological behavior of bioactive glasses and their composites. RSC Adv. 2016, 6, 70197–70214. [Google Scholar] [CrossRef]
- Huang, W.; Yang, J.; Feng, Q.; Shu, Y.; Liu, C.; Zeng, S.; Guan, H.; Ge, L.; Pathak, J.L.; Zeng, S. Mesoporous Bioactive Glass Nanoparticles Promote Odontogenesis and Neutralize Pathophysiological Acidic pH. Front. Mater. 2020, 7, 241. [Google Scholar] [CrossRef]
- Neščáková, Z.; Zheng, K.; Liverani, L.; Nawaz, Q.; Galusková, D.; Kaňková, H.; Michálek, M.; Galusek, D.; Boccaccini, A.R. Multifunctional zinc ion doped sol–gel derived mesoporous bioactive glass nanoparticles for biomedical applications. Bioact. Mater. 2019, 4, 312–321. [Google Scholar] [CrossRef]
- Tabia, Z.; El Mabrouk, K.; Bricha, M.; Nouneh, K. Mesoporous bioactive glass nanoparticles doped with magnesium: Drug delivery and acellular in vitro bioactivity. RSC Adv. 2019, 9, 12232–12246. [Google Scholar] [CrossRef] [Green Version]
- Bano, S.; Akhtar, M.; Yasir, M.; Maqbool, M.S.; Niaz, A.; Wadood, A.; Rehman, M.A.U. Synthesis and Characterization of Silver–Strontium (Ag-Sr)-Doped Mesoporous Bioactive Glass Nanoparticles. Gels 2021, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Heimann, R.B.J.S.; Technology, C. Thermal spraying of biomaterials. Surf. Coat. Technol. 2006, 201, 2012–2019. [Google Scholar] [CrossRef]
- Heimann, R.B. Plasma-Spray Coating: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Bourne, R.B.; Rorabeck, C.H.; Burkart, B.C.; Kirk, P.G. Ingrowth surfaces. Plasma spray coating to titanium alloy hip replacements. Clin. Orthop. Relat. Res. 1994, 298, 37–46. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.-H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process—An alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef]
- Ong, J.; Lucas, L.; Lacefield, W.; Rigney, E. Structure, solubility and bond strength of thin calcium phosphate coatings produced by ion beam sputter deposition. Biomaterials 1992, 13, 249–254. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A Comprehensive Review of Bioactive Glass Coatings: State of the Art, Challenges and Future Perspectives. Coatings 2020, 10, 757. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Keim, S.; Ma, R.; Li, Y.; Zhitomirsky, I. Electrophoretic deposition of biomaterials. J. R. Soc. Interface 2010, 7, S581–S613. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, Y.; Nawaz, A.; Virk, R.S.; Wadood, A.; Rehman, M.A.U. Fabrication and characterization of zein/bioactive glass deposited on pre-treated magnesium via electrophoretic deposition. Int. J. Ceram. Eng. Sci. 2020, 2, 254–263. [Google Scholar] [CrossRef]
- Ramos-Rivera, L.; Dippel, J.; Boccaccini, A.R. Formation of Zein/Bioactive Glass Layers Using Electrophoretic Deposition Technique. ECS Trans. 2018, 82, 73–80. [Google Scholar] [CrossRef]
- Demir, M.; Ramos-Rivera, L.; Silva, R.; Nazhat, S.N.; Boccaccini, A.R. Zein-based composites in biomedical applications. J. Biomed. Mater. Res. Part A 2017, 105, 1656–1665. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Cheng, C.J.; Jones, O.G. Stabilizing zein nanoparticle dispersions with ι-carrageenan. Food Hydrocoll. 2017, 69, 28–35. [Google Scholar] [CrossRef]
- Kaya, S.; Boccaccini, A.R. Electrophoretic deposition of zein coatings. J. Coat. Technol. Res. 2017, 14, 683–689. [Google Scholar] [CrossRef]
- Pishbin, F.; Simchi, A.; Ryan, M.; Boccaccini, A. Electrophoretic deposition of chitosan/45S5 Bioglass® composite coatings for orthopaedic applications. Surf. Coatings Technol. 2011, 205, 5260–5268. [Google Scholar] [CrossRef]
- Rehman, M.A.U. Zein/Bioactive Glass Coatings with Controlled Degradation of Magnesium under Physiological Conditions: Designed for Orthopedic Implants. Prosthesis 2020, 2, 211–224. [Google Scholar] [CrossRef]
- Speight, J.G. (Ed.) Industrial Organic Chemistry. In Environmental Organic Chemistry for Engineers; Butterworth-Heinemann: Oxford, UK, 2017; Chapter 3; pp. 87–151. [Google Scholar]
- Hamaker, H.C. Formation of a deposit by electrophoresis. Trans. Faraday Soc. 1940, 35, 279–287. [Google Scholar] [CrossRef]
- Nawaz, Q.; Fastner, S.; Rehman, M.A.U.; Ferraris, S.; Perero, S.; di Confiengo, G.G.; Yavuz, E.; Ferraris, M.; Boccaccini, A.R. Multifunctional stratified composite coatings by electrophoretic deposition and RF co-sputtering for orthopaedic implants. J. Mater. Sci. 2021, 56, 7920–7935. [Google Scholar] [CrossRef]
