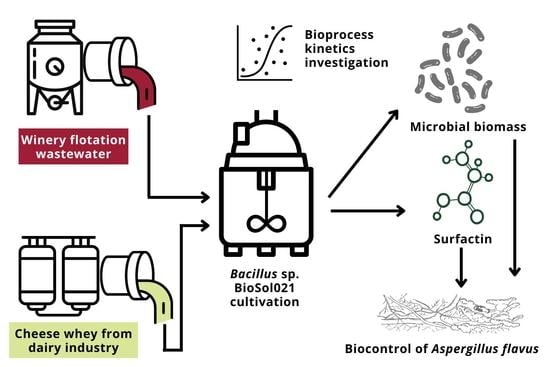
Dairy and Wine Industry Effluents as Alternative Media for the Production of Bacillus-Based Biocontrol Agents
Abstract
:1. Introduction
1.1. Biological Control as a Possible Route for Increased Sustainability of Agricultural Production
1.2. Alternative Media for Biocontrol Agents Production
1.2.1. Dairy Industry Effluents: A Brief Overview
1.2.2. Wine Industry Effluents: A Brief Overview
1.3. Hypothesis and the Main Objective of the Research
2. Materials and Methods
2.1. Microorganisms
2.2. Cultivation Media
2.3. Inoculum Preparation and Cultivation Parameters
2.4. Analytical Methods
2.4.1. Gravimetric Method for Biomass Dry Weight Measurement
2.4.2. Well-Diffusion Assay for Antimicrobial Activity Testing
2.4.3. CPC-BTB Method for Surfactin Quantification
2.4.4. HPLC Method for Sugar Concentration Determination
2.5. Investigation of Bioprocess Kinetics
3. Results and Discussion
3.1. Bioprocess Course Monitoring—Temperature, pH Value and DO Content
3.2. Microbial Growth Kinetics
3.3. Sugar Substrate Consumption Kinetics
3.4. Surfactin Production Kinetics
3.5. Antimicrobial Activity against the Aflatoxigenic Aspergillus flavus Strains
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shuping, D.S.S.; Eloff, J.N. The use of plants to protect plants and food against fungal pathogens: A review. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens-the IARC monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Savić, Z.; Dudaš, T.; Loc, M.; Grahovac, M.; Budakov, D.; Jajić, I.; Krstović, S.; Barošević, T.; Krska, R.; Sulyok, M.; et al. Biological Control of Aflatoxin in Maize Grown in Serbia. Toxins 2020, 12, 162. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Ghazali, F.M.; Mahyudin, N.A.; Samsudin, N.I.P. Biocontrol of aflatoxins using non-aflatoxigenic Aspergillus flavus: A literature review. J. Fungi 2021, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Amare, M.G.; Keller, N.P. Molecular mechanisms of Aspergillus flavus secondary metabolism and development. Fungal Genet. Biol. 2014, 66, 11–18. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Current Status and Recent Developments in Biopesticide Use. Agriculture 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Czaja, K.; Góralczyk, K.; Struciński, P.; Hernik, A.; Korcz, W.; Minorczyk, M.; Łyczewska, M.; Ludwicki, J.K. Biopesticides-towards increased consumer safety in the European Union. Pest Manag. Sci. 2015, 71, 3–6. [Google Scholar] [CrossRef]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Köhl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A. Biopesticides: Present status and the future prospects. J. Biofertilizers Biopestic. 2015, 6, 2. [Google Scholar] [CrossRef]
- Koul, O.K.O. Microbial biopesticides: Opportunities and challenges. CAB Rev. 2011, 2011, 1–26. [Google Scholar] [CrossRef]
- 11. Cawoy, H.; Bettiol, W.; Fickers, P.; Ongena, M. Bacillus-Based Biological Control of Plant Diseases. In Pesticides in the Modern World—Pesticides Use and Management; Stoytcheva, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 274–302. [Google Scholar]
- Penha, R.O.; Vandenberghe, L.P.S.; Faulds, C.; Soccol, V.T.; Soccol, C.R. Bacillus lipopeptides as powerful pest control agents for a more sustainable and healthy agriculture: Recent studies and innovations. Planta 2020, 251, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhi, Y.; Wu, Q.; Xu, Y. Production of surfactin from waste distillers’ grains by co-culture fermentation of two Bacillus amyloliquefaciens strains. Bioresour. Technol. 2017, 235, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Théatre, A.; Cano-Prieto, C.; Bartolini, M.; Laurin, Y.; Deleu, M.; Niehren, J.; Fida, T.; Gerbinet, S.; Alanjary, M.; Medema, M.H.; et al. The Surfactin-Like Lipopeptides From Bacillus spp.: Natural Biodiversity and Synthetic Biology for a Broader Application Range. Front. Bioeng. Biotechnol. 2021, 9, 623701. [Google Scholar] [CrossRef] [PubMed]
