Mechanical Characteristics and Bioactivity of Nanocomposite Hydroxyapatite/Collagen Coated Titanium for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characteristics of HAp
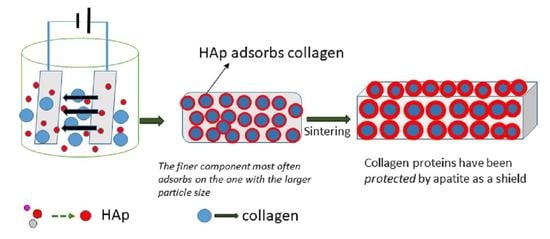
2.3. Coating Ti substrates by EPD Method
2.3.1. Ti Metal Preparations
2.3.2. Preparation of Solution for EPD
2.4. Compressive Strength Testing and Statistical Analysis
2.5. Bioactivity Assay Using SBF Solution
3. Results
3.1. Characteristics of Hydroxyapatite
3.2. Characteristics of Ti Substrates Coating
3.3. Compressive Strength
3.4. SEM Morphology of Coating Ti
3.5. Bioactivity of Ti Coating in SBF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kheirallah, M.; Almeshaly, H. Bone Graft Substitutes for Bone Defect Regeneration. A Collective Review. Int. J. Dent. Oral Sci. 2016, 3, 247–255. [Google Scholar] [CrossRef]
- Wang, X.; Nyman, J.S.; Dong, X.; Leng, H.; Reyes, M. Fundamental Biomechanics in Bone Tissue Engineering. Synth. Lect. Tissue Eng. 2010, 2, 1–225. [Google Scholar]
- Thrivikraman, G.; Madras, G.; Basu, B. In Vitro/In Vivo Assessment and Mechanisms of Toxicity of Bioceramic Materials and Its Wear Particulates. RSC Adv. 2014, 4, 12763–12781. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, K.; Kon, M.; Tenshin, S.; Kuwayama, N. Effects of Preparation Conditions in Aqueous Solution on Properties of Hydroxyapatites. Dent. Mater. J. 1990, 9, 58–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hench, L.L. An Introduction to Bioceramics, 2nd ed.; Imperial College Press: Tampa, FL, USA, 2013; ISBN 9781908977168. [Google Scholar]
- Dudek, A.; Adamczyk, L. Properties of Hydroxyapatite Layers Used for Implant Coatings. Opt. Appl. 2013, 43, 143–151. [Google Scholar] [CrossRef]
- Rizkayanti, Y.; Yusuf, Y. Effect of Temperature on Syntesis of Hydroxyapatite from Cockle Shells (Anadara Granosa). Int. J. Nanoelectron. Mater. 2018, 11, 43–50. [Google Scholar] [CrossRef]
- Januariyasa, I.K.; Yusuf, Y. Carbonated Hydroxyapatite Derived from Snail Shells (Pilla Ampulacea): The Influence of Sintering Temperature on Purity and Crystallography Properties. Mater. Sci. Forum 2020, 975 MSF, 82–87. [Google Scholar] [CrossRef]
- Syafaat, F.Y.; Yusuf, Y. Influence of ca/p Concentration on Hydroxyapatite (Hap) from Asian Moon Scallop Shell (Amusium Pleuronectes). Int. J. Nanoelectron. Mater. 2019, 12, 357–362. [Google Scholar]
- Patty, D.J.; Nugraheni, A.D.; Ana, I.D.; Yusuf, Y. Dual Functional Carbonate-Hydroxyapatite Nanocomposite from Pinctada Maxima and Egg-White for Bone Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2022, 33, 1043–1062. [Google Scholar] [CrossRef]
- Brundavanam, R.K.; Fawcett, D.; Poinern, G.E.J. Synthesis of a Bone like Composite Material Derived from Waste Pearl Oyster Shells for Potential Bone Tissue Bioengineering Applications. Int. J. Res. Med. Sci. 2017, 5, 2454. [Google Scholar] [CrossRef] [Green Version]
- Libouban, H.; Pascaretti-Grizon, F.; Camprasse, G.; Camprasse, S.; Chappard, D. In Vivo Erosion of Orthopedic Screws Prepared from Nacre (Mother of Pearl). Orthop. Traumatol. Surg. Res. 2016, 102, 913–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.N.; Zhu, X.Q.; Yan, X.H.; Deng, J.F.; Wang, R.; Wang, X.X. Nanostructured Individual Nacre Tablet: A Subtle Designed Organic-Inorganic Composite. CrystEngComm 2015, 17, 2964–2968. [Google Scholar] [CrossRef]
