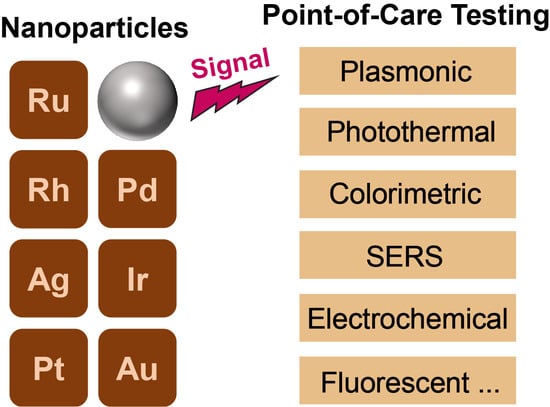
Noble Metal Nanoparticles for Point-of-Care Testing: Recent Advancements and Social Impacts
Abstract
:1. Introduction
2. The Unique Features of Noble Metal Nanoparticles (NM NPs)
3. Recent Advancements in NM NPs-Based POC Testing
3.1. Catalytically Active NM NPs-Based POC Tests
3.2. Plasmonically Active NM NPs-Based POC Tests

3.3. Photothermally Active NM NPs-Based POC Tests
3.4. SERS Active NM NPs-Based POC Tests
3.5. Label-Free Colorimetric NM NPs-Based POC Tests
3.6. NM NPs-Based POC Tests of Other Mechanisms
4. Social Impact
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Liu, K.; Li, Z.; Wang, P. Point of care testing for infectious diseases. Clin. Chim. Acta 2019, 493, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Syedmoradi, L.; Daneshpour, M.; Alvandipour, M.; Gomez, F.A.; Hajghassem, H.; Omidfar, K. Point of care testing: The impact of nanotechnology. Biosens. Bioelectron. 2017, 87, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Goble, J.A.; Rocafort, P.T. Point-of-Care Testing. J. Pharm. Pract. 2017, 30, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.Y.; Lee, S.-Y. Glucose Biosensors: An Overview of Use in Clinical Practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.S. Clinical tests with the dextrostix. A new method for rapid blood sugar determination. Ugeskr. Laeger. 1965, 127, 709–712. [Google Scholar] [PubMed]
- Xiang, Y.; Lan, T.; Lu, Y. Using the Widely Available Blood Glucose Meter to Monitor Insulin and HbA1c. J. Diabetes. Sci. Technol. 2014, 8, 855–888. [Google Scholar] [CrossRef] [Green Version]
- Leuvering, J.H.; Thal, P.J.; van der Waart, M.; Schuurs, A.H. Sol particle immunoassay (SPIA). J. Immunoass. 1980, 1, 77–91. [Google Scholar] [CrossRef]
- Vanamerongen, A.; Wichers, J.H.; Berendsen, L.B.J.M.; Timmermans, A.J.M.; Keizer, G.D.; Vandoorn, A.W.J.; Bantjes, A.; Vangelder, W.M.J. Colloidal carbon particles as a new label for rapid immunochemical test methods: Quantitative computer image analysis of results. J. Biotechnol. 1993, 30, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Glynou, K.; Ioannou, P.C.; Christopoulos, T.K.; Syriopoulou, V. Oligonucleotide-functionalized gold nanoparticles as probes in a dry-reagent strip biosensor for DNA analysis by hybridization. Anal. Chem. 2003, 75, 4155–4160. [Google Scholar] [CrossRef]
- Kozel, T.R.; Burnham-Marusich, A.R. Point-of-Care Testing for Infectious Diseases: Past, Present, and Future. J. Clin. Microbiol. 2017, 55, 2313–2320. [Google Scholar] [CrossRef]
- Nayak, S.; Blumenfeld, N.R.; Laksanasopin, T.; Sia, S.K. Point-of-Care Diagnostics: Recent Developments in a Connected Age. Anal. Chem. 2017, 89, 102–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Q.; Sun, X.; Dai, Z.; Gao, Y.; Gong, X.; Zhou, B.; Wu, J.; Wen, W. Point-of-care testing detection methods for COVID-19. Lab Chip 2021, 21, 1634–1660. [Google Scholar] [CrossRef] [PubMed]
- May, L.; Tran, N.; Ledeboer, N.A. Point-of-care COVID-19 testing in the emergency department: Current status and future prospects. Expert Rev. Mol. Diagn. 2021, 21, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Harun-Ur-Rashid, M.; Foyez, T.; Jahan, I.; Pal, K.; Imran, A.B. Rapid diagnosis of COVID-19 via nano-biosensor-implemented biomedical utilization: A systematic review. RSC Adv. 2022, 12, 9445–9465. [Google Scholar] [CrossRef]
