Standardization of Animal Models and Techniques for Platelet-Rich Fibrin Production: A Narrative Review and Guideline
Abstract
:1. Introduction
2. Definitions and Search Process
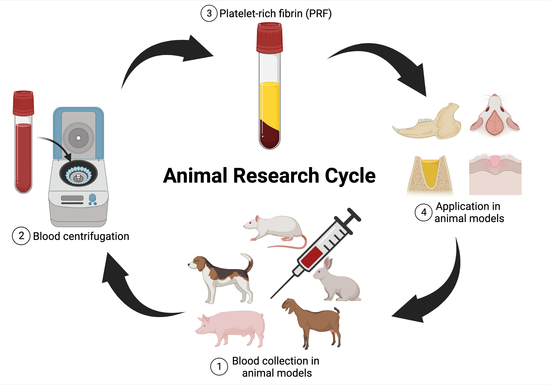
3. Overview of Animal Research
4. Considerations on Blood Collection
5. Venipuncture of Rats
6. Venipuncture of Rabbits
7. Venipuncture of Dogs
8. Venipuncture of Pigs and Mini-Pigs
9. Venipuncture of Goats
10. Considerations on the Relative Centrifugal Force
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butler, D. Crossing the valley of death: A chasm has opened up between biomedical researchers and the patients who need their discoveries. Declan Butler asks how the ground shifted and whether the US National Institutes of Health can bridge the gap. Nature 2008, 453, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Viergever, R.F.; Li, K. Trends in global clinical trial registration: An analysis of numbers of registered clinical trials in different parts of the world from 2004 to 2013. BMJ Open 2015, 5, e008932. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.S.; Tabak, L.A. Policy: NIH plans to enhance reproducibility. Nature 2014, 505, 612–613. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Adda, F.; Schoeffler, C.; Vervelle, A. Une opportunité en paro-implantologie: Le PRF. Implantodontie 2001, 42, e62. [Google Scholar]
- da Silva, M.T.; Mourão, C.F.d.A.B.; Mello-Machado, R.C.; Montemezzi, P.; Barbosa, R.d.L.; Sartoretto, S.C.; Leite, P.E.C.; Javid, K.; Kawase, T.; Alves, G.G. Effects of Leukocyte-Platelet-Rich Fibrin (L–PRF) on Pain, Soft Tissue Healing, Growth Factors, and Cytokines after Third Molar Extraction: A Randomized, Split-Mouth, Double-Blinded Clinical Trial. Appl. Sci. 2021, 11, 1666. [Google Scholar] [CrossRef]
- Mourão, C.F.d.A.B.; de Mello-Machado, R.C.; Javid, K.; Moraschini, V. The use of leukocyte-and platelet-rich fibrin in the management of soft tissue healing and pain in post-extraction sockets: A randomized clinical trial. J. Cranio-Maxillofac. Surg. 2020, 48, 452–457. [Google Scholar] [CrossRef]
- Mourão, C.F.d.A.B.; Valiense, H.; Melo, E.R.; Mourão, N.B.M.F.; Maia, M.D.-C. Obtention of injectable platelets rich-fibrin (i-PRF) and its polymerization with bone graft. Rev. Colégio Bras. Cir. 2015, 42, 421–423. [Google Scholar] [CrossRef]
- de Almeida Barros Mourao, C.F.; Lourenco, E.S.; Nascimento, J.R.B.; Machado, R.C.M.; Rossi, A.M.; Leite, P.E.C.; Granjeiro, J.M.; Alves, G.G.; Calasans-Maia, M.D. Does the association of blood-derived growth factors to nanostructured carbonated hydroxyapatite contributes to the maxillary sinus floor elevation? A randomized clinical trial. Clin. Oral Investig. 2019, 23, 369–379. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e51–e55. [Google Scholar] [CrossRef]
- Lourenco, E.S.; Mourao, C.; Leite, P.E.C.; Granjeiro, J.M.; Calasans-Maia, M.D.; Alves, G.G. The in vitro release of cytokines and growth factors from fibrin membranes produced through horizontal centrifugation. J. Biomed. Mater. Res. Part A 2018, 106, 1373–1380. [Google Scholar] [CrossRef]
- da Silva, L.M.P.; Sávio, D.S.F.; de Ávila, F.C.; Vicente, R.M.; Reis, G.G.D.; Denardi, R.J.; da Costa, N.M.M.; Silva, P.H.F.; Mourão, C.; Miron, R.J.; et al. Comparison of the effects of platelet concentrates produced by high and low-speed centrifugation protocols on the healing of critical-size defects in rat calvaria: A microtomographic and histomorphometric study. Platelets 2022, 33, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Mourão, C.F.d.A.B.; Gheno, E.; Lourenço, E.S.; de Lima Barbosa, R.; Kurtzman, G.M.; Javid, K.; Mavropoulos, E.; Benedicenti, S.; Calasans-Maia, M.D.; de Mello Machado, R.C. Characterization of a new membrane from concentrated growth factors associated with denaturized Albumin (Alb-CGF) for clinical applications: A preliminary study. Int. J. Growth Factors Stem Cells Dent. 2018, 1, 64. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Schaller, B.; Mourão, C.; Zhang, Y.; Sculean, A.; Miron, R.J. Biological characterization of an injectable platelet-rich fibrin mixture consisting of autologous albumin gel and liquid platelet-rich fibrin (Alb-PRF). Platelets 2020, 31, 74–81. [Google Scholar] [CrossRef]
- Şentürk, F.; Bahadır, O.; Aktaş, O.; Bıyık, A.F.; Ercan, E. Effects of titanium prepared platelet rich fibrin on facial nerve regeneration: An experimental study. Braz. J. Otorhinolaryngol. 2022, 88, 867–874. [Google Scholar] [CrossRef]
- Shanei, F.; Khoshzaban, A.; Taleghani, F.; Tehranchi, M.; Tayeed, M.H. The Effect of Low-Level Laser Therapy in Combination with Leukocyte- and Platelet- Rich Fibrin on Bone Regeneration in Rabbits’ Calvarial Defects: Histologic and Histomorphometric Studies. Cell J. 2022, 24, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Taufik, S.A.; Dirja, B.T.; Utomo, D.N.; Usman, M.A.; Sakti, M.; Saleh, M.R.; Hatta, M.; Budu. Double membrane platelet-rich fibrin (PRF)—Synovium succeeds in regenerating cartilage defect at the knee: An experimental study on rabbit. Heliyon 2023, 9, e13139. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Liu, Y.; Yu, J.; Sun, X.; Wang, L.; Zhou, Y. Effects of platelet-rich fibrin on osteogenic differentiation of Schneiderian membrane derived mesenchymal stem cells and bone formation in maxillary sinus. Cell Commun. Signal 2022, 20, 88. [Google Scholar] [CrossRef]