- Zhang, G.; Brion, A.; Willemin, A.S.; Piet, M.H.; Moby, V.; Bianchi, A.; Mainard, D.; Galois, L.; Gillet, P.; Rousseau, M. Nacre, a Natural, Multi-Use, and Timely Biomaterial for Bone Graft Substitution. J. Biomed. Mater. Res. A 2017, 105, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Nissan, B.B.; Choi, A.H.; Green, D.W. Marine-Derived Biomaterials for Tissue Engineering Applications; Choi, A.H., Nissan, B.B., Eds.; Springer: Singapore, 2019; ISBN 9789811388545. [Google Scholar]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Uezono, M.; Takakuda, K.; Kikuchi, M.; Suzuki, S.; Moriyama, K. Hydroxyapatite/Collagen Nanocomposite-Coated Titanium Rod for Achieving Rapid Osseointegration onto Bone Surface. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101 B, 1031–1038. [Google Scholar] [CrossRef]
- Li, T.; Li, J.S.; Kou, H.C.; Li, F.P.; Lu, T.L. Preparation of Collagen/Hydroxyapatite Composite Coating on Porous Titanium Substrate and Its Cellular Response. Mater. Sci. Forum 2015, 815, 429–433. [Google Scholar] [CrossRef]
- Zhitomirsky, I. Electrophoretic Hydroxyapatite Coatings and Fibers. Mater. Lett. 2000, 42, 262–271. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Keim, S.; Ma, R.; Li, Y.; Zhitomirsky, I. Electrophoretic Deposition of Biomaterials. J. R. Soc. Interface 2010, 7, S581–S613. [Google Scholar] [CrossRef] [Green Version]
- Patty, D.J.; Nugraheni, A.D.; Ana, I.D.; Yusuf, Y. In Vitro Bioactivity of 3D Microstructure Hydroxyapatite/Collagen Based-Egg White as an Antibacterial Agent. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1412–1424. [Google Scholar] [CrossRef]
- Barooghi, B.; Sheikhi, M.; Amiri, A. Effect of Nano-Hydroxyapatite and Dutycycle on the Structure and Corrosion Performance of Plasma Electrolyte Oxidation Coatings in Simulated Body Fluid on Ti–6Al–4 V. Proc. Inst. Mech. Eng. C J. Mech. Eng. Sci. 2018, 232, 4229–4236. [Google Scholar] [CrossRef]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-Gel Derived Hydroxyapatite Coatings for Titanium Implants: A Review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Gallinetti, S.; Canal, C.; Ginebra, M. Development and Characterization of Biphasic Hydroxyapatite/β-TCP Cements. J. Am. Ceram. Soc. 2014, 97, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Markovic, M.; Fowler, B.O.; Tung, M.S. Preparation and Comprehensive Characterization of Calcium Hydroxyapatite Reference Material. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 553–568. [Google Scholar] [CrossRef]
- Braga, N.A.; Ferreira, N.G.; Piorino Neto, F.; Baldan, M.R.; Cairo, C.A.A. Hydrogen Addition Effect on 3D Porous Titanium Produced by Powder Metallurgy. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2008; Volume 591–593, pp. 289–293. [Google Scholar]
- Han, M.K.; Im, J.B.; Hwang, M.J.; Kim, B.J.; Kim, H.Y.; Park, Y.J. Effect of Indium Content on the Microstructure, Mechanical Properties and Corrosion Behavior of Titanium Alloys. Metals 2015, 5, 850–862. [Google Scholar] [CrossRef] [Green Version]
- Saeed, W.; Al-Odayni, A.-B.; Alghamdi, A.; Alrahlah, A.; Aouak, T. Thermal Properties and Non-Isothermal Crystallization Kinetics of Poly (δ-Valerolactone) and Poly (δ-Valerolactone)/Titanium Dioxide Nanocomposites. Crystals 2018, 8, 452. [Google Scholar] [CrossRef] [Green Version]
- Safi, I.N.; Hussein, B.M.A.; al Shammari, A.M.; Tawfiq, T.A. Implementation and Characterization of Coating Pure Titanium Dental Implant with Sintered β-TCP by Using Nd:YAG Laser. Saudi Dent. J. 2019, 31, 242–250. [Google Scholar] [CrossRef]