- Sen, R. Surfactin: Biosynthesis, genetics and potential applications. In Biosurfactants; Sen, R., Ed.; Springer: New York, NY, USA, 2010; Volume 672, pp. 316–323. [Google Scholar] [CrossRef]
- Dimkić, I.; Živković, S.; Berić, T.; Ivanović, Ž.; Gavrilović, V.; Stanković, S.; Fira, Đ. Characterization and evaluation of two Bacillus strains, SS-12.6 and SS-13.1, as potential agents for the control of phytopathogenic bacteria and fungi. Biol. Control 2013, 65, 312–321. [Google Scholar] [CrossRef]
- Sarwar, A.; Hassan, M.N.; Imran, M.; Iqbal, M.; Majeed, S.; Brader, G.; Sessitsch, A.; Hafeez, F.Y. Biocontrol activity of surfactin A purified from Bacillus NH-100 and NH-217 against rice bakanae disease. Microbiol. Res. 2018, 209, 1–13. [Google Scholar] [CrossRef]
- Stoll, A.; Salvatierra-Martínez, R.; González, M.; Araya, M. The role of surfactin production by Bacillus velezensis on colonization, biofilm formation on tomato root and leaf surfaces and subsequent protection (ISR) against Botrytis cinerea. Microorganisms 2021, 9, 2251. [Google Scholar] [CrossRef]
- Fenibo, E.O.; Ijoma, G.N.; Matambo, T. Biopesticides in sustainable agriculture: A critical sustainable development driver governed by green chemistry principles. Front. Sustain. Food Syst. 2021, 5, 619058. [Google Scholar] [CrossRef]
- Yi, H.; Li, M.; Huo, X.; Zeng, G.; Lai, C.; Huang, D.; An, Z.; Qin, L.; Liu, X.; Li, B.; et al. Recent development of advanced biotechnology for wastewater treatment. Crit. Rev. Biotechnol. 2019, 40, 99–118. [Google Scholar] [CrossRef]
- Neves, A.; Godina, R.; Azevedo, S.G.; Matias, J.C.O. A comprehensive review of industrial symbiosis. J. Clean. Prod. 2020, 247, 119113. [Google Scholar] [CrossRef]
- Ioannou, L.A.; Puma, G.L.; Fatta-Kassinos, D. Treatment of winery wastewater by physicochemical, biological and advanced processes: A review. J. Hazard. Mater. 2015, 286, 343–368. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Gaffey, J.; Sharma, M.; Dewhurst, R.J.; Moreau, B.; Newbold, J.; Clark, W.; Kumar Thakur, V.; Kumar Gupta, V. Valorization of dairy waste and by-products through microbial bioprocesses. Bioresour. Technol. 2022, 346, 126444. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy by-products: A review on the valorization of whey and second cheese whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Asunis, F.; De Gioannis, G.; Dessì, P.; Isipato, M.; Lens, P.N.L.; Muntoni, A.; Polettini, A.; Pomi, R.; Rossi, A.; Spiga, D. The dairy biorefinery: Integrating treatment processes for cheese whey valorisation. J. Environ. Manag. 2020, 276, 111240. [Google Scholar] [CrossRef] [PubMed]
- Sar, T.; Harirchi, S.; Ramezani, M.; Bulkan, G.; Yesilcimen Akbas, M.; Pandey, A.; Taherzadeh, M.J. Potential utilization of dairy industries by-products and wastes through microbial processes: A critical review. Sci. Total Environ. 2022, 810, 152253. [Google Scholar] [CrossRef]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Sci. Total Environ. 2013, 445–446, 385–396. [Google Scholar] [CrossRef]
- Costa, J.R.; Tonon, R.V.; Gottschalk, L.M.F.; Santiago, M.C.P.D.; Mellinger-Silva, C.; Pastrana, L.; Pintado, M.M.; Cabral, L.M.C. Enzymatic production of xylooligosaccharides from Brazilian Syrah grape pomace flour: A green alternative to conventional methods for adding value to agricultural by-products. J. Sci. Food Agric. 2019, 99, 1250–1257. [Google Scholar] [CrossRef]
- FAO, Food and Agriculture Organization of the United Nations. FAO Statistical Database (2022). Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 26 September 2022).