- Zalama, E.; Karrouf, G.; Rizk, A.; Salama, B.; Samy, A. Does zinc oxide nanoparticles potentiate the regenerative effect of platelet-rich fibrin in healing of critical bone defect in rabbits? BMC Vet. Res. 2022, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Rady, D.; Mubarak, R.; Moneim, R.A.A. Healing capacity of bone marrow mesenchymal stem cells versus platelet-rich fibrin in tibial bone defects of albino rats: An in vivo study. F1000Research 2018, 7, 1573. [Google Scholar] [CrossRef]
- Özçay, N.; Özdemir, H.; Besim, H. Role of platelet-rich fibrin on intestinal anastomosis wound healing in a rat. Biomed. Mater. 2018, 13, 045006. [Google Scholar] [CrossRef]
- Huang, M.-L.; Zhai, Z.; Chen, Z.-X.; Yang, X.-N.; Qi, Z.-L. Platelet-rich fibrin membrane nerve guidance conduit: A potentially promising method for peripheral nerve injuries. Chin. Med. J. 2020, 133, 999–1001. [Google Scholar] [PubMed]
- Dohan Ehrenfest, D.M.; Bielecki, T.; Jimbo, R.; Barbe, G.; Del Corso, M.; Inchingolo, F.; Sammartino, G. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte-and platelet-rich fibrin (L-PRF). Curr. Pharm. Biotechnol. 2012, 13, 1145–1152. [Google Scholar] [CrossRef]
- Cieslik-Bielecka, A.; Choukroun, J.; Odin, G.; Ehrenfest, M.D. L-PRP/L-PRF in esthetic plastic surgery, regenerative medicine of the skin and chronic wounds. Curr. Pharm. Biotechnol. 2012, 13, 1266–1277. [Google Scholar] [PubMed]
- Andreone, A.; den Hollander, D. A retrospective study on the use of dermis micrografts in platelet-rich fibrin for the resurfacing of massive and chronic full-thickness burns. Stem Cells Int. 2019, 2019, 8636079. [Google Scholar] [CrossRef]
- Yamakawa, S.; Hayashida, K. Advances in surgical applications of growth factors for wound healing. Burn. Trauma 2019, 7, 10. [Google Scholar] [CrossRef]
- Weisel, J.W.; Litvinov, R.I. Fibrin Formation, Structure and Properties. Subcell. Biochem. 2017, 82, 405–456. [Google Scholar] [CrossRef]
- Beristain-Covarrubias, N.; Perez-Toledo, M.; Thomas, M.R.; Henderson, I.R.; Watson, S.P.; Cunningham, A.F. Understanding Infection-Induced Thrombosis: Lessons Learned From Animal Models. Front. Immunol. 2019, 10, 2569. [Google Scholar] [CrossRef]
- Hernandez, E.; Fawcett, A.; Brouwer, E.; Rau, J.; Turner, P.V. Speaking Up: Veterinary Ethical Responsibilities and Animal Welfare Issues in Everyday Practice. Animals 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J. Pharmacol. Pharmacother. 2010, 1, 94–99. [Google Scholar] [CrossRef]
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. PREPARE: Guidelines for planning animal research and testing. Lab. Anim. 2018, 52, 135–141. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
- Arifin, W.N.; Zahiruddin, W.M. Sample size calculation in animal studies using resource equation approach. Malays. J. Med. Sci. MJMS 2017, 24, 101. [Google Scholar] [PubMed]
- Albus, U. Guide for the Care and Use of Laboratory Animals, 8th ed.; SAGE Publications Sage: London, UK, 2012. [Google Scholar]
- Brouwer, G.J. Formulary for Laboratory Animals. Lab. Anim. 2005, 39, 457. [Google Scholar]
- Ren, Z.Q.; Du, B.; Dong, H.J.; Duan, G.H.; Du, A.C.; Wang, Y.; Zhao, L.X.; Shao, W. Autologous Platelet-Rich Plasma Repairs Burn Wound and Reduces Burn Pain in Rats. J. Burn. Care Res. 2022, 43, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, K.; Arkoumanis, T.; Liakea, A.; Nikiteas, N.; Zargaran, D.; Zargaran, A.; Kontzoglou, K.; Kyriakopoulou, P.; Perrea, D. Platelet-rich Plasma Gel versus Hyaluronic Acid on Prevention of Peritoneal Abdominal Adhesion Formation in Rats. Chirurgia 2022, 117, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Lee, K.H.; Chung, S.D.; Chen, K.C.; Praveen Rajneesh, C.; Chen, B.H.; Cheng, J.H.; Lin, W.Y.; Chiang, H.S.; Wu, Y.N. Intracavernous Injection of Platelet-Rich Plasma Therapy Enhances Erectile Function and Decreases the Mortality Rate in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2022, 23, 3017. [Google Scholar] [CrossRef]
- Aboutalebi, H.; Alipour, F.; Ebrahimzadeh-Bideskan, A. The protective effect of co-administration of platelet-rich plasma (PRP) and pentoxifylline (PTX) on cyclophosphamide-induced premature ovarian failure in mature and immature rats. Toxicol. Mech. Methods 2022, 32, 588–596. [Google Scholar] [CrossRef]
- Torul, D.; Bereket, M.C.; Onger, M.E.; Altun, G. Comparison of the regenerative effects of platelet-rich fibrin and plasma rich in growth factors on injured peripheral nerve: An experimental study. J. Oral Maxillofac. Surg. 2018, 76, 1823.e1821–1823.e1812. [Google Scholar] [CrossRef]
- Ozcay, N.; Ozant, A.; Arslan, K.; Ozkayalar, H.; Besim, H. Platelet-rich fibrin can accelerate the healing of common bile duct anastomosis in a rat/Trombositten zengin fibrin sicanlarda safra yolu anastomoz yara iyilesmesini hizlandirabilir. Turk. J. Surg. 2020, 36, 256–264. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Mao, L.; Cui, L.; Bai, W. Therapeutic Effect of Platelet-Rich Fibrin Transplant on Formation of Thin Endometrium. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2021, 19, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Engler-Pinto, A.; Siessere, S.; Calefi, A.; Oliveira, L.; Ervolino, E.; de Souza, S.; Furlaneto, F.; Messora, M.R. Effects of leukocyte-and platelet-rich fibrin associated or not with bovine bone graft on the healing of bone defects in rats with osteoporosis induced by ovariectomy. Clin. Oral Implant. Res. 2019, 30, 962–976. [Google Scholar] [CrossRef] [PubMed]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef] [PubMed]
