Effect of Passive Ultrasonic Irrigation, Er,Cr:YSGG Laser, and Photon-Induced Photoacoustic Streaming against Enterococcus faecalis Biofilms in the Apical Third of Root Canals
Abstract
:1. Introduction
2. Materials and Methods
Sample Selection, Preparation, and Inoculation
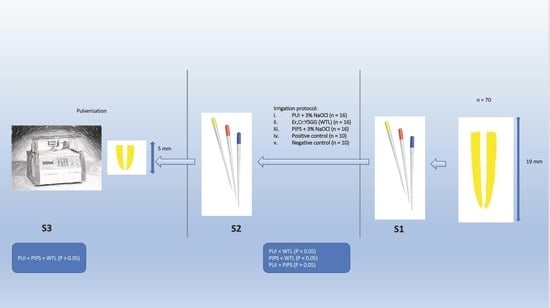
3. Grouping and Irrigation Protocols
- i.
- PUI + 3% NaOCl (n = 16)
- ii.
- Er,Cr:YSGG (WTL) (n = 16)
- iii.
- PIPS + 3% NaOCl (n = 16)
- iv.
- Positive control group (PC) (n = 10)
- v.
- Negative control group (NC) (n = 10)
4. Microbiological Analysis
4.1. Sampling of Root Canal Content
4.2. Pulverisation of the Apical Third of the Root
5. Data Analysis
6. Results
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peters, O.A.; Schönenberger, K.; Laib, A. Effects of four Ni-Ti preparation techniques on root canal geometry assessed by micro computed tomography. Int. Endod. J. 2001, 34, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Tonini, R.; Salvadori, M.; Audino, E.; Sauro, S.; Garo, M.L.; Salgarello, S. Irrigating Solutions and Activation Methods Used in Clinical Endodontics: A Systematic Review. Front. Oral Health 2022, 3, 838043. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, P.; Mariswamy, A.B. A scanning electron microscope evaluation of efficacy of sodium hypochlorite and Allium sativum in smear layer removal in root canals with the use of modified evacuation system: An ex vivo study. J. Conserv. Dent. 2018, 21, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Boutsioukis, C.; Arias-Moliz, M.T. Present status and future directions—Irrigants and irrigation methods. Int. Endod. J. 2022, 55 (Suppl. 3), 588–612. [Google Scholar] [CrossRef] [PubMed]
- Adcock, J.M.; Sidow, S.J.; Looney, S.W.; Liu, Y.; McNally, K.; Lindsey, K.; Tay, F.R. Histologic evaluation of canal and isthmus debridement efficacies of two different irrigant delivery techniques in a closed system. J. Endod. 2011, 37, 544–548. [Google Scholar] [CrossRef]
- van der Sluis, L.W.; Versluis, M.; Wu, M.K.; Wesselink, P.R. Passive ultrasonic irrigation of the root canal: A review of the literature. Int. Endod. J. 2007, 40, 415–426. [Google Scholar] [CrossRef]
- Orlowski, N.B.; Schimdt, T.F.; Teixeira, C.D.S.; Garcia, L.; Savaris, J.M.; Tay, F.R.; Bortoluzzi, E.A. Smear Layer Removal Using Passive Ultrasonic Irrigation and Different Concentrations of Sodium Hypochlorite. J. Endod. 2020, 46, 1738–1744. [Google Scholar] [CrossRef]
- Galler, K.M.; Grubmüller, V.; Schlichting, R.; Widbiller, M.; Eidt, A.; Schuller, C.; Wölflick, M.; Hiller, K.A.; Buchalla, W. Penetration depth of irrigants into root dentine after sonic, ultrasonic and photoacoustic activation. Int. Endod. J. 2019, 52, 1210–1217. [Google Scholar] [CrossRef]
- Zhu, X.; Yin, X.; Chang, J.W.; Wang, Y.; Cheung, G.S.; Zhang, C. Comparison of the antibacterial effect and smear layer removal using photon-initiated photoacoustic streaming aided irrigation versus a conventional irrigation in single-rooted canals: An in vitro study. Photomed. Laser Surg. 2013, 31, 371–377. [Google Scholar] [CrossRef]
- Balić, M.; Lucić, R.; Mehadžić, K.; Bago, I.; Anić, I.; Jakovljević, S.; Plečko, V. The efficacy of photon-initiated photoacoustic streaming and sonic-activated irrigation combined with QMiX solution or sodium hypochlorite against intracanal E. faecalis biofilm. Lasers Med. Sci. 2016, 31, 335–342. [Google Scholar] [CrossRef]
- Eldeeb, I.M.; Nawar, N.N.; Saber, S.M.; Hassanein, E.E.; Schäfer, E. Smear layer removal and sealer penetration with different tapers after using photon-initiated photoacoustic streaming technique. Clin. Oral Investig. 2021, 25, 5025–5032. [Google Scholar] [CrossRef]
