Effect of Inoculum Microbial Diversity in Ex Situ Biomethanation of Hydrogen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Analytical Methods
2.3. Microbial Community Analysis
2.4. Statistical Analysis
3. Results
3.1. Process Performance
3.2. Organic Acids Profiles
3.3. pH Profiles during Autotrophic Cultivation
3.4. Changes in Diversity of Microbial Communities
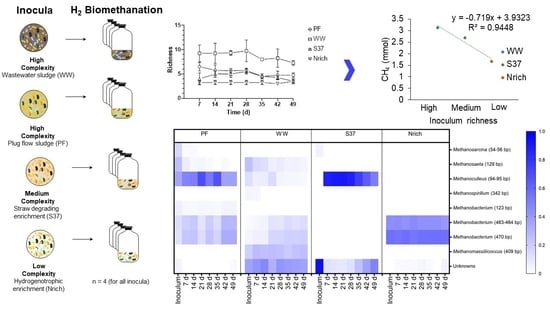
3.5. Relationship between the Richness of Methanogenic Communities and Methane Production
3.6. Changes in the Structure of the Microbial Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schiebahn, S.; Grube, T.; Robinius, M.; Tietze, V.; Kumar, B.; Stolten, D. Power to Gas: Technological Overview, Systems Analysis and Economic Assessment for a Case Study in Germany. Int. J. Hydrog. Energy 2015, 40, 4285–4294. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona, P.; Romeo, L.M.; Espatolero, S. Power to Gas Projects Review: Lab, Pilot and Demo Plants for Storing Renewable Energy and CO2. Renew. Sustain. Energy Rev. 2017, 69, 292–312. [Google Scholar] [CrossRef]
- Blanco, H.; Nijs, W.; Ruf, J.; Faaij, A. Potential of Power-to-Methane in the EU Energy Transition to a Low Carbon System Using Cost Optimization. Appl. Energy 2018, 232, 323–340. [Google Scholar] [CrossRef]
- Schaaf, T.; Grünig, J.; Schuster, M.R.; Rothenfluh, T.; Orth, A. Methanation of CO2-Storage of Renewable Energy in a Gas Distribution System. Energy Sustain. Soc. 2014, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Rittmann, S.; Seifert, A.; Herwig, C. Essential Prerequisites for Successful Bioprocess Development of Biological CH4 Production from CO2 and H2. Crit. Rev. Biotechnol. 2015, 35, 141–151. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas Upgrading and Utilization: Current Status and Perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef] [Green Version]
- Kougias, P.G.; Treu, L.; Benavente, D.P.; Boe, K.; Campanaro, S.; Angelidaki, I. Ex-Situ Biogas Upgrading and Enhancement in Different Reactor Systems. Bioresour. Technol. 2017, 225, 429–437. [Google Scholar] [CrossRef]
- Aryal, N.; Kvist, T.; Ammam, F.; Pant, D.; Ottosen, L.D.M. An Overview of Microbial Biogas Enrichment. Bioresour. Technol. 2018, 264, 359–369. [Google Scholar] [CrossRef]
- Fu, S.; Angelidaki, I.; Zhang, Y. In Situ Biogas Upgrading by CO2-to-CH4 Bioconversion. Trends Biotechnol. 2020, 39, 336–347. [Google Scholar] [CrossRef]
- Vechi, N.T.; Agneessens, L.M.; Feilberg, A.; Ottosen, L.D.M.; Kofoed, M.V.W. In Situ Biomethanation: Inoculum Origin Influences Acetate Consumption Rate during Hydrogen Addition. Bioresour. Technol. Rep. 2021, 14, 100656. [Google Scholar] [CrossRef]
- Agneessens, L.M.; Ottosen, L.D.M.; Andersen, M.; Berg Olesen, C.; Feilberg, A.; Kofoed, M.V.W. Parameters Affecting Acetate Concentrations during In-Situ Biological Hydrogen Methanation. Bioresour. Technol. 2018, 258, 33–40. [Google Scholar] [CrossRef]
- Agneessens, L.M.; Ottosen, L.D.M.; Voigt, N.V.; Nielsen, J.L.; de Jonge, N.; Fischer, C.H.; Kofoed, M.V.W. In-Situ Biogas Upgrading with Pulse H2 Additions: The Relevance of Methanogen Adaption and Inorganic Carbon Level. Bioresour. Technol. 2017, 233, 256–263. [Google Scholar] [CrossRef]