- Prando, D.; Brenna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.; Ormellese, M.; Pedeferri, M.P. Corrosion of Titanium: Part 2: Effects of Surface Treatments. J. Appl. Biomater. Funct. Mater. 2018, 16, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Stoch, A.; Jastrzębski, W.; Brożek, A.; Stoch, J.; Szaraniec, J.; Trybalska, B.; Kmita, G. FTIR Absorption–Reflection Study of Biomimetic Growth of Phosphates on Titanium Implants. J. Mol. Struct. 2000, 555, 375–382. [Google Scholar] [CrossRef]
- Miculescu, M.; Raditoiu, V.; Sirbu, I.; Corobea, M.S.; Stoenescu, M.; Miculescu, M.; Raditoiu, V.; Fierascu, R.C.; Sirbu, I.; Vuluga, Z.; et al. Titanium Functionalizing and Derivatizing for Implantable Materials Osseointegration Properties Enhancing. Dig. J. Nanomater. Biostruct. 2014, 9, 1339–1347. [Google Scholar]
- Tan, K.J.; Idris, M.I.; Abdullah, H.Z. Investigation of Surface Properties of Titanium Treated with Combined Alkali and Sodium Removal Treatments. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2016; Volume 840, pp. 225–230. [Google Scholar]
- Suchý, T.; Bartoš, M.; Sedláček, R.; Šupová, M.; Žaloudková, M.; Martynková, G.S.; Foltán, R. Various Simulated Body Fluids Lead to Significant Differences in Collagen Tissue Engineering Scaffolds. Materials 2021, 14, 4388. [Google Scholar] [CrossRef]
- Barralet, J.; Best, S.; Bonfield, W. Carbonate Substitution in Precipitated Hydroxyapatite: An Investigation into the Effects of Reaction Temperature and Bicarbonate Ion Concentration. J. Biomed. Mater. Res. 1998, 41, 79–86. [Google Scholar] [CrossRef]
- Ye, H.; Liu, X.Y.; Hong, H. Characterization of Sintered Titanium/Hydroxyapatite Biocomposite Using FTIR Spectroscopy. J. Mater. Sci. Mater. Med. 2009, 20, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öhman, M. Development of ATR-FTIR Kretschmann Spectroscopy for In Situ Studies of Metal/Polymer Interfaces: And Its Intergration with EIS for Exposure to Corrosive Conditions. Ph.D. Dissertation, KTH, Stockholm, Sweden, 2010. [Google Scholar]
- Al-Amin, M.; Chandra Dey, S.; Rashid, T.U.; Ashaduzzaman, M.; Shamsuddin, S.M. Solar Assisted Photocatalytic Degradation of Reactive Azo Dyes in Presence of Anatase Titanium Dioxide. Int. J. Latest Res. Eng. Technol. IJLRET 2016, 2, 14–21. [Google Scholar]
- Liao, S.; Wang, W.; Uo, M.; Ohkawa, S.; Akasaka, T.; Tamura, K.; Cui, F.; Watari, F. A Three-Layered Nano-Carbonated Hydroxyapatite/Collagen/PLGA Composite Membrane for Guided Tissue Regeneration. Biomaterials 2005, 26, 7564–7571. [Google Scholar] [CrossRef]
- Pang, X.; Zhitomirsky, I. Electrodeposition of Composite Hydroxyapatite-Chitosan Films. Mater. Chem. Phys. 2005, 94, 245–251. [Google Scholar] [CrossRef]
- Bakhshandeh, S.; Amin Yavari, S. Electrophoretic Deposition: A Versatile Tool against Biomaterial Associated Infections. J. Mater. Chem. B 2018, 6, 1128–1148. [Google Scholar] [CrossRef]
- Zhitomirsky, I. Cathodic Electrodeposition of Ceramic and Organoceramic Materials. Fundamental Aspects. Adv. Colloid Interface Sci. 2002, 97, 279–317. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Pazarceviren, A.E.; Tezcaner, A.; Evis, Z. Historical Development of Simulated Body Fluids Used in Biomedical Applications: A Review. Microchem. J. 2020, 155, 104713. [Google Scholar] [CrossRef]