- Wen, C.; Yan, L.; Kong, Y.; Zhao, J.; Li, Y.; Jiang, Q. The antibacterial efficacy of photon-initiated photoacoustic streaming in root canals with different diameters or tapers. BMC Oral Health 2021, 21, 542. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zou, T.; Li, D.; Chang, J.W.; Huang, X.; Zhang, C. Effectiveness of Sonic, Ultrasonic, and Photon-Induced Photoacoustic Streaming Activation of NaOCl on Filling Material Removal Following Retreatment in Oval Canal Anatomy. Photomed. Laser Surg. 2016, 34, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kosarieh, E.; Bolhari, B.; Sanjari Pirayvatlou, S.; Kharazifard, M.J.; Sattari Khavas, S.; Jafarnia, S.; Saberi, S. Effect of Er:YAG laser irradiation using SWEEPS and PIPS technique on dye penetration depth after root canal preparation. Photodiagn. Photodyn. Ther. 2021, 33, 102136. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.T.; Torabinejad, M.; Shabahang, S.; Retamozo, B.; Aprecio, R.M.; Chen, J.W. Comparison of efficacy of pulverization and sterile paper point techniques for sampling root canals. J. Endod. 2013, 39, 1057–1059. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.R.; Siqueira, J.F., Jr.; Carmo, F.L.; Santos, A.L.; Peixoto, R.S.; Rôças, I.N.; Rosado, A.S. Bacterial community profiling of cryogenically ground samples from the apical and coronal root segments of teeth with apical periodontitis. J. Endod. 2009, 35, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Rôças, I.N.; Siqueira, J.F., Jr.; Santos, K.R. Association of Enterococcus faecalis with different forms of periradicular diseases. J. Endod. 2004, 30, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Pelozo, L.L.; Silva-Neto, R.D.; de Oliveira, L.P.B.; Salvador, S.L.; Corona, S.A.M.; Souza-Gabriel, A.E. Comparison of the methods of disinfection/sterilization of extracted human roots for research purposes. Dent. Med. Probl. 2022, 59, 381–387. [Google Scholar] [CrossRef]
- Jensen, S.A.; Walker, T.L.; Hutter, J.W.; Nicoll, B.K. Comparison of the cleaning efficacy of passive sonic activation and passive ultrasonic activation after hand instrumentation in molar root canals. J. Endod. 1999, 25, 735–738. [Google Scholar] [CrossRef]
- Martins, M.R.; Carvalho, M.F.; Pina-Vaz, I.; Capelas, J.A.; Martins, M.A.; Gutknecht, N. Outcome of Er,Cr:YSGG laser-assisted treatment of teeth with apical periodontitis: A blind randomized clinical trial. Photomed. Laser Surg. 2014, 32, 3–9. [Google Scholar] [CrossRef]
- Rôças, I.N.; Provenzano, J.C.; Neves, M.A.; Siqueira, J.F., Jr. Disinfecting Effects of Rotary Instrumentation with Either 2.5% Sodium Hypochlorite or 2% Chlorhexidine as the Main Irrigant: A Randomized Clinical Study. J. Endod. 2016, 42, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jung, H.; Kim, S.; Shin, S.J.; Kim, E. The Influence of an Isthmus on the Outcomes of Surgically Treated Molars: A Retrospective Study. J. Endod. 2016, 42, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr. Aetiology of root canal treatment failure: Why well-treated teeth can fail. Int. Endod. J. 2001, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Villalta-Briones, N.; Baca, P.; Bravo, M.; Solana, C.; Aguado-Pérez, B.; Ruiz-Linares, M.; Arias-Moliz, M.T. A laboratory study of root canal and isthmus disinfection in extracted teeth using various activation methods with a mixture of sodium hypochlorite and etidronic acid. Int. Endod. J. 2021, 54, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Rödig, T.; Koberg, C.; Baxter, S.; Konietschke, F.; Wiegand, A.; Rizk, M. Micro-CT evaluation of sonically and ultrasonically activated irrigation on the removal of hard-tissue debris from isthmus-containing mesial root canal systems of mandibular molars. Int. Endod. J. 2019, 52, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Peciuliene, V.; Balciuniene, I.; Eriksen, H.M.; Haapasalo, M. Isolation of Enterococcus faecalis in previously root-filled canals in a Lithuanian population. J. Endod. 2000, 26, 593–595. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rocas, I.N. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 85–94. [Google Scholar] [CrossRef]