- Luo, G.; Johansson, S.; Boe, K.; Xie, L.; Zhou, Q.; Angelidaki, I. Simultaneous Hydrogen Utilization and in situ Biogas Upgrading in an Anaerobic Reactor. Biotechnol. Bioeng. 2012, 109, 1088–1094. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Angelidaki, I. In-Situ Biogas Upgrading in Thermophilic Granular UASB Reactor: Key Factors Affecting the Hydrogen Mass Transfer Rate. Bioresour. Technol. 2016, 221, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Logroño, W.; Popp, D.; Kleinsteuber, S.; Straeuber, H.; Harms, H.; Nikolausz, M. Microbial Resource Management for Ex Situ Biomethanation of Hydrogen at Alkaline pH. Microorganisms 2020, 8, 614. [Google Scholar] [CrossRef] [Green Version]
- Voelklein, M.A.; Rusmanis, D.; Murphy, J.D. Biological Methanation: Strategies for in-situ and ex-situ Upgrading in Anaerobic Digestion. Appl. Energy 2019, 235, 1061–1071. [Google Scholar] [CrossRef]
- Braga Nan, L.; Trably, E.; Santa-Catalina, G.; Bernet, N.; Delgenès, J.-P.; Escudié, R. Biomethanation Processes: New Insights on the Effect of a High H2 Partial Pressure on Microbial Communities. Biotechnol. Biofuels 2020, 13, 141. [Google Scholar] [CrossRef]
- Strübing, D.; Moeller, A.B.; Mößnang, B.; Lebuhn, M.; Drewes, J.E.; Koch, K. Anaerobic Thermophilic Trickle Bed Reactor as a Promising Technology for Flexible and Demand-Oriented H2/CO2 Biomethanation. Appl. Energy 2018, 232, 543–554. [Google Scholar] [CrossRef]
- Corbellini, V.; Kougias, P.G.; Treu, L.; Bassani, I.; Malpei, F.; Angelidaki, I. Hybrid Biogas Upgrading in a Two-Stage Thermophilic Reactor. Energy Convers. Manag. 2018, 168, 1–10. [Google Scholar] [CrossRef]
- Logroño, W.; Nikolausz, M.; Harms, H.; Kleinsteuber, S. Physiological Effects of 2-Bromoethanesulfonate on Hydrogenotrophic Pure and Mixed Cultures. Microorganisms 2022, 10, 355. [Google Scholar] [CrossRef]
- Hattori, S. Syntrophic Acetate-Oxidizing Microbes in Methanogenic Environments. Microbes Environ. 2008, 23, 118–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strübing, D.; Moeller, A.B.; Mößnang, B.; Lebuhn, M.; Drewes, J.E.; Koch, K. Load Change Capability of an Anaerobic Thermophilic Trickle Bed Reactor for Dynamic H2/CO2 Biomethanation. Bioresour. Technol. 2019, 289, 121735. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, M.; Jordan, I.; Heinrich, S.; Behrens, J.; Ziesche, A.; Busch, G. Long Term and Demand-Oriented Biocatalytic Synthesis of Highly Concentrated Methane in a Trickle Bed Reactor. Appl. Energy 2019, 240, 818–826. [Google Scholar] [CrossRef]
- Braga Nan, L.; Trably, E.; Santa-Catalina, G.; Bernet, N.; Delgenes, J.-P.; Escudie, R. Microbial Community Redundance in Biomethanation Systems Lead to Faster Recovery of Methane Production Rates after Starvation. Sci. Total Environ. 2022, 804, 150073. [Google Scholar] [CrossRef] [PubMed]
- Bassani, I.; Kougias, P.G.; Treu, L.; Porté, H.; Campanaro, S.; Angelidaki, I. Optimization of Hydrogen Dispersion in Thermophilic Up-Flow Reactors for Ex Situ Biogas Upgrading. Bioresour. Technol. 2017, 234, 310–319. [Google Scholar] [CrossRef]
- Szuhaj, M.; Ács, N.; Tengölics, R.; Bodor, A.; Rákhely, G.; Kovács, K.L.; Bagi, Z. Conversion of H2 and CO2 to CH4 and Acetate in Fed-Batch Biogas Reactors by Mixed Biogas Community: A Novel Route for the Power-to-Gas Concept. Biotechnol. Biofuels 2016, 9, 102. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.B.; Kofoed, M.V.W.; Fischer, K.; Voigt, N.V.; Agneessens, L.M.; Batstone, D.J.; Ottosen, L.D.M. Venturi-Type Injection System as a Potential H2 Mass Transfer Technology for Full-Scale in Situ Biomethanation. Appl. Energy 2018, 222, 840–846. [Google Scholar] [CrossRef]