- Jorge, N.; Teixeira, A.R.; Guimarães, V.; Lucas, M.S.; Peres, J.A. Treatment of winery wastewater with a combination of adsorption and thermocatalytic processes. Processes 2022, 10, 75. [Google Scholar] [CrossRef]
- Oliveira, M.; Queda, C.; Duarte, E. Aerobic treatment of winery wastewater with the aim of water reuse. Water Sci. Technol. 2009, 60, 1217–1223. [Google Scholar] [CrossRef] [Green Version]
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable green processing of grape pomace for the production of value-added products: An overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar] [CrossRef]
- Allison, B.J.; Simmons, C.W. Obtaining multiple coproducts from red grape pomace via anthocyanin extraction and biogas production. J. Agric. Food Chem. 2018, 66, 8045–8053. [Google Scholar] [CrossRef]
- Caldas, T.W.; Mazza, K.E.L.; Teles, A.S.C.; Mattos, G.N.; Brígida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Phenolic compounds recovery from grape skin using conventional and non-conventional extraction methods. Ind. Crop. Prod. 2018, 111, 86–91. [Google Scholar] [CrossRef]
- Soceanu, A.; Dobrinas, S.; Sirbu, A.; Manea, N.; Popescu, V. Economic aspects of waste recovery in the wine industry. A multidisciplinary approach. Sci. Total. Environ. 2021, 10, 759. [Google Scholar] [CrossRef]
- Calheiros, C.S.C.; Pereira, S.I.A.; Castro, P.M.L. Culturable bacteria associated to the rhizosphere and tissues of Iris pseudacorus plants growing in a treatment wetland for winery wastewater discharge. Ecol. Eng. 2018, 115, 67–74. [Google Scholar] [CrossRef]
- Vlyssides, A.; Barampouti, E.; Mai, S. Wastewater characteristics from Greek wineries and distilleries. Water Sci. Technol. 2005, 51, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, C.S.; Patil, D.V.; Sarode, D.B.; Jadhav, R.N.; Attarde, S.B. Biodegradation of pollutants from winery wastewater by using fungi Aspergillus fumigatus and bacterium Bacillus Subtilis. J. Int. Environ. Appl. Sci. 2012, 7, 324–330. [Google Scholar]
- Dmitrović, S.; Pajčin, I.; Lukić, N.; Vlajkov, V.; Grahovac, M.; Grahovac, J.; Jokić, A. Taguchi grey relational analysis for multi-response optimization of Bacillus bacteria flocculation recovery from fermented broth by chitosan to enhance biocontrol efficiency. Polymers 2022, 14, 3282. [Google Scholar] [CrossRef]
- Vlajkov, V.; Grahovac, M.; Budakov, D.; Loc, M.; Pajčin, I.; Milić, D.; Novaković, T.; Grahovac, J. Distribution, genetic diversity and biocontrol of aflatoxigenic Aspergillus flavus in Serbian maize fields. Toxins 2021, 13, 687. [Google Scholar] [CrossRef]
- LabPlot: A Free, Open Source, Cross-Platform Data Visualization and Analysis Software Accessible to Everyone (v. 2.9). Available online: https://labplot.kde.org (accessed on 15 September 2022).