- Sari, M.; Hening, P.; Ana, I.D.; Yusuf, Y. Bioceramic Hydroxyapatite-Based Scaffold with a Porous Structure Using Honeycomb as a Natural Polymeric Porogen for Bone Tissue Engineering. Biomater. Res. 2021, 25, 1–13. [Google Scholar] [CrossRef]
- Abdullah, M.; Khairurrijal, K. A Simple Method for Determining Surface Porosity Based on SEM Images Using OriginPro Software. Indones. J. Phys. 2016, 20, 37–40. [Google Scholar] [CrossRef]
- Stock, S.R. The Mineral–Collagen Interface in Bone. Calcif. Tissue Int. 2015, 97, 262–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.K.; Gautieri, A.; Chang, S.W.; Buehler, M.J. Molecular Mechanics of Mineralized Collagen Fibrils in Bone. Nat. Commun. 2013, 4, 1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutini, M.; Corno, M.; Costa, D.; Ugliengo, P. How Does Collagen Adsorb on Hydroxyapatite? Insights from Ab Initio Simulations on a Polyproline Type II Model. J. Phys. Chem. C 2019, 123, 7540–7550. [Google Scholar] [CrossRef] [Green Version]
- Tavafoghi, M.; Cerruti, M. The Role of Amino Acids in Hydroxyapatite Mineralization. J. R. Soc. Interface 2016, 13, 20160462. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.M.; Kalantar-Zadeh, K.; Markewich, T.; Colman, S.; Benner, D.; Kovesdy, C.P. Dietary egg whites for phosphorus control in maintenance haemodialysis patients: A pilot study. J. Ren. Care—Wiley Online Libr. 2011, 37, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.H.; Lee, J.H.; Jung, H.S.; Shin, H.; Shin, H. Biomineralization of Bone Tissue: Calcium Phosphate-Based Inorganics in Collagen Fibrillar Organic Matrices. Biomater. Res. 2022, 26, 42. [Google Scholar] [CrossRef]
- Nudelman, F.; Pieterse, K.; George, A.; Bomans, P.H.H.; Friedrich, H.; Brylka, L.J.; Hilbers, P.A.J.; de With, G.; Sommerdijk, N.A.J.M. The Role of Collagen in Bone Apatite Formation in the Presence of Hydroxyapatite Nucleation Inhibitors. Nat. Mater. 2010, 9, 1004–1009. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Jacquet, R.; Lowder, E.; Landis, W.J. Refinement of Collagen–Mineral Interaction: A Possible Role for Osteocalcin in Apatite Crystal Nucleation, Growth and Development. Bone 2015, 71, 7–16. [Google Scholar] [CrossRef]
- Aniołek, K.; Łosiewicz, B.; Kubisztal, J.; Osak, P.; Stróż, A.; Barylski, A.; Kaptacz, S. Mechanical Properties, Corrosion Resistance and Bioactivity of Oxide Layers Formed by Isothermal Oxidation of Ti-6al-7nb Alloy. Coatings 2021, 11, 505. [Google Scholar] [CrossRef]
- Sari, M.; Kristianto, N.A.; Ana, I.D.; Yusuf, Y. Carbonated Hydroxyapatite-Based Honeycomb Scaffold Coatings on a Titanium Alloy for Bone Implant Application—Physicochemical and Mechanical Properties Analysis. Coatings 2021, 11, 941. [Google Scholar] [CrossRef]
- Sari, M.; Ana, I.D.; Yusuf, Y. Cell Viability Assay and Surface Morphology Analysis of Carbonated Hydroxyapatite/Honeycomb/Titanium Alloy Coatings for Bone Implant Applications. Bioengineering 2022, 9, 325. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.Q.; Wahafu, T.; Jiang, G.F.; Liu, W.; Qiao, Y.Q.; Peng, X.C.; Cheng, T.; Zhang, X.L.; He, G.; Liu, X.Y. A Novel Open-Porous Magnesium Scaffold with Controllable Microstructures and Properties for Bone Regeneration. Sci. Rep. 2016, 6, 24134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaddon, M.; Samizadeh, S.; Wang, L.; Blunn, G.; Liu, C. Intrinsic Osteoinductivity of Porous Titanium Scaffold for Bone Tissue Engineering. Int. J. Biomater. 2017, 2017, 5093063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristianto, N.A.; Sari, M.; Yusuf, Y. Hydroxyapatite Based on Abalone Mussel Shells Coating on Titanium Alloy Using Electrophoretic Deposition Dip Coating as a Bone Implant Candidate. Chiang Mai Univ. J. Nat. Sci. 2022, 21, e2022021. [Google Scholar] [CrossRef]