- Jensen, M.B.; Jensen, B.; Ottosen, L.D.M.; Kofoed, M.V.W. Integrating H2 Injection and Reactor Mixing for Low-Cost H2 Gas-Liquid Mass Transfer in Full-Scale in Situ Biomethanation. Biochem. Eng. J. 2021, 166, 107869. [Google Scholar] [CrossRef]
- Figeac, N.; Trably, E.; Bernet, N.; Delgenès, J.-P.; Escudié, R. Temperature and Inoculum Origin Influence the Performance of Ex-Situ Biological Hydrogen Methanation. Molecules 2020, 25, 5665. [Google Scholar] [CrossRef]
- Ju, D.H.; Shin, J.H.; Lee, H.K.; Kong, S.H.; Kim, J.-I.; Sang, B.I. Effects of pH Conditions on the Biological Conversion of Carbon Dioxide to Methane in a Hollow-Fiber Membrane Biofilm Reactor (Hf-MBfR). Desalination 2008, 234, 409–415. [Google Scholar] [CrossRef]
- Chen, L.; Du, S.; Xie, L. Effects of pH on Ex-Situ Biomethanation with Hydrogenotrophic Methanogens under Thermophilic and Extreme-Thermophilic Conditions. J. Biosci. Bioeng. 2021, 131, 168–175. [Google Scholar] [CrossRef]
- Luo, G.; De Francisci, D.; Kougias, P.G.; Laura, T.; Zhu, X.; Angelidaki, I. New Steady-State Microbial Community Compositions and Process Performances in Biogas Reactors Induced by Temperature Disturbances. Biotechnol. Biofuels 2015, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Maus, I.; Klocke, M.; Derenkó, J.; Stolze, Y.; Beckstette, M.; Jost, C.; Wibberg, D.; Blom, J.; Henke, C.; Willenbücher, K.; et al. Impact of Process Temperature and Organic Loading Rate on Cellulolytic/Hydrolytic Biofilm Microbiomes during Biomethanation of Ryegrass Silage Revealed by Genome-Centered Metagenomics and Metatranscriptomics. Environ. Microbiome 2020, 15, 7. [Google Scholar] [CrossRef] [Green Version]
- Ryue, J.; Lin, L.; Liu, Y.; Lu, W.; McCartney, D.; Dhar, B.R. Comparative Effects of GAC Addition on Methane Productivity and Microbial Community in Mesophilic and Thermophilic Anaerobic Digestion of Food Waste. Biochem. Eng. J. 2019, 146, 79–87. [Google Scholar] [CrossRef]
- Høj, L.; Olsen, R.A.; Torsvik, V.L. Effects of Temperature on the Diversity and Community Structure of Known Methanogenic Groups and Other Archaea in High Arctic Peat. ISME J. 2008, 2, 37–48. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; He, Z.; Luo, S.; Su, D.; Jiang, H.; Zhou, H.; Xu, Q. Effects of Temperature, Hydrogen/Carbon Monoxide Ratio and Trace Element Addition on Methane Production Performance from Syngas Biomethanation. Bioresour. Technol. 2020, 295, 122296. [Google Scholar] [CrossRef]
- Andreides, D.; Stransky, D.; Bartackova, J.; Pokorna, D.; Zabranska, J. Syngas Biomethanation in Countercurrent Flow Trickle-Bed Reactor Operated under Different Temperature Conditions. Renew. Energy 2022, 199, 1329–1335. [Google Scholar] [CrossRef]
- Jønson, B.D.; Mortensen, L.O.L.; Schmidt, J.E.; Jeppesen, M.; Bastidas-Oyanedel, J.-R. Flexibility as the Key to Stability: Optimization of Temperature and Gas Feed during Downtime towards Effective Integration of Biomethanation in an Intermittent Energy System. Energies 2022, 15, 5827. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, F.; Wang, Y.; Feng, L.; Zhou, H.; Li, Y. Characteristics of In-Situ Hydrogen Biomethanation at Mesophilic and Thermophilic Temperatures. Bioresour. Technol. 2021, 337, 125455. [Google Scholar] [CrossRef]