- Biby, A.; Wang, X.; Liu, X.; Roberson, O.; Henry, A.; Xia, X. Rapid testing for coronavirus disease 2019 (COVID-19). MRS Commun. 2022, 12, 12–23. [Google Scholar] [CrossRef]
- Baker, A.N.; Hawker-Bond, G.W.; Georgiou, P.G.; Dedola, S.; Field, R.A.; Gibson, M.I. Glycosylated gold nanoparticles in point of care diagnostics: From aggregation to lateral flow. Chem. Soc. Rev. 2022, 51, 7238–7259. [Google Scholar] [CrossRef]
- Hansen, G.T. Point-of-Care Testing in Microbiology: A Mechanism for Improving Patient Outcomes. Clin. Chem. 2020, 66, 124–137. [Google Scholar] [CrossRef]
- Swierczewska, M.; Liu, G.; Lee, S.; Chen, X. High-sensitivity nanosensors for biomarker detection. Chem. Soc. Rev. 2012, 41, 2641–2655. [Google Scholar] [CrossRef]
- Kim, Y.S.; Raston, N.H.; Gu, M.B. Aptamer-based nanobiosensors. Biosens. Bioelectron. 2016, 76, 2–19. [Google Scholar]
- Crawford, B.M.; Wang, H.N.; Stolarchuk, C.; von Furstenberg, R.J.; Strobbia, P.; Zhang, D.; Qin, X.; Owzar, K.; Garman, K.S.; Vo-Dinh, T. Plasmonic nanobiosensors for detection of microRNA cancer biomarkers in clinical samples. Analyst 2020, 145, 4587–4594. [Google Scholar] [CrossRef]
- Aykaç, A.; Gergeroglu, H.; Beşli, B.; Akkaş, E.Ö.; Yavaş, A.; Güler, S.; Güneş, F.; Erol, M. An Overview on Recent Progress of Metal Oxide/Graphene/CNTs-Based Nanobiosensors. Nanoscale Res. Lett. 2021, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jing, C.; Zhang, L.; Long, Y.-T. Resonance scattering particles as biological nanosensors in vitro and in vivo. Chem. Soc. Rev. 2012, 41, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Parolo, C.; Merkoçi, A. Paper-based nanobiosensors for diagnostics. Chem. Soc. Rev. 2013, 42, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dao, T.N.T.; Koo, B.; Jang, Y.O.; Shin, Y. Trends and challenges of nanotechnology in self-test at home. TrAC—Trends Anal. Chem. 2021, 144, 116438. [Google Scholar] [CrossRef]
- Goldoni, R.; Farronato, M.; Connelly, S.T.; Tartaglia, G.M.; Yeo, W.H. Recent advances in graphene-based nanobiosensors for salivary biomarker detection. Biosens. Bioelectron. 2021, 171, 112723. [Google Scholar] [CrossRef]
- Xi, Z.; Ye, H.; Xia, X. Engineered noble-metal nanostructures for in vitro diagnostics. Chem. Mater. 2018, 30, 8391–8414. [Google Scholar] [CrossRef]
- Malekzad, H.; Zangabad, P.S.; Mirshekari, H.; Karimi, M.; Hamblin, M.R. Noble metal nanoparticles in biosensors: Recent studies and applications. Nanotechnol. Rev. 2017, 6, 301–329. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, X.; Liu, D.; Chen, X. Gold Nanoparticles for In Vitro Diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef] [Green Version]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [Green Version]
- Rycenga, M.; Xia, X.; Moran, C.; Zhou, F.; Qin, D.; Li, Z.-Y.; Xia, Y. Generation of hot spots with silver nanocubes for single-molecule detection by surface-enhanced Raman scattering. Angew. Chem. Int. Ed. 2011, 50, 5473–5477. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Matsubara, S.; Xiong, L.; Hayakawa, T. Nogami, M. Solvothermal Synthesis of Multiple Shapes of Silver Nanoparticles and Their SERS Properties. J. Phys. Chem. C 2007, 111, 9095–9104. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem. Int. Ed. 2008, 48, 60–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, A.R.; Habas, S.; Yang, P. Shape Control of Colloidal Metal Nanocrystals. Small 2008, 4, 310–325. [Google Scholar] [CrossRef]