- Yang, H.; Yu, H.; Shen, Z. A novel high-throughput and quantitative method based on visible color shifts for screening Bacillus subtilis THY-15 for surfactin production. J. Ind. Microbiol. Biotechnol. 2015, 42, 1139–1147. [Google Scholar] [CrossRef]
- Valenzuela-Ávila, L.; Miliar, Y.; Moya-Ramirez, I.; Chyhyrynets, O.; Garcia-Román, M.; Altmajer-Vaz, D. Effect of emulsification and hydrolysis pretreatments of waste frying oil on surfactin production. J. Chem. Technol. Biotechnol. 2019, 95, 223–231. [Google Scholar] [CrossRef]
- Mohsin, A.; Zhang, K.; Hu, J.; Rehman, S.; Tariq, M.; Zaman, W.Q.; Khan, I.M.; Zhuang, Y.; Guo, M. Optimized biosynthesis of xanthan via effective valorization of orange peels using response surface methodology: A kinetic model approach. Carbohydr. Polym. 2018, 181, 793–800. [Google Scholar] [CrossRef]
- GraphPad Prism Software; v. 8.4.3; GraphPad Software Inc.: San Diego, CA, USA, 2020.
- Dias, D.R.; Vilela, D.M.; Silvestre, M.P.C.; Schwan, R.F. Alkaline protease from Bacillus sp. isolated from coffee bean grown on cheese whey. World J. Microbiol. Biotechnol. 2008, 24, 2027–2034. [Google Scholar] [CrossRef]
- Pokhrel, C.; Ohga, S. Submerged culture conditions for mycelial yield and polysaccharides production by Lyophyllum decastes. Food Chem. 2007, 105, 641–646. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Qi, Q.; Hua, Q. Quantification and analysis of metabolic characteristics of aerobic succinate-producing Escherichia coli under different aeration conditions. Process Biochem. 2012, 47, 1532–1538. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef]
- Oliveira, C.S.S.; Silva, M.O.D.; Silva, C.E.; Carvalho, G.; Reis, M.A.M. Assessment of protein-rich cheese whey waste stream as a nutrients source for low-cost mixed microbial PHA production. Appl. Sci. 2018, 8, 1817. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.; Zhan, Z.; Saakes, M.; van der Weijden, R.D.; Buisman, C.J.N. Electrochemical recovery of phosphorus from acidic cheese wastewater: Feasibility, quality of products, and comparison with chemical precipitation. ACS ES&T Water 2021, 1, 1002–1013. [Google Scholar] [CrossRef]
- Tjørve, K.M.C.; Tjørve, E. The use of Gompertz models in growth analyses, and new Gompertz-model approach: An addition to the Unified-Richards family. PLoS ONE 2017, 12, e0178691. [Google Scholar] [CrossRef]
- Rangarajan, V.; Clarke, K.G. Process development and intensification for enhanced production of Bacillus lipopeptides. Biotechnol. Genet. Eng. Rev. 2015, 31, 46–68. [Google Scholar] [CrossRef]
- Barale, S.S.; Ghane, S.G.; Sonawane, K.D. Purification and characterization of antibacterial surfactin isoforms produced by Bacillus velezensis SK. AMB Express 2022, 12, 7. [Google Scholar] [CrossRef]
- Duque, A.F.; Campo, R.; Val del Rio, A.; Amorim, C.L. Wastewater valorization: Practice around the world at pilot-and full-scale. Int. J. Environ. Res. Public Health 2021, 18, 9466. [Google Scholar] [CrossRef]
- Li, L.; Ge, Y.; Xiao, M. Towards biofuel generation III+: A sustainable industrial symbiosis design of co-producing algal and cellulosic biofuels. J. Clean. Prod. 2021, 306, 127144. [Google Scholar] [CrossRef]
- Mantese, G.C.; Amaral, D.C. Agent-based simulation to evaluate and categorize industrial symbiosis indicators. J. Clean. Prod. 2018, 186, 450–464. [Google Scholar] [CrossRef]
- Liguori, R.; Amore, A.; Faraco, V. Waste valorization by biotechnological conversion into added value products. Appl. Microbiol. Biotechnol. 2013, 97, 6129–6147. [Google Scholar] [CrossRef]