- Kozak, M.; Köroğlu, E.O.; Cirik, K.; Zaimoğlu, Z. Evaluation of Ex-Situ Hydrogen Biomethanation at Mesophilic and Thermophilic Temperatures. Int. J. Hydrog. Energy 2022, 47, 15434–15441. [Google Scholar] [CrossRef]
- Asimakopoulos, K.; Łężyk, M.; Grimalt-Alemany, A.; Melas, A.; Wen, Z.; Gavala, H.N.; Skiadas, I.V. Temperature Effects on Syngas Biomethanation Performed in a Trickle Bed Reactor. Chem. Eng. J. 2020, 393, 124739. [Google Scholar] [CrossRef]
- Bu, F.; Dong, N.; Kumar Khanal, S.; Xie, L.; Zhou, Q. Effects of CO on Hydrogenotrophic Methanogenesis under Thermophilic and Extreme-Thermophilic Conditions: Microbial Community and Biomethanation Pathways. Bioresour. Technol. 2018, 266, 364–373. [Google Scholar] [CrossRef]
- Grimalt-Alemany, A.; Łężyk, M.; Kennes-Veiga, D.M.; Skiadas, I.V.; Gavala, H.N. Enrichment of Mesophilic and Thermophilic Mixed Microbial Consortia for Syngas Biomethanation: The Role of Kinetic and Thermodynamic Competition. Waste Biomass Valor 2020, 11, 465–481. [Google Scholar] [CrossRef] [Green Version]
- Grimalt-Alemany, A.; Asimakopoulos, K.; Skiadas, I.V.; Gavala, H.N. Modeling of Syngas Biomethanation and Catabolic Route Control in Mesophilic and Thermophilic Mixed Microbial Consortia. Appl. Energy 2020, 262, 114502. [Google Scholar] [CrossRef]
- Liu, C.; Luo, G.; Wang, W.; He, Y.; Zhang, R.; Liu, G. The Effects of pH and Temperature on the Acetate Production and Microbial Community Compositions by Syngas Fermentation. Fuel 2018, 224, 537–544. [Google Scholar] [CrossRef]
- Kakuk, B.; Wirth, R.; Maróti, G.; Szuhaj, M.; Rakhely, G.; Laczi, K.; Kovács, K.L.; Bagi, Z. Early Response of Methanogenic Archaea to H2 as Evaluated by Metagenomics and Metatranscriptomics. Microb. Cell Factories 2021, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Ács, N.; Szuhaj, M.; Wirth, R.; Bagi, Z.; Maróti, G.; Rákhely, G.; Kovács, K.L. Microbial Community Rearrangements in Power-to-Biomethane Reactors Employing Mesophilic Biogas Digestate. Front. Energy Res. 2019, 7, 132. [Google Scholar] [CrossRef]
- Tsapekos, P.; Treu, L.; Campanaro, S.; Centurion, V.B.; Zhu, X.; Peprah, M.; Zhang, Z.; Kougias, P.G.; Angelidaki, I. Pilot-Scale Biomethanation in a Trickle Bed Reactor: Process Performance and Microbiome Functional Reconstruction. Energy Convers. Manag. 2021, 244, 114491. [Google Scholar] [CrossRef]
- De Bernardini, N.; Basile, A.; Zampieri, G.; Kovalovszki, A.; De Diego Diaz, B.; Offer, E.; Wongfaed, N.; Angelidaki, I.; Kougias, P.G.; Campanaro, S.; et al. Integrating Metagenomic Binning with Flux Balance Analysis to Unravel Syntrophies in Anaerobic CO2 Methanation. Microbiome 2022, 10, 117. [Google Scholar] [CrossRef]
- Ebrahimian, F.; De Bernardini, N.; Tsapekos, P.; Treu, L.; Zhu, X.; Campanaro, S.; Karimi, K.; Angelidaki, I. Effect of Pressure on Biomethanation Process and Spatial Stratification of Microbial Communities in Trickle Bed Reactors under Decreasing Gas Retention Time. Bioresour. Technol. 2022, 361, 127701. [Google Scholar] [CrossRef]
- Fontana, A.; Kougias, P.G.; Treu, L.; Kovalovszki, A.; Valle, G.; Cappa, F.; Morelli, L.; Angelidaki, I.; Campanaro, S. Microbial Activity Response to Hydrogen Injection in Thermophilic Anaerobic Digesters Revealed by Genome-Centric Metatranscriptomics. Microbiome 2018, 6, 194. [Google Scholar] [CrossRef]