- Murphy, C.J.; Sau, T.K.; Gole, A.M.; Orendorff, C.J.; Gao, J.; Gou, L.; Hunyadi, S.E.; Li, T. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications. J. Phys. Chem. B 2005, 109, 13857–13870. [Google Scholar] [CrossRef] [PubMed]
- Brioude, A.; Jiang, X.C.; Pileni, M.P. Optical Properties of Gold Nanorods: DDA Simulations Supported by Experiments. J. Phys. Chem. B 2005, 109, 13138–13142. [Google Scholar] [CrossRef]
- Huang, X.; Tang, S.; Mu, X.; Dai, Y.; Chen, G.; Zhou, Z.; Ruan, F.; Yang, Z.; Zheng, N. Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat. Nanotechnol. 2011, 6, 28–32. [Google Scholar] [CrossRef]
- Shi, Y.; Lyu, Z.; Zhao, M.; Chen, R.; Nguyen, Q.N.; Xia, Y. Noble-Metal Nanocrystals with Controlled Shapes for Catalytic and Electrocatalytic Applications. Chem. Rev. 2021, 121, 649–735. [Google Scholar] [CrossRef]
- Habibullah, G.; Viktorova, J.; Ruml, T. Current Strategies for Noble Metal Nanoparticle Synthesis. Nanoscale Res. Lett. 2021, 16, 47. [Google Scholar] [CrossRef]
- Sau, T.K.; Rogach, A.L. Nonspherical Noble Metal Nanoparticles: Colloid-Chemical Synthesis and Morphology Control. Adv. Mater. 2010, 22, 1781–1804. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, X.; Peng, H.-C. Shape-controlled synthesis of colloidal metal nanocrystals: Thermodynamic versus kinetic products. J. Am. Chem. Soc. 2015, 137, 7947–7966. [Google Scholar] [CrossRef]
- Xia, Y.; Gilroy, K.D.; Peng, H.-C.; Xia, X. Seed-mediated growth of colloidal metal nanocrystals. Angew. Chem. Int. Ed. 2017, 56, 60–95. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, M.H.; Amani, H.; Pourfatollah, A.A.; Pazoki-Toroudi, H.; Sedighimoghaddam, B. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens. Bio-Sens. Res. 2016, 9, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Peng, X.H.; Ansari, D.O.; Yin-Goen, Q.; Chen, G.Z.; Shin, D.M.; Yang, L.; Young, A.N.; Wang, M.D.; Nie, S. In vivo tumor targeting and spectroscopic detection with surface-enhanced raman nanoparticle tags. Nat. Biotechnol. 2008, 26, 83–90. [Google Scholar] [CrossRef]
- Weisbecker, C.S.; Merritt, M.V.; Whitesides, G.M. Molecular Self-Assembly of Aliphatic Thiols on Gold Colloids. Langmuir 1996, 12, 3763–3772. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, J.; Lu, N.; Kim, M.; Ghale, K.; Xu, Y.; McKenzie, E.; Liu, J.; Ye, H. Pd-Ir core-shell nanocubes: A type of highly efficient and versatile peroxidase mimic. ACS Nano 2015, 9, 9994–10004. [Google Scholar] [CrossRef]
- Wang, P.; Ahn, J.; Gao, R.; Qin, Q. Preserving the Shape of Silver Nanocubes under Corrosive Environment by Covering Their Edges and Corners with Iridium. Nanoscale 2020, 12, 20859–20867. [Google Scholar] [CrossRef]
- Lu, N.; Wang, J.; Xie, S.; Xia, Y.; Kim, M.J. Enhanced shape stability of Pd–Rh core–frame nanocubes at elevated temperature: In situ heating transmission electron microscopy. Chem. Commun. 2013, 49, 11806–11808. [Google Scholar] [CrossRef]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno)assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef] [Green Version]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Ye, H.; Tang, D.; Tao, J.; Habibi, S.; Minerick, A.; Tang, D.; Xia, X. Platinum decorated gold nanoparticles with dual functionalities for ultrasensitive colorimetric in vitro diagnostics. Nano Lett. 2017, 17, 5572–5579. [Google Scholar] [CrossRef]