- Mitra, R.; Dutta, D. Growth profiling, kinetics and substrate utilization of low-cost dairy waste for production of β-cryptoxanthin by Kocuria marina DAGII. R. Soc. Open Sci. 2018, 5, 172318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panikov, N.S. Microbial Growth Kinetics, 1st ed.; Chapman & Hall: London, UK, 1995; pp. 15–16. [Google Scholar]
- Heuson, E.; Etchegaray, A.; Filipe, S.L.; Beretta, D.; Chevalier, M.; Phalip, V.; Coutte, F. Screening of lipopeptide-producing strains of Bacillus sp. using a new automated and sensitive fluorescence detection method. Biotechnol. J. 2019, 14, 1800314. [Google Scholar] [CrossRef]
- Ong, S.A.; Wu, J.C. A simple method for rapid screening of biosurfactant-producing strains using bromothymol blue alone. Biocatal. Agric. Biotechnol. 2018, 16, 121–125. [Google Scholar] [CrossRef]
- Yeh, M.S.; Wei, Y.H.; Chang, J.S. Enhanced production of surfactin from Bacillus subtilis by addition of solid carriers. Biotechnol. Prog. 2005, 21, 1329–1334. [Google Scholar] [CrossRef]
- Zanotto, A.W.; Valério, A.; de Andrade, C.J.; Pastore, G.M. New sustainable alternatives to reduce the production costs for surfactin 50 years after the discovery. Appl. Microbiol. Biotechnol. 2019, 103, 8647–8656. [Google Scholar] [CrossRef]
- Sousa, M.; Melo, V.M.M.; Rodrigues, S.; Sant’ana, H.B.; Gonçalves, L.R.B. Screening of biosurfactant-producing Bacillus strains using glycerol from the biodiesel synthesis as main carbon source. Bioprocess Biosyst. Eng. 2012, 35, 897–906. [Google Scholar] [CrossRef]
- de Andrade, C.J.; Simiqueli, A.P.R.; de Andrade, L.M.; Mendes, A.M.; Jauregi, P.; Pastore, G.M. Comparative study: Bench-scale surfactin production from Bacillus subtilis using analytical grade and concentrated glycerol from the biodiesel industry. Int. J. Sci. World 2016, 5, 28–37. [Google Scholar] [CrossRef]
- Paraszkiewicz, K.; Bernat, P.; Kusmierka, A.; Chojniak, J.; Plaza, G. Structural identification of lipopeptide biosurfactants produced by Bacillus subtilis strains grown on the media obtained from renewable natural resources. J. Environ. Manag. 2018, 209, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Cagri-Mehmetoglu, A.; Kusakli, S.; van de Ventert, M. Production of polysaccharide and surfactin by Bacillus subtilis ATCC 6633 using rehydrated whey powder as the fermentation medium. J. Dairy Sci. 2012, 95, 3643–3649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitschke, M.; Pastore, G.M. Biosurfactant production by Bacillus subtilis using cassava-processing effluent. Appl. Biochem. Biotechnol. 2004, 112, 163–172. [Google Scholar] [CrossRef]
- de Andrade, C.J.; de Andrade, L.M.; Bution, M.L.; Dolder, M.A.H.; Barros, F.F.C.; Pastore, G.M. Optimizing alternative substrate for simultaneous production of surfactin and 2, 3-butanediol by Bacillus subtilis LB5a. Biocatal. Agric. Biotechnol. 2016, 6, 209–218. [Google Scholar] [CrossRef]
- de Andrade, C.J.; Barros, F.F.C.; de Andrade, L.M.; Rocco, S.A.; Sforça, M.L.; Pastore, G.M.; Jauregi, P. Ultrafiltration based purification strategies for surfactin produced by Bacillus subtilis LB5A using cassava wastewater as substrate. J. Chem. Technol. Biotechnol. 2016, 91, 3018–3027. [Google Scholar] [CrossRef] [Green Version]
- Gaden, E.L. Fermentation process kinetics. Biotechnol. Bioeng. 2000, 67, 629–635. [Google Scholar] [CrossRef]
- Kirishnan, N.; Velramar, B.; Velu, R.K. Investigation of antifungal activity of surfactin against mycotoxigenic phytopathogenic fungus Fusarium moniliforme and its impact in seed germination and mycotoxicosis. Pestic. Biochem. Physiol. 2019, 155, 101–107. [Google Scholar] [CrossRef]