- Rehman, M.A.U.; Bastan, F.E.; Haider, B.; Boccaccini, A.R. Electrophoretic deposition of PEEK/bioactive glass composite coatings for orthopedic implants: A design of experiments (DoE) study. Mater. Des. 2017, 130, 223–230. [Google Scholar] [CrossRef]
- Rehman, M.A.U.; Munawar, M.A.; Schubert, D.W.; Boccaccini, A.R. Electrophoretic deposition of chi-tosan/gelatin/bioactive glass composite coatings on 316L stainless steel: A design of experiment study. Surf. Coat. Technol. 2019, 358, 976–986. [Google Scholar] [CrossRef]
- Aqib, R.; Kiani, S.; Bano, S.; Wadood, A.; Rehman, M.A.U. Ag–Sr doped mesoporous bioactive glass nanoparticles loaded chitosan/gelatin coating for orthopedic implants. Int. J. Appl. Ceram. Technol. 2021, 18, 544–562. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Wiser, R.; Elder, S.H.; Jouett, R.; Yang, Y.; Ong, J.L. Contact angle, protein adsorption and osteoblast precursor cell attachment to chitosan coatings bonded to titanium. J. Biomater. Sci. Polym. Ed. 2003, 14, 1401–1409. [Google Scholar] [CrossRef]
- Wei, J.; Igarashi, T.; Okumori, N.; Igarashi, T.; Maetani, T.; Liu, B.; Yoshinari, M. Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed. Mater. 2009, 4, 045002. [Google Scholar] [CrossRef]
- Laskowski, J.; Kitchener, J.A. The hydrophilic—hydrophobic transition on silica. J. Colloid Interface Sci. 1969, 29, 670–679. [Google Scholar] [CrossRef]
- Wu, J.; Xue, K.; Li, H.; Sun, J.; Liu, K. Improvement of PHBV Scaffolds with Bioglass for Cartilage Tissue Engineering. PLoS ONE 2013, 8, e71563. [Google Scholar] [CrossRef] [Green Version]
- Bin Masripan, N.A.; Miyahira, Y.; Nishimura, H.; Tokoroyama, T.; Umehara, N.; Fuwa, Y. Effect of Transfer Layer on Ultra Low Friction of CNx Coating under Blowing Dry Ar. Tribol. Online 2013, 8, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Waqar, S.; Wadood, A.; Mateen, A.; Rehman, M.A.U. Effects of Ni and Cr addition on the wear performance of NiTi alloy. Int. J. Adv. Manuf. Technol. 2020, 108, 625–634. [Google Scholar] [CrossRef]
- Virk, R.S.; Rehman, M.A.U.; Munawar, M.A.; Schubert, D.W.; Goldmann, W.H.; Dusza, J.; Boccaccini, A.R. Curcumin-Containing Orthopedic Implant Coatings Deposited on Poly-Ether-Ether-Ketone/Bioactive Glass/Hexagonal Boron Nitride Layers by Electrophoretic Deposition. Coatings 2019, 9, 572. [Google Scholar] [CrossRef] [Green Version]
- Thormann, E. Negative friction coefficients. Nat. Mater. 2013, 12, 468. [Google Scholar] [CrossRef]
- Dedinaite, A.; Thormann, E.; Olanya, G.; Claesson, P.M.; Nyström, B.; Kjøniksen, A.-L.; Zhu, K. Friction in aqueous media tuned by temperature-responsive polymer layers. Soft Matter 2010, 6, 2489–2498. [Google Scholar] [CrossRef]
- Batool, S.A.; Ahmad, K.; Irfan, M.; Rehman, M.A.U. Zn–Mn-Doped Mesoporous Bioactive Glass Nanoparticle-Loaded Zein Coatings for Bioactive and Antibacterial Orthopedic Implants. J. Funct. Biomater. 2022, 13, 97. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Rehman, M.A.U.; Bastan, F.E.; Nawaz, A.; Nawaz, Q.; Wadood, A. Electrophoretic deposition of PEEK/bioactive glass composite coatings on stainless steel for orthopedic applications: An optimization for in vitro bioactivity and adhesion strength. Int. J. Adv. Manuf. Technol. 2020, 108, 1849–1862. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]








| Voltage (V) | Time (s) | Hardness Grade |
|---|---|---|
| 10 | 180 | 8B |
| 12 | 180 | 8B |
| 14 | 180 | 5B |
| 16 | 180 | 3B |
| 18 | 180 | 3B |
| 20 | 180 | 1B |
| 25 | 180 | F |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batool, S.A.; Liaquat, U.; Channa, I.A.; Gilani, S.J.; Makhdoom, M.A.; Yasir, M.; Ashfaq, J.; Jumah, M.N.b.; Rehman, M.A.u. Development and Characterization of Zein/Ag-Sr Doped Mesoporous Bioactive Glass Nanoparticles Coatings for Biomedical Applications. Bioengineering 2022, 9, 367. https://doi.org/10.3390/bioengineering9080367
Batool SA, Liaquat U, Channa IA, Gilani SJ, Makhdoom MA, Yasir M, Ashfaq J, Jumah MNb, Rehman MAu. Development and Characterization of Zein/Ag-Sr Doped Mesoporous Bioactive Glass Nanoparticles Coatings for Biomedical Applications. Bioengineering. 2022; 9(8):367. https://doi.org/10.3390/bioengineering9080367
Chicago/Turabian StyleBatool, Syeda Ammara, Ushna Liaquat, Iftikhar Ahmad Channa, Sadaf Jamal Gilani, Muhammad Atif Makhdoom, Muhammad Yasir, Jaweria Ashfaq, May Nasser bin Jumah, and Muhammad Atiq ur Rehman. 2022. "Development and Characterization of Zein/Ag-Sr Doped Mesoporous Bioactive Glass Nanoparticles Coatings for Biomedical Applications" Bioengineering 9, no. 8: 367. https://doi.org/10.3390/bioengineering9080367