- Josephy, P.D.; Eling, T.E.; Mason, R.P. The horseradish peroxidase-catalyzed oxidation of 3,5,3′,5′-tetramethylbenzidine: Free radical and charge-transfer complex intermediates. J. Biol. Chem. 1982, 257, 3669–3675. [Google Scholar] [CrossRef]
- Frey, A.; Meckelein, B.; Externest, D.; Schmidt, M.A. A stable and highly sensitive 3,3′,5,5′-tetramethylbenzidine-based substrate reagent for enzyme-linked immunosorbent assays. J. Immunol. Methods 2000, 233, 47–56. [Google Scholar] [CrossRef]
- Loynachan, C.N.; Thomas, M.R.; Gray, E.R.; Richards, D.A.; Kim, J.; Miller, B.S.; Brookes, J.C.; Agarwal, S.; Chudasama, V.; McKendry, R.A.; et al. Platinum Nanocatalyst Amplification: Redefining the Gold Standard for Lateral Flow Immunoassays with Ultrabroad Dynamic Range. ACS Nano 2018, 12, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.F.; Jia, S.; Ahmed, M.G.; Li, X.; Lin, L.; Chen, X.; Zhu, Z.; Yang, C. Visual Quantitative Detection of Circulating Tumor Cells with Single-Cell Sensitivity Using a Portable Microfluidic Device. Small 2019, 15, 1804890. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, F.; Zhu, Z.; Liu, D.; Chen, X.; Song, Y.; Zhou, L.; Yang, C. In Situ Pt Staining Method for Simple, Stable, and Sensitive Pressure-Based Bioassays. ACS Appl. Mater. Interfaces 2018, 10, 13390–13396. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-H.; Choi, E.; Ellis, E.; Lee, T.-C. Recent advances in gold nanoparticles for biomedical applications: From hybrid structures to multi-functionality. J. Mater. Chem. B 2019, 7, 3480–3496. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Li, W.; Cobley, C.M.; Chen, J.; Xia, X.; Zhang, Q.; Yang, M.; Cho, E.C.; Brown, P.K. Gold Nanocages: From synthesis to theranostic applications. Acc. Chem. Res. 2011, 44, 914–924. [Google Scholar] [CrossRef] [Green Version]
- Tu, M.H.; Sun, T.; Grattan, K.T.V. LSPR optical fibre sensors based on hollow gold nanostructures. Sens. Actuators B Chem. 2014, 191, 37–44. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Ruditskiy, A.; Xia, Y. 25th anniversary article: Galvanic replacement: A simple and versatile route to hollow nanostructures with tunable and well-controlled properties. Adv. Mater. 2013, 25, 6313–6333. [Google Scholar] [CrossRef]
- Gao, Z.; Ye, H.; Wang, Q.; Kim, M.; Tang, D.; Xi, Z.; Wei, Z.; Shao, S.; Xia, X. Template regeneration in galvanic replacement: A route to highly diverse hollow nanostructures. ACS Nano 2020, 14, 791–801. [Google Scholar] [CrossRef]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef]
- Boken, J.; Khurana, P.; Thatai, S.; Kumar, D.; Prasad, S. Plasmonic nanoparticles and their analytical applications: A review. Appl. Spectrosc. Rev. 2017, 52, 774–820. [Google Scholar] [CrossRef]
- Ye, H.; Nowak, C.; Liu, Y.; Li, Y.; Zhang, T.; Bleris, L.; Qin, Z. Plasmonic LAMP: Improving the Detection Specificity and Sensitivity for SARS-CoV-2 by Plasmonic Sensing of Isothermally Amplified Nucleic Acids. Small 2022, 18, 2107832. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, H.; Bayram, A.; Zhang, T.; Cai, Q.; Xie, C.; Huynh, H.; Peerzade, S.A.M.A.; Kahn, J.S.; Qin, Z. Gold Nanourchins Improve Virus Targeting and Plasmonic Coupling for Virus Diagnosis on a Smartphone Platform. medRxiv 2022. [Google Scholar] [CrossRef]