- Wong, D.T.; Cheung, G.S. Extension of bactericidal effect of sodium hypochlorite into dentinal tubules. J. Endod. 2014, 40, 825–829. [Google Scholar] [CrossRef]
- Shen, Y.; Stojicic, S.; Haapasalo, M. Antimicrobial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. J. Endod. 2011, 37, 657–661. [Google Scholar] [CrossRef]
- Lu, W.; Yao, J.; Zhu, X.; Qi, Y. Nanomedicines: Redefining traditional medicine. Biomed. Pharmacother. 2021, 134, 111103. [Google Scholar] [CrossRef]
- Teplitsky, P.E.; Chenail, B.L.; Mack, B.; Machnee, C.H. Endodontic irrigation—A comparison of endosonic and syringe delivery systems. Int. Endod. J. 1987, 20, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Al-Jadaa, A.; Paqué, F.; Attin, T.; Zehnder, M. Necrotic pulp tissue dissolution by passive ultrasonic irrigation in simulated accessory canals: Impact of canal location and angulation. Int. Endod. J. 2009, 42, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, P.; Merlos, A.; Sierra, J.M.; Arnabat-Dominguez, J.; Viñas, M. Er,Cr:YSGG Laser-Activated Irrigation and Passive Ultrasonic Irrigation: Comparison of Two Strategies for Root Canal Disinfection. Photobiomodul. Photomed. Laser Surg. 2020, 38, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Gordon, W.; Atabakhsh, V.A.; Meza, F.; Doms, A.; Nissan, R.; Rizoiu, I.; Stevens, R.H. The antimicrobial efficacy of the erbium, chromium:yttrium-scandium-gallium-garnet laser with radial emitting tips on root canal dentin walls infected with Enterococcus faecalis. J. Am. Dent. Assoc. 2007, 138, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Pedullà, E.; Genovese, C.; Campagna, E.; Tempera, G.; Rapisarda, E. Decontamination efficacy of photon-initiated photoacoustic streaming (PIPS) of irrigants using low-energy laser settings: An ex vivo study. Int. Endod. J. 2012, 45, 865–870. [Google Scholar] [CrossRef]
- da Fonseca Alvarez, A.; Moura-Netto, C.; Daliberto Frugoli, A.; Fernando, C.; Aranha, A.C.; Davidowicz, H. Temperature changes on the root surfaces of mandibular incisors after an 810-nm high-intensity intracanal diode laser irradiation. J. Biomed. Opt. 2012, 17, 015006. [Google Scholar] [CrossRef]
- Golob, B.S.; Olivi, G.; Vrabec, M.; El Feghali, R.; Parker, S.; Benedicenti, S. Efficacy of Photon-induced Photoacoustic Streaming in the Reduction of Enterococcus faecalis within the Root Canal: Different Settings and Different Sodium Hypochlorite Concentrations. J. Endod. 2017, 43, 1730–1735. [Google Scholar] [CrossRef]
- George, R.; Walsh, L.J. Apical extrusion of root canal irrigants when using Er:YAG and Er,Cr:YSGG lasers with optical fibers: An in vitro dye study. J. Endod. 2008, 34, 706–708. [Google Scholar] [CrossRef]
- Yost, R.A.; Bergeron, B.E.; Kirkpatrick, T.C.; Roberts, M.D.; Roberts, H.W.; Himel, V.T.; Sabey, K.A. Evaluation of 4 Different Irrigating Systems for Apical Extrusion of Sodium Hypochlorite. J. Endod. 2015, 41, 1530–1534. [Google Scholar] [CrossRef]
- Peters, O.A.; Bardsley, S.; Fong, J.; Pandher, G.; Divito, E. Disinfection of root canals with photon-initiated photoacoustic streaming. J. Endod. 2011, 37, 1008–1012. [Google Scholar] [CrossRef]
- Al Shahrani, M.; DiVito, E.; Hughes, C.V.; Nathanson, D.; Huang, G.T. Enhanced removal of Enterococcus faecalis biofilms in the root canal using sodium hypochlorite plus photon-induced photoacoustic streaming: An in vitro study. Photomed. Laser Surg. 2014, 32, 260–266. [Google Scholar] [CrossRef]