- Akyildiz, S.; Soluk-Tekkesin, M.; Keskin-Yalcin, B.; Unsal, G.; Yildiz, S.O.; Ozcan, I.; Cakarer, S. Acceleration of fracture healing in experimental model: Platelet-rich fibrin or hyaluronic acid? J. Craniofacial Surg. 2018, 29, 1794–1798. [Google Scholar] [CrossRef]
- Alizadeh, M.; Salehi, S.; Tavakoli, M.; Mirhaj, M.; Varshosaz, J.; Kazemi, N.; Salehi, S.; Mehrjoo, M.; Abadi, S.A.M. PDGF and VEGF-releasing bi-layer wound dressing made of sodium tripolyphosphate crosslinked gelatin-sponge layer and a carrageenan nanofiber layer. Int. J. Biol. Macromol. 2023, 223, 123491. [Google Scholar] [CrossRef] [PubMed]
- Alsherif, A.A.; Eltokhey, H.M.; Taiema, D.A. Platelet rich fibrin versus ozone gel for periodontal regeneration in induced rats’ intrabony three-wall periodontal defects. J. Oral Biol. Craniofacial Res. 2020, 10, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Awadeen, M.A.; Al-Belasy, F.A.; Ameen, L.E.; Helal, M.E.; Grawish, M.E. Early therapeutic effect of platelet-rich fibrin combined with allogeneic bone marrow-derived stem cells on rats’ critical-sized mandibular defects. World J. Stem Cells 2020, 12, 55. [Google Scholar] [CrossRef]
- Demirel, E.; Yildiz, K.; Çadirci, K.; Aygün, H.; Şenocak, E.; Gündoğdu, B. Effect of platelet-rich fibrin on epidural fibrosis and comparison to ADCON® Gel and hyaluronic acid. Acta Orthop. Et Traumatol. Turc. 2018, 52, 469–474. [Google Scholar] [CrossRef]
- Grecu, A.F.; Popa, D.G.; Lungulescu, C.V.; Ciucă, E.M.; Camen, A.; Marinescu, D.; Nica, O.; Busuioc, C.J.; Chen, F.I.; Ciurea, M.E. Histological findings from rat calvaria defect augmented with platelet-rich fibrin by using two consecutive periosteal incisions. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2019, 60, 111–118. [Google Scholar]
- Grecu, A.F.; Grecu, D.; Nica, O.; Ciuca, E.M.; Camen, A.; Ciurea, M.E. A novel method of obtaining platelet rich fibrin from rats and quantifying platelet count. Curr. Health Sci. J. 2019, 45, 104. [Google Scholar]
- Jamalpour, M.R.; Shahabi, S.; Baghestani, M.; Shokri, A.; Jamshidi, S.; Khazaei, S. Complementarity of surgical therapy, photobiomodulation, A-PRF and L-PRF for management of medication-related osteonecrosis of the jaw (MRONJ): An animal study. BMC Oral. Health 2022, 22, 241. [Google Scholar] [CrossRef]
- Mirhaj, M.; Tavakoli, M.; Varshosaz, J.; Labbaf, S.; Jafarpour, F.; Ahmaditabar, P.; Salehi, S.; Kazemi, N. Platelet rich fibrin containing nanofibrous dressing for wound healing application: Fabrication, characterization and biological evaluations. Biomater. Adv. 2022, 134, 112541. [Google Scholar] [CrossRef]
- Mourad, S.; Al-Dubai, S.; Elsayed, S.; El-Zehary, R. Efficacy of platelet-rich fibrin and tacrolimus on facial nerve regeneration: An animal study. Int. J. Oral Maxillofac. Surg. 2022, 51, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Atti, V.N.; Fernandes, M.; de Lima Figueiredo, G.S.; Roth, F.; Valente, S.G.; Nakachima, L.; Fernandes, C.; Dos Santos, J.G. Peripheral nerve regeneration in rats using nerve graft in a vein conduit pre-filled with platelet-rich fibrin (PRF). Hand Surg. Rehabil. 2023, 42, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Nica, O.; Popa, D.G.; Grecu, A.F.; Ciucă, E.M.; Ciurea, M.E. Effects of platelet rich fibrin on full thickness skin grafts in the rat model-planimetry results. Curr. Health Sci. J. 2019, 45, 278. [Google Scholar] [PubMed]
- Nugraha, A.P.; Narmada, I.B.; Ernawati, D.S.; Dinaryanti, A.; Hendrianto, E.; Ihsan, I.S.; Riawan, W.; Rantam, F.A. Osteogenic potential of gingival stromal progenitor cells cultured in platelet rich fibrin is predicted by core-binding factor subunit-α1/Sox9 expression ratio (in vitro). F1000Research 2018, 7, 1134. [Google Scholar] [CrossRef] [PubMed]
- Motta Padilha, W.S.; Borges Soares, A.; Navarro-Junior, H.; César Joly, J.; Cristina Peruzzo, D.; Henrique Napimoga, M.; Ferreira Martinez, E. Histologic Evaluation of Leucocyte-and Platelet-Rich Fibrin in the Inflammatory Process and Repair of Noncritical Bone Defects in the Calvaria of Rats. Int. J. Oral Maxillofac. Implant. 2018, 33, 1206–1212. [Google Scholar] [CrossRef]
- Silveira, B.B.B.; Teixeira, L.N.; Miron, R.J.; Martinez, E.F. Effect of platelet-rich fibrin (PRF) membranes on the healing of infected skin wounds. Arch. Dermatol. Res. 2022, 315, 559–567. [Google Scholar] [CrossRef]
- Sumida, R.; Maeda, T.; Kawahara, I.; Yusa, J.; Kato, Y. Platelet-rich fibrin increases the osteoprotegerin/receptor activator of nuclear factor-κB ligand ratio in osteoblasts. Exp. Ther. Med. 2019, 18, 358–365. [Google Scholar] [CrossRef]
- Tavakoli, M.; Mirhaj, M.; Salehi, S.; Varshosaz, J.; Labbaf, S.; Golshirazi, A.; Kazemi, N.; Haghighi, V. Coaxial electrospun angiogenic nanofiber wound dressing containing advanced platelet rich-fibrin. Int. J. Biol. Macromol. 2022, 222, 1605–1618. [Google Scholar] [CrossRef]
- Tayşi, M.; Atalay, B.; Çankaya, B.; Yıldırım, S. Effects of single-and double-layered resorbable membranes and platelet-rich fibrin on bone healing. Clin. Oral Investig. 2018, 22, 1689–1695. [Google Scholar] [CrossRef]