- Treu, L.; Campanaro, S.; Kougias, P.G.; Sartori, C.; Bassani, I.; Angelidaki, I. Hydrogen-Fueled Microbial Pathways in Biogas Upgrading Systems Revealed by Genome-Centric Metagenomics. Front. Microbiol. 2018, 9, 1079. [Google Scholar] [CrossRef] [Green Version]
- Szuhaj, M.; Wirth, R.; Bagi, Z.; Maróti, G.; Rákhely, G.; Kovács, K.L. Development of Stable Mixed Microbiota for High Yield Power to Methane Conversion. Energies 2021, 14, 7336. [Google Scholar] [CrossRef]
- Jiang, B.; Hu, X.; Söderlind, U.; Göransson, K.; Zhang, W.; Yu, C. Identification of the Biomethanation Pathways during Biological CO2 Fixation with Exogenous H2 Addition. Fuel Process. Technol. 2022, 238, 107478. [Google Scholar] [CrossRef]
- Tsapekos, P.; Alvarado-Morales, M.; Angelidaki, I. H2 Competition between Homoacetogenic Bacteria and Methanogenic Archaea during Biomethanation from a Combined Experimental-Modelling Approach. J. Environ. Chem. Eng. 2022, 10, 107281. [Google Scholar] [CrossRef]
- Cheng, G.; Gabler, F.; Pizzul, L.; Olsson, H.; Nordberg, Å.; Schnürer, A. Microbial Community Development during Syngas Methanation in a Trickle Bed Reactor with Various Nutrient Sources. Appl. Microbiol. Biotechnol. 2022, 106, 5317–5333. [Google Scholar] [CrossRef]
- Dong, D.; Kyung Choi, O.; Woo Lee, J. Influence of the Continuous Addition of Zero Valent Iron (ZVI) and Nano-Scaled Zero Valent Iron (NZVI) on the Anaerobic Biomethanation of Carbon Dioxide. Chem. Eng. J. 2022, 430, 132233. [Google Scholar] [CrossRef]
- Ghofrani-Isfahani, P.; Tsapekos, P.; Peprah, M.; Kougias, P.; Zervas, A.; Zhu, X.; Yang, Z.; Jacobsen, C.S.; Angelidaki, I. Ex-Situ Biogas Upgrading in Thermophilic Trickle Bed Reactors Packed with Micro-Porous Packing Materials. Chemosphere 2022, 296, 133987. [Google Scholar] [CrossRef]
- Palù, M.; Peprah, M.; Tsapekos, P.; Kougias, P.; Campanaro, S.; Angelidaki, I.; Treu, L. In-Situ Biogas Upgrading Assisted by Bioaugmentation with Hydrogenotrophic Methanogens during Mesophilic and Thermophilic Co-Digestion. Bioresour. Technol. 2022, 348, 126754. [Google Scholar] [CrossRef]
- Khan, A.; Akbar, S.; Okonkwo, V.; Smith, C.; Khan, S.; Ali Shah, A.; Adnan, F.; Zeeshan Ijaz, U.; Ahmed, S.; Badshah, M. Enrichment of the hydrogenotrophic methanogens for, in-situ biogas up-gradation by recirculation of gases and supply of hydrogen in methanogenic reactor. Bioresour. Technol. 2022, 345, 126219. [Google Scholar] [CrossRef]
- Jiang, H.; Hao, W.; Li, Y.; Zhou, H. Biological methanation of H2 and CO2 in a continuous stirred tank reactor. J. Clean. Prod. 2022, 370, 133518. [Google Scholar] [CrossRef]
- Logroño, W.; Popp, D.; Nikolausz, M.; Kluge, P.; Harms, H.; Kleinsteuber, S. Microbial Communities in Flexible Biomethanation of Hydrogen Are Functionally Resilient Upon Starvation. Front. Microbiol. 2021, 12, 619632. [Google Scholar] [CrossRef] [PubMed]
- Porsch, K.; Wirth, B.; Tóth, E.M.; Schattenberg, F.; Nikolausz, M. Characterization of Wheat Straw-Degrading Anaerobic Alkali-Tolerant Mixed Cultures from Soda Lake Sediments by Molecular and Cultivation Techniques. Microb. Biotechnol. 2015, 8, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, L.M.; Regan, J.M. Phylogenetic Comparison of the Methanogenic Communities from an Acidic, Oligotrophic Fen and an Anaerobic Digester Treating Municipal Wastewater Sludge. Appl. Environ. Microbiol. 2008, 74, 6663–6671. [Google Scholar] [CrossRef] [Green Version]