- Abudalazez, A.M.A.; Kasim, S.R.; Ariffin, A.B.; Ahmad, Z.A. Effect of the Solid Concentration in the Suspension on Electrophoretic Deposition (EPD) Coating Parameters. Int. J. Eng. Res. Afr. 2012, 8, 47–54. [Google Scholar] [CrossRef]
- Rustom, L.E.; Poellmann, M.J.; Wagoner Johnson, A.J. Mineralization in Micropores of Calcium Phosphate Scaffolds. Acta Biomater. 2019, 83, 435–455. [Google Scholar] [CrossRef]
- Al-Munajjed, A.A.; Plunkett, N.A.; Gleeson, J.P.; Weber, T.; Jungreuthmayer, C.; Levingstone, T.; Hammer, J.; O’Brien, F.J. Development of a Biomimetic Collagen-Hydroxyapatite Scaffold for Bone Tissue Engineering Using a SBF Immersion Technique. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90B, 584–591. [Google Scholar] [CrossRef]
- Spanos, N.; Misirlis, D.Y.; Kanellopoulou, D.G.; Koutsoukos, P.G. Seeded Growth of Hydroxyapatite in Simulated Body Fluid. J. Mater. Sci. 2006, 41, 1805–1812. [Google Scholar] [CrossRef]
- Gu, Y.W.; Khor, K.A.; Cheang, P. In Vitro Studies of Plasma-Sprayed Hydroxyapatite/Ti-6Al-4V Composite Coatings in Simulated Body Fluid (SBF). Biomaterials 2003, 24, 1603–1611. [Google Scholar] [CrossRef]
- Zhangabcde, F.; Zhangcdf, C.-F.; Yinef, M.-N.; Renef, L.-F.; Linde, H.-S.; Shiacdeg, G.-S. Effect of Heat Treatment on H2O2/HCl Etched Pure Titanium Dental Implant: An in Vitro Study. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012, 18, BR265. [Google Scholar]
- Hung, K.Y.; Lin, Y.C.; Feng, H.P. The Effects of Acid Etching on the Nanomorphological Surface Characteristics and Activation Energy of Titanium Medical Materials. Materials 2017, 10, 1164. [Google Scholar] [CrossRef] [PubMed]









| No | Reagent | Amount |
|---|---|---|
| 1 | NaCl | 8.035 g |
| 2 | NaHCO3 | 0.355 g |
| 3 | KCl | 0.225 g |
| 4 | 0.231 g | |
| 5 | 0.311 g | |
| 6 | 1.0 M HCl | 39 mL |
| 7 | CaCl2 | 0.292 g |
| 8 | Na2SO4 | 0.072 g |
| 9 | 1.0 M HCl | 0–5 mL |
| 10 | Tris (hydroxymethyl) aminomethane | 6118 g |
| No | Sample | Force (kN) | Surface Area (mm2) | Compressive Strength (MPa) |
|---|---|---|---|---|
| 1 | Control (Uncoated Ti) | 0.56 | 11.99 | 46.71 |
| 2 | Ti/HAp-1 | 1.07 | 12.26 | 87.28 |
| 3 | Ti/HAp-3 | 1.08 | 12.53 | 86.19 |
| 4 | Ti/HAp-coll | 0.85 | 12.26 | 69.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patty, D.J.; Nugraheni, A.D.; Dewi Ana, I.; Yusuf, Y. Mechanical Characteristics and Bioactivity of Nanocomposite Hydroxyapatite/Collagen Coated Titanium for Bone Tissue Engineering. Bioengineering 2022, 9, 784. https://doi.org/10.3390/bioengineering9120784
Patty DJ, Nugraheni AD, Dewi Ana I, Yusuf Y. Mechanical Characteristics and Bioactivity of Nanocomposite Hydroxyapatite/Collagen Coated Titanium for Bone Tissue Engineering. Bioengineering. 2022; 9(12):784. https://doi.org/10.3390/bioengineering9120784
Chicago/Turabian StylePatty, Diana Julaidy, Ari Dwi Nugraheni, Ika Dewi Ana, and Yusril Yusuf. 2022. "Mechanical Characteristics and Bioactivity of Nanocomposite Hydroxyapatite/Collagen Coated Titanium for Bone Tissue Engineering" Bioengineering 9, no. 12: 784. https://doi.org/10.3390/bioengineering9120784