- Claesson, R.; Johansson, A.; Belibasakis, G.N. Clinical laboratory diagnostics in dentistry: Application of microbiological methods. Front. Oral Health 2022, 3, 983991. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.J.; Baylan, S.; Park, B.-W.; Richter, G.; Sitti, M. Hydrophobic pinning with copper nanowhiskers leads to bactericidal properties. PLoS ONE 2017, 12, e0175428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lv, B.; Sun, F.; Liu, J.; Wang, Y.; Gao, Y.; Qi, F.; Chang, Z.; Fu, X. Rapid Freezing Enables Aminoglycosides to Eradicate Bacterial Persisters via Enhancing Mechanosensitive Channel MscL-Mediated Antibiotic Uptake. mBio 2020, 11, e03239-19. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.C.; Cheung, G.S.P.; Chang, J.W.W.; Zhang, C.; Lee, A.H.C. Enterococcus faecalis Shields Porphyromonas gingivalis in Dual-Species Biofilm in Oxic Condition. Microorganisms 2022, 10, 1729. [Google Scholar] [CrossRef]
- Kharouf, N.; Pedullà, E.; La Rosa, G.R.M.; Bukiet, F.; Sauro, S.; Haikel, Y.; Mancino, D. In Vitro Evaluation of Different Irrigation Protocols on Intracanal Smear Layer Removal in Teeth with or without Pre-Endodontic Proximal Wall Restoration. J. Clin. Med. 2020, 9, 3325. [Google Scholar] [CrossRef]
- Do, Q.L.; Gaudin, A. The Efficiency of the Er: YAG Laser and PhotonInduced Photoacoustic Streaming (PIPS) as an Activation Method in Endodontic Irrigation: A Literature Review. J. Lasers Med. Sci. 2020, 11, 316–334. [Google Scholar] [CrossRef]

| Group | n | S1 (CFU/mL) | S2 (CFU/mL) | No. of Samples with No Bacteria Recovered | Bacterial Reduction | ||
|---|---|---|---|---|---|---|---|
| Mean | Medium | Mean | Medium | ||||
| PUI | 16 | 5.69 × 105 | 3.80 × 105 | 2.91 × 102 | 0.00 | 8 | 99.89% |
| PIPS | 16 | 6.14 × 105 | 3.00 × 105 | 6.06 × 101 | 6.00 | 7 | 99.98% |
| WTL | 16 | 3.83 × 105 | 3.12 × 105 | 2.69 × 104 | 9.48 × 103 | 0 | 92.06% |
| PC | 16 | 5.99 × 105 | 4.60 × 105 | - | - | - | - |
| Group | n | S1 (CFU/mL) | S2 (CFU/mL) | No. of Samples with No Bacteria Recovered | Bacterial Reduction | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Mean | Medium | Mean | Medium | |||||
| PUI | 16 | 5.55 × 102 | 5.25 × 102 | 5.50 × 101 | 0.00 | 10 | 84% | p < 0.00 |
| PIPS | 16 | 7.34 × 102 | 4.50 × 102 | 1.61 × 102 | 2.00 × 101 | 7 | 63% | p < 0.01 |
| WTL | 16 | 7.24 × 102 | 5.62 × 102 | 1.46 × 102 | 4.00 × 101 | 6 | 71% | p < 0.02 |
| PC | 16 | - | - | - | - | - | - | |
| Multiple Comparisons | Mean Rank Difference | p-Value |
|---|---|---|
| PUI vs. WTL | −23.00 | p < 0.001 |
| PUI vs. PIPS | 0.6875 | p > 0.05 |
| PIPS vs. WTL | 23.688 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seghayer, I.; Lee, A.H.C.; Cheung, G.S.P.; Zhang, C. Effect of Passive Ultrasonic Irrigation, Er,Cr:YSGG Laser, and Photon-Induced Photoacoustic Streaming against Enterococcus faecalis Biofilms in the Apical Third of Root Canals. Bioengineering 2023, 10, 490. https://doi.org/10.3390/bioengineering10040490
Seghayer I, Lee AHC, Cheung GSP, Zhang C. Effect of Passive Ultrasonic Irrigation, Er,Cr:YSGG Laser, and Photon-Induced Photoacoustic Streaming against Enterococcus faecalis Biofilms in the Apical Third of Root Canals. Bioengineering. 2023; 10(4):490. https://doi.org/10.3390/bioengineering10040490
Chicago/Turabian StyleSeghayer, Ibrahim, Angeline H. C. Lee, Gary S. P. Cheung, and Chengfei Zhang. 2023. "Effect of Passive Ultrasonic Irrigation, Er,Cr:YSGG Laser, and Photon-Induced Photoacoustic Streaming against Enterococcus faecalis Biofilms in the Apical Third of Root Canals" Bioengineering 10, no. 4: 490. https://doi.org/10.3390/bioengineering10040490