- Vares, P.; Dehghan, M.M.; Bastami, F.; Biazar, E.; Shamloo, N.; Keshel, S.H.; Khojasteh, A. Effects of platelet-rich fibrin/collagen membrane on sciatic nerve regeneration. J. Craniofacial Surg. 2021, 32, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, Y.; Xue, Y.; Shi, J.; Zhang, X.; Liu, Y.; Midgley, A.C.; Wang, S. Multifunctional triple-layered composite scaffolds combining platelet-rich fibrin promote bone regeneration. ACS Biomater. Sci. Eng. 2019, 5, 6691–6702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, S.; Lei, C.; Li, G.; Wang, B. Experimental study of negative pressure wound therapy combined with platelet-rich fibrin for bone-exposed wounds. Regen. Med. 2022, 17, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Lemini, C.; Jaimez, R.; Franco, Y. Gender and inter-species influence on coagulation tests of rats and mice. Thromb. Res. 2007, 120, 415–419. [Google Scholar] [CrossRef]
- Lewis, J.H.; Van Thiel, D.H.; Hasiba, U.; Spero, J.A.; Gavaler, J. Comparative hematology and coagulation: Studies on rodentia (rats). Comp. Biochem. Physiol. A Comp. Physiol. 1985, 82, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Siller-Matula, J.M.; Plasenzotti, R.; Spiel, A.; Quehenberger, P.; Jilma, B. Interspecies differences in coagulation profile. Thromb. Haemost. 2008, 100, 397–404. [Google Scholar] [CrossRef]
- Costa, N.O.; Forcato, S.; Cavichioli, A.M.; Pereira, M.R.F.; Gerardin, D.C.C. In utero and lactational exposure to triclocarban: Age-associated changes in reproductive parameters of male rat offspring. Toxicol. Appl. Pharmacol. 2020, 401, 115077. [Google Scholar] [CrossRef]
- Somogyi, V.; Peto, K.; Deak, A.; Tanczos, B.; Nemeth, N. Effects of aging and gender on micro-rheology of blood in 3 to 18 months old male and female Wistar (Crl:WI) rats. Biorheology 2018, 54, 127–140. [Google Scholar] [CrossRef]
- Pertsov, S.S.; Abramova, A.Y.; Chekhlov, V.V. Effect of Repeated Stress Exposures on the Blood Cytokine Profile in Rats with Different Behavioral Parameters. Bull. Exp. Biol. Med. 2022, 172, 397–401. [Google Scholar] [CrossRef]
- Mu, Z.; He, Q.; Xin, L.; Li, Y.; Yuan, S.; Zou, H.; Shu, L.; Song, J.; Huang, Y.; Chen, T. Effects of injectable platelet rich fibrin on bone remodeling in combination with DBBM in maxillary sinus elevation: A randomized preclinical study. Am. J. Transl. Res. 2020, 12, 7312–7325. [Google Scholar]
- Liu, Z.; Jin, H.; Xie, Q.; Jiang, Z.; Guo, S.; Li, Y.; Zhang, B. Controlled Release Strategies for the Combination of Fresh and Lyophilized Platelet-Rich Fibrin on Bone Tissue Regeneration. BioMed Res. Int. 2019, 2019, 4923767. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, H.; Duan, Q.; Bao, H.; Li, A.; Li, W.; Chen, J.; He, Y. A comparative study of the effects of platelet-rich fibrin, concentrated growth factor and platelet-poor plasma on the healing of tooth extraction sockets in rabbits. BMC Oral. Health 2022, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, M.; Fujioka-Kobayashi, M.; Yoneyama, Y.; Inada, R.; Satomi, T. Regenerative Potential of Solid Bone Marrow Aspirate Concentrate Compared with Platelet-Rich Fibrin. Tissue Eng. Part A 2022, 28, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, C.; Li, J.; Liu, C.; Mi, K.; Zhang, X. Platelet-rich fibrin combined with new bone graft material for mandibular defect repair: A in vivo study on rabbits. Dent. Mater. J. 2023, 42, 241–247. [Google Scholar] [CrossRef]
- Mogharehabed, A.; Torabinia, N.; Sharifi Darani, S.; Afshari, Z.; Yaghini, J. Effect of leukocyte and platelet-rich fibrin on free gingival graft healing: A clinical and histological study in rabbits. J. Adv. Periodontol. Implant. Dent. 2022, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.H.; Kim, Y.D.; Song, J.M.; Shin, S.H. Comparative study of bone regeneration using fibrin sealant with xenograft in rabbit sinus: Pilot study. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 5. [Google Scholar] [CrossRef] [PubMed]
- Damayanti, M.M.; Rachmawati, M. Pre-Clinical Study: Immunohistochemical evaluation of matrix metalloproteinase-13 on rabbit (Oryctolagus cuniculus) socket healing after application of platelet-rich fibrin with and without hydroxyapatite. F1000Research 2022, 11, 29. [Google Scholar] [CrossRef]
- Dereli Can, G.; Akcan, G.; Can, M.E.; Akdere, Ö.E.; Çaylı, S.; Şimşek, G.; Gümüşderelioğlu, M. Surgical and Immunohistochemical Outcomes of Scleral Reconstruction with Autogenic, Allogenic and Xenogenic Grafts: An Experimental Rabbit Model. Curr. Eye Res. 2020, 45, 1572–1582. [Google Scholar] [CrossRef]
- Karayürek, F.; Kadiroğlu, E.T.; Nergiz, Y.; Coşkun Akçay, N.; Tunik, S.; Ersöz Kanay, B.; Uysal, E. Combining platelet rich fibrin with different bone graft materials: An experimental study on the histopathological and immunohistochemical aspects of bone healing. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2019, 47, 815–825. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, S.K.; Lee, J.H. Bone regeneration of demineralized dentin matrix with platelet-rich fibrin and recombinant human bone morphogenetic protein-2 on the bone defects in rabbit calvaria. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 34. [Google Scholar] [CrossRef]
- Kinoshita, T.; Hashimoto, Y.; Orita, K.; Nishida, Y.; Nishino, K.; Nakamura, H. Autologous Platelet-Rich Fibrin Membrane to Augment Healing of Microfracture Has Better Macroscopic and Histologic Grades Compared with Microfracture Alone on Chondral Defects in a Rabbit Model. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2022, 38, 417–426. [Google Scholar] [CrossRef]