- Qin, Z.; Chan, W.C.W.; Boulware, D.R.; Akkin, T.; Butler, E.K.; Bischof, J.C. Significantly improved analytical sensitivity of lateral flow immunoassays by using thermal contrast. Angew. Chem. Int. Ed. 2012, 51, 4358–4361. [Google Scholar] [CrossRef] [Green Version]
- Zhan, L.; Guo, S.Z.; Song, F.; Gong, Y.; Xu, F.; Boulware, D.R.; McAlpine, M.C.; Chan, W.C.W.; Bischof, J.C. The Role of Nanoparticle Design in Determining Analytical Performance of Lateral Flow Immunoassays. Nano Lett. 2017, 17, 7207–7212. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ye, H.; Huynh, H.; Xie, C.; Kang, P.; Kahn, J.S.; Qin, Z. Digital plasmonic nanobubble detection for rapid and ultrasensitive virus diagnostics. Nat. Commun. 2022, 13, 1687. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [Green Version]
- Li, J.F.; Zhang, Y.J.; Ding, S.Y.; Panneerselvam, R.; Tian, Z.Q. Core-Shell Nanoparticle-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Haynes, C.L.; McFarland, A.D.; Van Duyne, R.P. Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2005, 77, 338A–346A. [Google Scholar] [CrossRef] [Green Version]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.K.; Lee, Y.H.; Koh, C.S.L.; Phan-Quang, G.C.; Han, X.; Lay, C.L.; Sim, H.Y.F.; Kao, Y.C.; An, Q.; Ling, X.Y. Designing surface-enhanced Raman scattering (SERS) platforms beyond hotspot engineering: Emerging opportunities in analyte manipulations and hybrid materials. Chem. Soc. Rev. 2019, 48, 731–756. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Sun, X.; Yin, D.; Li, X.; Yang, M.; Han, X.; Yang, L.; Zhao, B. Recyclable Au–TiO2 nanocomposite SERS-active substrates contributed by synergistic charge-transfer effect. Phys. Chem. Chem. Phys. 2017, 19, 11212–11219. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Si, Y.; Wang, D.; Wu, Z.; Li, J.; Yin, Y. Nanoconjugates of Ag/Au/Carbon Nanotube for Alkyne-Meditated Ratiometric SERS Imaging of Hypoxia in Hepatic Ischemia. Anal. Chem. 2019, 91, 4529–4536. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dai, E.; Xiao, R.; Zhou, Z.; Zhang, M.; Bai, Z.; Shao, Y.; Qi, K.; Tu, J.; Wang, C.; et al. Development of a SERS-based lateral flow immunoassay for rapid and ultra-sensitive detection of anti-SARS-CoV-2 IgM/IgG in clinical samples. Sens. Actuators B Chem. 2021, 329, 129196. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, J.; Kim, K.; Jeon, J.; Lee, S.; Ko, J.; Lee, J.; Kang, M.; Chung, D.R.; Choo, J. Quantitative Serodiagnosis of Scrub Typhus Using Surface-Enhanced Raman Scattering-Based Lateral Flow Assay Platforms. Anal. Chem. 2019, 91, 12275–12282. [Google Scholar] [CrossRef]
- Sharifi, M.; Attar, F.; Saboury, A.A.; Akhtari, K.; Hooshmand, N.; Hasan, A.; El-Sayed, M.A.; Falahati, M. Plasmonic gold nanoparticles: Optical manipulation, imaging, drug delivery and therapy. J. Control. Release 2019, 311-312, 170–189. [Google Scholar] [CrossRef]
- Ha, M.; Kim, J.-H.; You, M.; Li, Q.; Fan, C.; Nam, J.-M. Multicomponent Plasmonic Nanoparticles: From Heterostructured Nanoparticles to Colloidal Composite Nanostructures. Chem. Rev. 2019, 119, 12208–12278. [Google Scholar] [CrossRef]