- Popp, D.; Harms, H.; Sträuber, H. The Alkaloid Gramine in the Anaerobic Digestion Process—Inhibition and Adaptation of the Methanogenic Community. Appl. Microbiol. Biotechnol. 2016, 100, 7311–7322. [Google Scholar] [CrossRef]
- Popp, D.; Schrader, S.; Kleinsteuber, S.; Harms, H.; Sträuber, H. Biogas Production from Coumarin-Rich Plants—Inhibition by Coumarin and Recovery by Adaptation of the Bacterial Community. FEMS Microbiol. Ecol. 2015, 91, fiv103. [Google Scholar] [CrossRef]
- Lucas, R.; Kuchenbuch, A.; Fetzer, I.; Harms, H. Long-Term Monitoring Reveals Stable and Remarkably Similar Microbial Communities in Parallel Full-Scale Biogas Reactors Digesting Energy Crops. FEMS Microbiol. Ecol. 2015, 91, fiv004. [Google Scholar] [CrossRef] [Green Version]
- Bühligen, F.; Lucas, R.; Nikolausz, M.; Kleinsteuber, S. A T-RFLP Database for the Rapid Profiling of Methanogenic Communities in Anaerobic Digesters. Anaerobe 2016, 39, 114–116. [Google Scholar] [CrossRef]
- Rusmanis, D.; Shea, R.O.; Wall, D.M.; Murphy, J.D.; Rusmanis, D.; Shea, R.O.; Wall, D.M.; Murphy, J.D.; Rusmanis, D. Biological Hydrogen Methanation Systems—An Overview of Design and Efficiency Efficiency. Bioengineered 2019, 10, 604–634. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.; Angelidaki, I. Integrated Biogas Upgrading and Hydrogen Utilization in an Anaerobic Reactor Containing Enriched Hydrogenotrophic Methanogenic Culture. Biotechnol. Bioeng. 2012, 109, 2729–2736. [Google Scholar] [CrossRef]
- Rachbauer, L.; Beyer, R.; Bochmann, G.; Fuchs, W. Characteristics of Adapted Hydrogenotrophic Community during Biomethanation. Sci. Total Environ. 2017, 595, 912–919. [Google Scholar] [CrossRef]
- Liew, F.; Martin, M.E.; Tappel, R.C.; Heijstra, B.D.; Mihalcea, C.; Köpke, M. Gas Fermentation—A Flexible Platform for Commercial Scale Production of Low-Carbon-Fuels and Chemicals from Waste and Renewable Feedstocks. Front. Microbiol. 2016, 7, 694. [Google Scholar] [CrossRef]
- Baleeiro, F.C.F.; Kleinsteuber, S.; Sträuber, H. Hydrogen as a Co-Electron Donor for Chain Elongation with Complex Communities. Front. Bioeng. Biotechnol. 2021, 9, 650631. [Google Scholar] [CrossRef]
- Baleeiro, F.C.F.; Kleinsteuber, S.; Neumann, A.; Sträuber, H. Syngas-Aided Anaerobic Fermentation for Medium-Chain Carboxylate and Alcohol Production: The Case for Microbial Communities. Appl. Microbiol. Biotechnol. 2019, 103, 8689–8709. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Treu, L.; Angelidaki, I. Biogas Upgrading via Hydrogenotrophic Methanogenesis in Two-Stage Continuous Stirred Tank Reactors at Mesophilic and Thermophilic Conditions. Environ. Sci. Technol. 2015, 49, 12585–12593. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logroño, W.; Kluge, P.; Kleinsteuber, S.; Harms, H.; Nikolausz, M. Effect of Inoculum Microbial Diversity in Ex Situ Biomethanation of Hydrogen. Bioengineering 2022, 9, 678. https://doi.org/10.3390/bioengineering9110678
Logroño W, Kluge P, Kleinsteuber S, Harms H, Nikolausz M. Effect of Inoculum Microbial Diversity in Ex Situ Biomethanation of Hydrogen. Bioengineering. 2022; 9(11):678. https://doi.org/10.3390/bioengineering9110678
Chicago/Turabian StyleLogroño, Washington, Paul Kluge, Sabine Kleinsteuber, Hauke Harms, and Marcell Nikolausz. 2022. "Effect of Inoculum Microbial Diversity in Ex Situ Biomethanation of Hydrogen" Bioengineering 9, no. 11: 678. https://doi.org/10.3390/bioengineering9110678