- Kızıldağ, A.; Tasdemir, U.; Arabacı, T.; Kızıldağ, C.A.; Albayrak, M.; Şahin, B. Effects of Autogenous Tooth Bone Graft and Platelet-Rich Fibrin in Peri-Implant Defects: An Experimental Study in an Animal Model. J. Oral Implantol. 2020, 46, 221–226. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, Y. Effect of Co-transplanting Stromal Vascular Fraction-Gelatin and Platelet-Rich Fibrin on the Long-Term Maintenance of Fat Volume. Aesthetic. Plast. Surg. 2021, 45, 1853–1859. [Google Scholar] [CrossRef]
- Mudalal, M.; Sun, X.; Li, X.; Zhou, Y. The evaluation of leukocyte-platelet rich fibrin as an anti-inflammatory autologous biological additive. A novel in vitro study. Saudi Med. J. 2019, 40, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Rezuc, A.; Saavedra, C.; Maass, R.; Poblete, C.; Nappe, C. Histological comparison of DBBM and platelet rich fibrin for guided bone regeneration in a rabbit model. J. Oral Biol. Craniofacial Res. 2020, 10, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Salih, S.I.; Al-Falahi, N.H.; Saliem, A.H.; Abedsalih, A.N. Effectiveness of platelet-rich fibrin matrix treated with silver nanoparticles in fracture healing in rabbit model. Vet. World 2018, 11, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Yeh, Y.Y.; Chen, C.H.; Manga, Y.B.; Jheng, P.R.; Lu, C.X.; Chuang, E.Y. Effectiveness of treating segmental bone defects with a synergistic co-delivery approach with platelet-rich fibrin and tricalcium phosphate. Mater. Sci. Engineering. C Mater. Biol. Appl. 2021, 129, 112364. [Google Scholar] [CrossRef] [PubMed]
- Abade dos Santos, F.A.; Carvalho, C.L.; Peleteiro, M.C.; Gabriel, S.I.; Patrício, R.; Carvalho, J.; Cunha, M.V.; Duarte, M.D. Blood collection from the external jugular vein of Oryctolagus cuniculus algirus sedated with midazolam: Live sampling of a subspecies at risk. Wildl. Biol. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Cooper, T.K.; Meyerholz, D.K.; Beck, A.P.; Delaney, M.A.; Piersigilli, A.; Southard, T.L.; Brayton, C.F. relevant conditions and pathology of laboratory mice, rats, gerbils, Guinea pigs, hamsters, naked mole rats, and rabbits. ILAR J. 2021, 62, 77–132. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, S. Care and Handling of Laboratory Animals. In Introduction to Basics of Pharmacology and Toxicology: Experimental Pharmacology: Research Methodology and Biostatistics; Springer: Berlin, Germany, 2022; Volume 3, pp. 37–43. [Google Scholar]
- Grgurevic, L.; Oppermann, H.; Pecin, M.; Erjavec, I.; Capak, H.; Pauk, M.; Karlovic, S.; Kufner, V.; Lipar, M.; Bubic Spoljar, J. Recombinant human bone morphogenetic protein 6 delivered within autologous blood coagulum restores critical size segmental defects of ulna in rabbits. JBMR Plus 2019, 3, e10085. [Google Scholar] [CrossRef]
- Handtke, S.; Thiele, T. Large and small platelets—(When) do they differ? J. Thromb. Haemost. 2020, 18, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Kinlough-Rathbone, R.; Mustard, J.; Perry, D.; Dejana, E.; Cazenave, J.; Packham, M.; Harfenist, E. Factors influencing the deaggregation of human and rabbit platelets. Thromb. Haemost. 1983, 49, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Alenazy, M.S.; Al-Nazhan, S.; Mosadomi, H.A. Histologic, radiographic, and micro-computed tomography evaluation of experimentally enlarged root apices in dog teeth with apical periodontitis after regenerative treatment. Curr. Ther. Res. 2021, 94, 100620. [Google Scholar] [CrossRef]
- Anwar, S.K.; Hamid, H.M.A. Immuno-histopathologic evaluation of mineralized plasmatic matrix in the management of horizontal ridge defects in a canine model (a split-mouth comparative study). Odontology 2022, 110, 523–534. [Google Scholar] [CrossRef]
- Benalcazar Jalkh, E.B.; Tovar, N.; Arbex, L.; Kurgansky, G.; Torroni, A.; Gil, L.F.; Wall, B.; Kohanbash, K.; Bonfante, E.A.; Coelho, P.G. Effect of leukocyte-platelet-rich fibrin in bone healing around dental implants placed in conventional and wide osteotomy sites: A pre-clinical study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2705–2713. [Google Scholar] [CrossRef]
- El Halaby, H.M.; Abu-Seida, A.M.; Fawzy, M.I.; Farid, M.H.; Bastawy, H.A. Evaluation of the regenerative potential of dentin conditioning and naturally derived scaffold for necrotic immature permanent teeth in a dog model. Int. J. Exp. Pathol. 2020, 101, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.-I.; Kim, S.-G.; Oh, J.-S.; Lee, S.-Y.; Cho, Y.-S.; Yang, S.-S.; Park, S.-C.; You, J.-S.; Lim, S.-C.; Jeong, M. Effect of platelet-rich plasma and platelet-rich fibrin on peri-implant bone defects in dogs. J. Biomed. Nanotechnol. 2013, 9, 535–537. [Google Scholar] [CrossRef]
- Ji, B.; Sheng, L.; Chen, G.; Guo, S.; Xie, L.; Yang, B.; Guo, W.; Tian, W. The combination use of platelet-rich fibrin and treated dentin matrix for tooth root regeneration by cell homing. Tissue Eng. Part A 2015, 21, 26–34. [Google Scholar] [CrossRef]
- Kazemi, D.; Fakhrjou, A.; Mirzazadeh Dizaji, V.; Khanzadeh Alishahi, M. Effect of autologous platelet rich fibrin on the healing of experimental articular cartilage defects of the knee in an animal model. BioMed Res. Int. 2014, 2014, 486436. [Google Scholar] [CrossRef]