- de la Rica, R.; Stevens, M.M. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat. Nanotechnol. 2012, 7, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Shao, S.; Gao, W.; Tang, D.; Tang, D.; Zou, S.; Kim, M.J.; Xia, X. Morphology-Invariant Metallic Nanoparticles with Tunable Plasmonic Properties. ACS Nano 2021, 15, 2428–2438. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, Y.; Zhu, M.; Yu, Z.; Ma, X.; Song, Y.; Zhou, S.; Yang, C. Microfluidic-Integrated Multicolor Immunosensor for Visual Detection of HIV-1 p24 Antigen with the Naked Eye. Anal. Chem. 2020, 92, 11826–11833. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Liu, X.; Coutts, J.; Austin, L.; Huo, Q. A One-Step Highly Sensitive Method for DNA Detection Using Dynamic Light Scattering. J. Am. Chem. Soc. 2008, 130, 8138–8139. [Google Scholar] [CrossRef]
- Pingarrón, J.M.; Yáñez-Sedeño, P.; González-Cortés, A. Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Ding, L.; Bond, A.M.; Zhai, J.; Zhang, J. Utilization of nanoparticle labels for signal amplification in ultrasensitive electrochemical affinity biosensors: A review. Anal. Chim. Acta. 2013, 797, 1–12. [Google Scholar] [CrossRef]
- Kang, X.; Zhu, M. Tailoring the photoluminescence of atomically precise nanoclusters. Chem. Soc. Rev. 2019, 48, 2422–2457. [Google Scholar] [CrossRef]
- Yu, Y.; New, S.Y.; Xie, J.; Su, X.; Tan, Y.N. Protein-based fluorescent metal nanoclusters for small molecular drug screening. Chem. Commun. 2014, 50, 13805–13808. [Google Scholar] [CrossRef]
- Xu, T.; Luo, Y.; Liu, C.; Zhang, X.; Wang, S. Integrated Ultrasonic Aggregation-Induced Enrichment with Raman Enhancement for Ultrasensitive and Rapid Biosensing. Anal. Chem. 2020, 92, 7816–7821. [Google Scholar] [CrossRef]
- Ndugga, N.; Artiga, S. Disparities in Health and Health Care: 5 Key Questions and Answers. Available online: https://www.kff.org/racial-equity-and-health-policy/issue-brief/disparities-in-health-and-health-care-5-key-question-and-answers/ (accessed on 3 October 2022).
- Agency for Healthcare Research and Quality. 2021 National Healthcare Quality and Disparities Report. Available online: https://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/nhqrdr/2021qdr.pdf (accessed on 3 October 2022).
- Institute of Medicine; Board on Health Sciences Policy; Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care; Smedley, B.D., Stith, A.Y., Nelson, A.R., Eds.; National Academies Press: Washington, DC, USA, 2003; ISBN 030908265X. [Google Scholar]
- Wheeler, S.M.; Bryant, A.S. Racial and Ethnic Disparities in Health and Health Care. Obstet. Gynecol. Clin. 2017, 44, 1–11. [Google Scholar] [CrossRef]
- Institute of Medicine; Committee on Monitoring Access to Personal Health Care Services. Access to Health Care in America; Millman, M., Ed.; National Academies Press: Washington, DC, USA, 1993; ISBN 0-309-04742-0. [Google Scholar]
- Nadeem, M.F.; Kaiser, L.R. Disparities in Health Care Delivery Systems. Thorac. Surg. Clin. 2022, 32, 13–21. [Google Scholar] [CrossRef]
- Kullgren, J.T.; McLaughlin, C.G.; Mitra, N.; Armstrong, K. Nonfinancial Barriers and Access to Care for U.S. Adults. Health Serv. Res. 2012, 47, 462–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, S.A.; Masters, R.M. Disparities in Health Care and the Digital Divide. Curr. Psychiatry Rep. 2021, 23, 61. [Google Scholar] [CrossRef] [PubMed]
- Turner, A. The Business Case for Racial Equity: A Strategy for Growth. Available online: http://www.nationalcivicleague.org/wp-content/uploads/2018/04/RacialEquityNationalReport-kellogg.pdf (accessed on 3 October 2022).