- Toral, L.; Rodriguez, M.; Béjar, V.; Sampedro, I. Antifungal activity of lipopeptides from Bacillus XT1 CECT 8661 against Botrytis cinerea. Front. Microbiol. 2018, 9, 1315. [Google Scholar] [CrossRef] [Green Version]
- Desmyttere, H.; Deweer, C.; Muchembled, J.; Sahmer, K.; Jacquin, J.; Coutte, F.; Jacques, P. Antifungal activities of Bacillus subtilis lipopeptides to two Venturia inaequalis strains possessing different tebuconazole sensitivity. Front. Microbiol. 2019, 10, 2327. [Google Scholar] [CrossRef]
- Pathak, K.V.; Keharia, H. Identification of surfactins and iturins produced by potent fungal antagonist, Bacillus subtilis K1 isolated from aerial roots of banyan (Ficus benghalensis) tree using mass spectrometry. 3 Biotech 2014, 4, 283–295. [Google Scholar] [CrossRef]
- Farzaneh, M.; Shi, Z.Q.; Ahmadzadeh, M.; Hu, L.B.; Ghassempour, A. Inhibition of the Aspergillus flavus growth and aflatoxin B1 contamination on pistachio nut by fengycin and surfactin-producing Bacillus subtilis UTBSP1. Plant Pathol. J. 2016, 32, 209–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadipour, M.; Mousivand, M.; Salehi Jouzani, G.; Abbasalizadeh, S. Molecular and biochemical characterization of Iranian surfactin-producing Bacillus subtilis isolates and evaluation of their biocontrol potential against Aspergillus flavus and Colletotrichum gloeosporioides. Can. J. Microbiol. 2009, 55, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, Y.; Shan, M.; Zhao, H.; Lu, Z.; Lu, Y. A mini-review: Mechanism of antimicrobial action and application of surfactin. World J. Microbiol. Biotechnol. 2022, 38, 143. [Google Scholar] [CrossRef] [PubMed]








| Parameter/Effluent | pH Value | Dry Matter/Water Content (%, w/v) | Sugar Content (g/L) |
|---|---|---|---|
| CW | 4.90 | 5.81/94.19 | 50.28 L |
| WFW | 3.60 | 17.01/82.99 | 57.36 F 66.33 G |
| B1 | B2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Gompertz Equation | R2 | Logistic Equation | R2 | Gompertz Equation | R2 | Logistic Equation | R2 | |
| Microbial growth | ||||||||
| X0 (g/L) | 0.2109 | 0.9984 | 0.3754 | 0.9954 | 0.1606 | 0.9956 | 0.2971 | 0.9958 |
| Xmax (g/L) | 4.223 | 4.113 | 3.652 | 3.516 | ||||
| µ (1/h) | 0.0722 | 0.1123 | 0.0544 | 0.0856 | ||||
| Substrate consumption | ||||||||
| α (gsubstrate/gbiomass) | 3.470 L | 0.9771 L | 1.410 L | 0.9908 L | 1.974 F 4.331 G | 0.9874 F 0.9991 G | 0.4179 F 0.7416 G | 0.9920 F 0.9961 G |
| β (1/h) | 0.0053 L | 0.0336 L | 0.0010 F 0.0015 G | 0.0211 F 0.0494 G | ||||
| Product formation | ||||||||
| γ (gsubstrate/gbiomass) | 0.1769 | 0.9841 | 0.6137 | 0.9897 | 0.7466 | 0.9923 | 0.1453 | 0.9913 |
| δ (1/h) | 0.0011 | 0.0025 | 0.0001 | 0.0081 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitrović, S.; Pajčin, I.; Vlajkov, V.; Grahovac, M.; Jokić, A.; Grahovac, J. Dairy and Wine Industry Effluents as Alternative Media for the Production of Bacillus-Based Biocontrol Agents. Bioengineering 2022, 9, 663. https://doi.org/10.3390/bioengineering9110663
Dmitrović S, Pajčin I, Vlajkov V, Grahovac M, Jokić A, Grahovac J. Dairy and Wine Industry Effluents as Alternative Media for the Production of Bacillus-Based Biocontrol Agents. Bioengineering. 2022; 9(11):663. https://doi.org/10.3390/bioengineering9110663
Chicago/Turabian StyleDmitrović, Selena, Ivana Pajčin, Vanja Vlajkov, Mila Grahovac, Aleksandar Jokić, and Jovana Grahovac. 2022. "Dairy and Wine Industry Effluents as Alternative Media for the Production of Bacillus-Based Biocontrol Agents" Bioengineering 9, no. 11: 663. https://doi.org/10.3390/bioengineering9110663