- Kazemi, D.; Asenjan, K.S.; Dehdilani, N.; Parsa, H. Canine articular cartilage regeneration using mesenchymal stem cells seeded on platelet rich fibrin: Macroscopic and histological assessments. Bone Jt. Res. 2017, 6, 98–107. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Elsherbini, A.M.; Ibrahim, F.M.; El-Meadawy, S.M.; Youssef, J.M. Biological effect of the nanocrystalline calcium sulfate bone graft in the periodontal regeneration. J. Oral Biol. Craniofacial Res. 2021, 11, 47–52. [Google Scholar] [CrossRef]
- Neiva, R.F.; Gil, L.F.; Tovar, N.; Janal, M.N.; Marao, H.F.; Bonfante, E.A.; Pinto, N.; Coelho, P.G. The synergistic effect of leukocyte platelet-rich fibrin and micrometer/nanometer surface texturing on bone healing around immediately placed implants: An experimental study in dogs. BioMed Res. Int. 2016, 2016, 9507342. [Google Scholar] [CrossRef]
- Park, G.; Jalkh, E.B.B.; Boczar, D.; Bergamo, E.T.; Kim, H.; Kurgansky, G.; Torroni, A.; Gil, L.F.; Bonfante, E.A.; Coelho, P.G. Bone regeneration at extraction sockets filled with leukocyte-platelet-rich fibrin: An experimental pre-clinical study. Med. Oral Patol. Oral Y Cirugía Bucal 2022, 27, e468. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-C.; Kim, S.-G.; Oh, J.-S.; You, J.-S.; Kim, J.-S.; Lim, S.-C.; Jeong, M.; Kim, J.-S.; Jung, C.; Kwon, Y.-S. Early bone formation at a femur defect using CGF and PRF grafts in adult dogs: A comparative study. Implant. Dent. 2016, 25, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Hong, K.J.; Ko, K.A.; Cha, J.K.; Gruber, R.; Lee, J.S. Platelet-rich fibrin combined with a particulate bone substitute versus guided bone regeneration in the damaged extraction socket: An in vivo study. J. Clin. Periodontol. 2023, 50, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Xuan, F.; Lee, C.-U.; Son, J.-S.; Jeong, S.-M.; Choi, B.-H. A comparative study of the regenerative effect of sinus bone grafting with platelet-rich fibrin-mixed Bio-Oss® and commercial fibrin-mixed Bio-Oss®: An experimental study. J. Cranio-Maxillofac. Surg. 2014, 42, e47–e50. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, Y.; Chen, Y.; Chen, S.; Lyu, H.; Cai, Z.; Huang, X. Radiographic, histologic, and biomechanical evaluation of combined application of platelet-rich fibrin with blood clot in regenerative endodontics. J. Endod. 2017, 43, 2034–2040. [Google Scholar] [CrossRef]
- Cortese, L.; Christopherson, P.W.; Pelagalli, A. Platelet function and therapeutic applications in dogs: Current status and future prospects. Animals 2020, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Creevy, K.E.; Akey, J.M.; Kaeberlein, M.; Promislow, D.E. An open science study of ageing in companion dogs. Nature 2022, 602, 51–57. [Google Scholar] [CrossRef]
- Yilmaz, D.; Dogan, N.; Ozkan, A.; Sencimen, M.; Ora, B.E.; Mutlu, I. Effect of platelet rich fibrin and beta tricalcium phosphate on bone healing. A histological study in pigs. Acta Cir. Bras. 2014, 29, 59–65. [Google Scholar] [CrossRef]
- Chen, Y.; Niu, Z.; Xue, Y.; Yuan, F.; Fu, Y.; Bai, N. Improvement in the repair of defects in maxillofacial soft tissue in irradiated minipigs by a mixture of adipose-derived stem cells and platelet-rich fibrin. Br. J. Oral Maxillofac. Surg. 2014, 52, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-C.; Chang, G.R.-L.; Fan, H.-C.; Ou-Yang, H.; Huang, L.-C.; Wu, S.-C.; Chen, C.-M. A mini-pig model for evaluating the efficacy of autologous platelet patches on induced acute full thickness wound healing. BMC Vet. Res. 2019, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Wang, C.H.; Chang, H.H.; Chan, W.P.; Chi, C.H.; Kuo, T.F. Fibrin glue mixed with platelet-rich fibrin as a scaffold seeded with dental bud cells for tooth regeneration. J. Tissue Eng. Regen. Med. 2012, 6, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Pan, S.; Dangaria, S.J.; Gopinathan, G.; Kolokythas, A.; Chu, S.; Geng, Y.; Zhou, Y.; Luan, X. Platelet-rich fibrin promotes periodontal regeneration and enhances alveolar bone augmentation. BioMed Res. Int. 2013, 2013, 638043. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Reed, D.A.; Min, L.; Gopinathan, G.; Li, S.; Dangaria, S.J.; Li, L.; Geng, Y.; Galang, M.-T.; Gajendrareddy, P. Lyophilized platelet-rich fibrin (PRF) promotes craniofacial bone regeneration through Runx2. Int. J. Mol. Sci. 2014, 15, 8509–8525. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.; Wang, C.; Pao, Y.; Fu, Y.; Liu, C.; Yao, C.; Kuo, T. The effect of platelet-rich fibrin on autologous osteochondral transplantation: An in vivo porcine model. Knee 2017, 24, 1392–1401. [Google Scholar] [CrossRef]
- Walters, E.M.; Prather, R.S. Advancing swine models for human health and diseases. Mo. Med. 2013, 110, 212–215. [Google Scholar]
- Marchelak, A.; Kolodziejczyk-Czepas, J.; Wasielewska, P.; Nowak, P.; Olszewska, M.A. The effects of Prunus spinosa L. flower extracts, model polyphenols and phenolic metabolites on oxidative/nitrative modifications of human plasma components with particular emphasis on fibrinogen in vitro. Antioxidants 2021, 10, 581. [Google Scholar] [CrossRef]
- Dre’Von, A.D.; Holle, L.A.; Lin, F.-C.; Huffman, J.E.; Luyendyk, J.P.; Flick, M.J.; Smith, N.L.; de Vries, P.S.; Morrison, A.C.; Wolberg, A.S. Novel Genetic Regulators of Fibrinogen Synthesis Identified by an In Vitro Experimental Platform. J. Thromb. Haemost. 2022, 21, 522–533. [Google Scholar]