- Baker, A.N.; Richards, S.J.; Guy, C.S.; Congdon, T.R.; Hasan, M.; Zwetsloot, A.J.; Gallo, A.; Lewandowski, J.R.; Stansfeld, P.J.; Straube, A.; et al. The SARS-COV-2 Spike Protein Binds Sialic Acids and Enables Rapid Detection in Lateral Flow Point of Care Diagnostic Device. ACS Cent. Sci. 2020, 6, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Degregory, P.R.; Tapia, J.; Wong, T.; Villa, J.; Richards, I.; Crooks, R.M. Managing Heart Failure at Home with Point-of-Care Diagnostics. IEEE J. Transl. Eng. Health Med. 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Peng, H.; Chen, I.A. Rapid Colorimetric Detection of Bacterial Species Through the Capture of Gold Nanoparticles by Chimeric Phages. ACS Nano 2019, 13, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.T.; Gerber, B.S.; Sharp, L.K. Traveling Towards Disease: Transportation Barriers to Health Care Access. J. Community Health 2013, 38, 976–993. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, M.K.; McDonald, N.C.; Holmes, G.M. Transportation Barriers to Health Care in the United States: Findings from the National Health Interview Survey, 1997–2017. Am. J. Public Health 2020, 110, 815–822. [Google Scholar] [CrossRef]
- Montero, A.; Kearney, A.; Hamel, L.; Brodie, M. Americans’ Challenges with Health Care Costs. Available online: https://www.kff.org/health-costs/issue-brief/americans-challenges-with-health-care-costs/# (accessed on 3 October 2022).
- Huang, L.; Ding, L.; Zhou, J.; Chen, S.; Chen, F.; Zhao, C.; Xu, J.; Hu, W.; Ji, J.; Xu, H.; et al. One-Step Rapid Quantification of SARS-CoV-2 Virus Particles via Low-Cost Nonplasmonic Sensors in Generic Microplate Reader and Point-of-Care Device. Biosens. Bioelectron. 2021, 171, 112685. [Google Scholar] [CrossRef]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020, 14, 3822–3835. [Google Scholar] [CrossRef] [Green Version]
- Dinnes, J.; Deeks, J.J.; Berhane, S.; Taylor, M.; Adriano, A.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2021, 3, CD013705. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, M.; Gloag, L.; Bennett, D.T.; Hoque, S.; Pardehkhorram, R.; Bakthavathsalam, P.; Gonçales, V.R.; Tilley, R.D.; Gooding, J.J. Synthesis of gold-coated magnetic conglomerate nanoparticles with a fast magnetic response for bio-sensing. J. Mater. Chem. C. 2021, 9, 1034–1043. [Google Scholar] [CrossRef]
- Gao, Z.; Song, Y.; Hsiao, T.Y.; He, J.; Wang, C.; Shen, J.; MacLachlan, A.; Dai, S.; Singer, B.H.; Kurabayashi, K.; et al. Machine-Learning-Assisted Microfluidic Nanoplasmonic Digital Immunoassay for Cytokine Storm Profiling in COVID-19 Patients. ACS Nano 2021, 15, 18023–18036. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luciano, K.; Wang, X.; Liu, Y.; Eyler, G.; Qin, Z.; Xia, X. Noble Metal Nanoparticles for Point-of-Care Testing: Recent Advancements and Social Impacts. Bioengineering 2022, 9, 666. https://doi.org/10.3390/bioengineering9110666
Luciano K, Wang X, Liu Y, Eyler G, Qin Z, Xia X. Noble Metal Nanoparticles for Point-of-Care Testing: Recent Advancements and Social Impacts. Bioengineering. 2022; 9(11):666. https://doi.org/10.3390/bioengineering9110666
Chicago/Turabian StyleLuciano, Keven, Xiaochuan Wang, Yaning Liu, Gabriella Eyler, Zhenpeng Qin, and Xiaohu Xia. 2022. "Noble Metal Nanoparticles for Point-of-Care Testing: Recent Advancements and Social Impacts" Bioengineering 9, no. 11: 666. https://doi.org/10.3390/bioengineering9110666