- Al-Maawi, S.; Dohle, E.; Kretschmer, W.; Rutkowski, J.; Sader, R.; Ghanaati, S. A Standardized g-Force Allows the Preparation of Similar Platelet-Rich Fibrin Qualities Regardless of Rotor Angle. Tissue Eng. Part A 2022, 28, 353–365. [Google Scholar] [CrossRef]
- Miron, R.J.; Xu, H.; Chai, J.; Wang, J.; Zheng, S.; Feng, M.; Zhang, X.; Wei, Y.; Chen, Y.; Mourão, C.F.d.A.B. Comparison of platelet-rich fibrin (PRF) produced using 3 commercially available centrifuges at both high (~700 g) and low (~200 g) relative centrifugation forces. Clin. Oral Investig. 2020, 24, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Castro Ana, B.; Catherine, A.; Xin, L.; Nelson, P.; Wim, T.; Marc, Q. Impact of g force and timing on the characteristics of PRF matrices. Sci. Rep. 2021, 11, 6038. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Mourão, C.F.; Adam, E.H.; Sader, R.; Zadeh, H.H.; Al-Maawi, S. The role of centrifugation process in the preparation of therapeutic blood concentrates: Standardization of the protocols to improve reproducibility. Int. J. Growth Factors Stem Cells Dent. 2019, 2, 41–44. [Google Scholar] [CrossRef]
- Miron, R.J.; Pinto, N.R.; Quirynen, M.; Ghanaati, S. Standardization of Relative Centrifugal Forces in Studies Related to Platelet-Rich Fibrin; Wiley Online Library: New York, NY, USA, 2019; Volume 90, pp. 817–820. [Google Scholar]





| Author and Year | Breed | Animal Size | Number of Animals | Blood Collected (mL) | Centrifuge (RPM/min or RCF) | Donor Area |
|---|---|---|---|---|---|---|
| Akyildiz et al. (2018) [44] | Sprague-Dawley rats | 340–380 g | 23 | 4 | 3000 rpm/10 min | Intracardiac puncture |
| Alizadeh et al. (2023) [45] | Wistar rats | 230–300 g | NR | NR | 2700 rpm/12 min | Orbital sinus |
| Alsherif et al. (2020) [46] | Wistar rats | 300–360 g | 10 (PRF group) | 4 | 2000 rpm/10 min (400 RCF) | Orbital sinus |
| Awadeen et al. (2020) [47] | Sprague-Dawley rats | NR | 63 | 10 | 3000 rpm/10 min | Orbital sinus |
| da Silva et al. (2022) [11] | Wistar rats | 350–450 g | 24 | 3 | 2700 rpm/12 min (701 RCF-max) and 1500 rpm/14 min (216 RCF-max) | Intracardiac puncture |
| Demirel et al. (2018) [48] | Sprague-Dawley rats | 400–450 g | 28 | 16 | 3000 rpm/13 min | Intracardiac puncture |
| Engler-Pinto et al. (2019) [42] | Wistar rats | 250–300 g | 8 | 3.5 | 2700 rpm/12 min | Intracardiac puncture |
| Grecu et al. (2019) [49] | Wistar rats | 220–420 g | 35 | 10 | 1300 rpm/8 min (400 RCF) | Intracardiac puncture |
| Grecu et al. (2019) [50] | Wistar rats | 220–420 g | 35 (scarified to produce PRF) | 10 | 1300 rpm/8 min (400 RCF) | Intracardiac puncture |
| Huang et al. (2020) [23] | Sprague-Dawley rats | 180–220 g | 24 (sacrificed to produce PRF) | 5 | 400 g/10 min | NR |
| Jamalpour et al. (2022) [51] | Wistar rats | 300–350 g | 60 | 2 | 1500 rpm/14 min and 2700 rpm/12 min | Orbital sinus |
| Mirhaj et al. (2022) [52] | Wistar rats | 250–300 g | 3 (PRF group) | NR | 2700 rpm/12 min | Orbital sinus |
| Mourad et al. (2022) [53] | Wistar rats | 250–300 g | 30 | 2 | 3000 rpm/10 min | Tail vein |
| Neves-Atti et al. (2022) [54] | Spontaneously hypertensive rats | 250 g | 40 | 6 | 3000 rpm/10 min | Intracardiac puncture |
| Nica et al. (2019) [55] | Wistar rats | 460–550 g | 40 | 9 to 12 | 450 g/12 min | Intracardiac puncture |
| Nugraha et al. (2018) [56] | Wistar rats | 250 g | 36 | 1.5 | 3000 rpm/10 min | Intracardiac puncture |
| Özçay et al. (2018) [22] | Sprague-Dawley rats | 250–300 g | 40 | 1 | 3000 rpm/10 min | Intracardiac puncture |
| Özçay et al. (2020) [40] | Sprague-Dawley rats | 250–300 g | 10 (PRF group) | 1 | 3000 rpm/10 min | Intracardiac puncture |
| Padilha et al. (2019) [57] | Wistar rats | 300–400 g | 1 animal scarified per group | 9 | 3000 rpm/12 min | Intracardiac puncture |
| Rady et al. (2022) [19] | Wistar rats | 175–200 g | 36 | 2 | 3000 rpm/10 min | Tail vein |
| Silveira et al. (2022) [58] | Wistar rats | 320 g | 54 (2 animals scarified for PRF) | 10 | 2700 rpm/12 min (857 RCF max) | Intracardiac puncture |
| Sumida et al. (2019) [59] | Wistar rats | 400–450 g | 23 | 6 | 890 g/13 min | Intracardiac puncture |
| Tavakoli et al. (2022) [60] | Wistar rats | 400 g | NR | 6 | 1500 rpm/14 min | Orbital sinus |
| Tayşi et al. (2018) [61] | Sprague-Dawley rats | 240–260 g | 60 (including a sacrigication group) | 10 to 15 | 3000 rpm/10 min (400 RCF) | Intracardiac puncture |
| Torul et al. (2018) [39] | Wistar rats | 200–250 g | 30 | 2 | 3000 rpm/10 min (400 RCF) | Tail vein |
| Vares et al. (2021) [62] | Wistar rats | 250–280 g | 32 | 2 | 3000 rpm/12 min (400 RCF) | Intracardiac puncture |
| Wang et al. (2021) [41] | Sprague-Dawley rats | NR | 30 | 5 | 400 RCF/10 min | Abdominal aortic |
| Zhang et al. (2019) [63] | Sprague-Dawley rats | 210–310 g | 20 | 5 | 3000 rpm/10 min | Abdominal aortic |
| Zhang et al. (2022) [64] | Sprague-Dawley rats | 250–300 g | 32 | 3.5 | 600 RCF | Intracardiac puncture |
| Author and Year | Breed | Animal Size | Number of Animals | Blood Collected (mL) | Centrifuge (RPM/min or RCF) | Donor Area |
|---|---|---|---|---|---|---|
| Choi et al. (2021) [77] | New Zealand | 2.5–3 kg | 33 | 5 | 2700 rpm/12 min | NR |
| Damayanti et al. (2022) [78] | New Zealand | 3–4 kg | 18 | 3 | 3200 rpm/10 min | Ear |
| Dereli-Can et al. (2020) [79] | New Zealand | 3.0–3.5 kg | 45 | 5 | 2700 rpm/12 min | Femoral vein |
| Karayürek et al. (2019) [80] | New Zealand | 2.6–3.9 kg | 28 | 8 | 3000 rpm/10 min | Ear |
| Kim et al. (2021) [81] | New Zealand | 3.0–4.0 kg | 12 | 10 | 3000 rpm/10 min | Ear |
| Kinoshita et al. (2021) [82] | New Zealand | 3.5–4.2 kg | 18 | 10 | 2400–3000 rpm/13 min | NR |
| Kızıldağ et al. (2020) [83] | New Zealand | 3.0–3.5 kg | 18 | 5 | 2700 rpm/12 min | Ear |
| Koyanagi et al. (2022) [74] | New Zealand | 3.5–4.0 kg | 5 | 2.5 | 700 RCF/12 min | Ear |
| Li et al. (2022) [73] | New Zealand | 2 ± 0.2 kg | 52 | 5 | 2000 RCF/5 min | Intracardiac puncture |
| Liu et al. (2021) [84] | New Zealand | 3–4 kg | 12 | 10 | 3000 rpm/10 min | Ear |
| Liu et al. (2019) [72] | New Zealand | 2.8 and 4 kg | 12 | 5 | 3000 rpm/10 min | Ear |
| Mogharehabed et al. (2022) [76] | New Zealand | 1.5 kg | 20 | 9 | 2700 rpm (408 RCF)/12 min | NR |
| Mu et al. (2020) [71] | New Zealand | 3.0–3.5 kg | 16 | 10 | 700 rpm/3 min | NR |
| Mudalal et al. (2019) [85] | New Zealand | 3.0–3.5 kg | 12 | 10 | 3000 rpm (1278 RCF)/12 min | NR |
| Rezuc et al. (2020) [86] | New Zealand | 2 kg | 12 | 8 | 3000 rpm/400 RCF/10 min | Ear |
| Salih et al. (2018) [87] | New Zealand | 1.5–2 kg | 20 | 3 | 3000 rpm/10 min | Intracardiac puncture |
| Şentürk et al. (2020) [14] | New Zealand | 2.0–3.0 kg | 27 | 10 | 3500 rpm/15 min | Ear |
| Shanei et al. (2022) [15] | New Zealand | 2.5–3 kg | 5 | 5 | 2700 rpm/8 min | Ear |
| Taufik et al. (2023) [16] | New Zealand | 2.0–3.5 kg | 15 | 10 | 3000 rpm/10 min | Ear |
| Wang et al. (2022) [17] | New Zealand | 3–3.5 kg | 10 | 10 | 3000 rpm/12 min | Ear |
| Wong et al. (2021) [88] | New Zealand | 2–2.5 kg | 24 | 8 | 2700 rpm/10 min | Ear |
| Zalama et al. (2022) [18] | New Zealand | N/D | 30 | NR | NR | NR |
| Zhang et al. (2023) [75] | New Zealand | 3.5 ± 0.5 kg | 9 | 5 | 1300 rpm/14 min | NR |
| Author and Year | Breed | Animal Size | Number of Animals | Blood Collected (mL) | Centrifuge (RPM/min or RCF) | Donor Area |
|---|---|---|---|---|---|---|
| Alenazy et al. (2021) [95] | Mixed breed dog | NR | 4 | 10 | 3000 rpm/10 min | NR |
| Anwar et al. (2022) [96] | Mixed breed dog | 9–14 kg | 9 | 20 | 2500 rpm/15 min | NR |
| Benalcázar et al. (2022) [97] | Beagle dog | NR | 13 | 18 | 2700 rpm/12 min | NR |
| El Halaby et al. (2020) [98] | Mixed breed dog | NR | 9 | 20 | 3000 rpm/10 min | Right cephalic vein |
| Jeong et al. (2013) [99] | NR | 10–15 kg | 6 | 10 | 400 RCF/10 min | NR |
| Ji et al. (2015) [100] | NR | 12–15 kg | NR | 5 | 3000 rpm/5 min | Lower limb vein |
| Kazemi et al. (2014) [101] | Mixed breed dog | 20–30 kg | 12 | 20 | 3000 rpm/10 min | Jugular vein |
| Kazemi et al. (2017) [102] | Mixed breed dog | 18–40 kg | 12 | 20 | 3000 rpm/10 min | Jugular vein |
| Mohammed et al. (2021) [103] | Mixed breed dog | 18–23 kg | 8 | 5 | 400 RCF/10 min | Lower limb vein |
| Neiva et al. (2016) [104] | Beagle dog | NR | 8 | NR | 2700 rpm/12 min | NR |
| Park et al. (2022) [105] | Beagle dog | NR | 7 | NR | 408 RCF—time NR | NR |
| Park et al. (2016) [106] | NR | 15 kg | 6 | 10 | 3000 rpm/12 min | NR |
| Park et al. (2023) [107] | Beagle dog | 15 kg | 7 | 20 | 1300 rpm/8 min | Jugular vein |
| Xuan et al. (2014) [108] | Mixed breed dog | 15–20 kg | 6 | 20 | 2400 rpm/10 min | NR |
| Zhou et al. (2017) [109] | Beagle dog | NR | 3 | 10 | NR | Antecubital vein |
| Author and Year | Breed | Animal Size | Number of Animals | Blood Collected (mL) | Centrifuge (RPM/min or RCF) | Donor Area |
|---|---|---|---|---|---|---|
| Chen et al. (2014) [113] | Mini-pig | 25–30 kg | 20 | 8 | 3000 rpm/10 min | Superior vena cava |
| Li et al. (2013) [116] | Pig | NR | NR | 10 | 2100 rpm/12 min | Precaval vein |
| Li et al. (2014) [117] | Pig | NR | NR | 10 | 2100 rpm/12 min | Precaval vein |
| Sheu et al. (2017) [118] | Mini-pig | 21.8 kg | 6 | 8 | 3000 rpm/10 min | Right jugular vein |
| Tsai et al. (2019) [114] | Mini-pig | 26.6 ± 4.1 kg | 6 | 40 | 1300 g/15 min | Internal jugular vein |
| Yang et al. (2012) [115] | Pig | 6.8–11.2 kg | 21 | 8 | 3000 rpm/10 min | Right jugular vein |
| Yilmaz et al. (2014) [112] | Pig | 60 ± 5 kg | 3 | 10 | 400 RCF/10 min | Ear vein |
| Animal | Platelet Count (Platelets/µL) | Coagulation Time (Min) |
|---|---|---|
| Human | 150,000–450,000 | 5 to 10 |
| Rat | 600,000–1,500,000 | 2 to 5 |
| Rabbit | 150,000–450,000 | 5 to 10 |
| Dog | 200,000–500,000 | 2 to 10 |
| Mini-pig | 250,000–600,000 | 2 to 5 |
| Goat | 100,000–500,000 | 3 to 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourão, C.F.; Lowenstein, A.; Mello-Machado, R.C.; Ghanaati, S.; Pinto, N.; Kawase, T.; Alves, G.G.; Messora, M.R. Standardization of Animal Models and Techniques for Platelet-Rich Fibrin Production: A Narrative Review and Guideline. Bioengineering 2023, 10, 482. https://doi.org/10.3390/bioengineering10040482
Mourão CF, Lowenstein A, Mello-Machado RC, Ghanaati S, Pinto N, Kawase T, Alves GG, Messora MR. Standardization of Animal Models and Techniques for Platelet-Rich Fibrin Production: A Narrative Review and Guideline. Bioengineering. 2023; 10(4):482. https://doi.org/10.3390/bioengineering10040482
Chicago/Turabian StyleMourão, Carlos Fernando, Adam Lowenstein, Rafael Coutinho Mello-Machado, Shahram Ghanaati, Nelson Pinto, Tomoyuki Kawase, Gutemberg Gomes Alves, and Michel Reis Messora. 2023. "Standardization of Animal Models and Techniques for Platelet-Rich Fibrin Production: A Narrative Review and Guideline" Bioengineering 10, no. 4: 482. https://doi.org/10.3390/bioengineering10040482