Electrical Stimulation in Cartilage Tissue Engineering
Abstract
:1. Introduction
2. Electrical Stimulation Overview
3. Electromechanics of Articular Cartilage
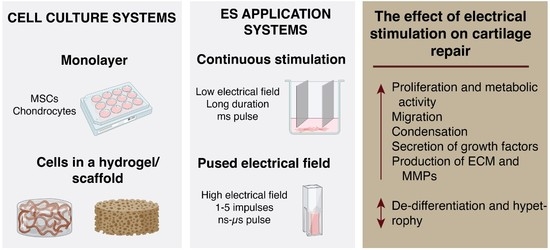
4. Types of ES Application and Their Effects Activating Cellular Mechanisms and Functions
5. The Effects of ES on Chondrogenesis In Vitro
6. Recommendations on Reporting ES in the Context of Chondrogenesis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.-S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 25. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Klein, J.D.; Hassounah, F.; Cai, H.; Zhang, C.; Xu, P.; Wang, X.H. Low-frequency electrical stimulation attenuates muscle atrophy in CKD—A potential treatment strategy. J. Am. Soc. Nephrol. 2015, 26, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Faingold, C.L. Electrical stimulation therapies for CNS disorders and pain are mediated by competition between different neuronal networks in the brain. Med. Hypotheses 2008, 71, 668–681. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Das, R.; Patel, A.; Nguyen, T.D. Physical Stimulations for Bone and Cartilage Regeneration. Regen. Eng. Transl. Med. 2018, 4, 216–237. [Google Scholar] [CrossRef]
- Yuan, X.; Arkonac, D.E.; Chao, P.H.; Vunjak-Novakovic, G. Electrical stimulation enhances cell migration and integrative repair in the meniscus. Sci. Rep. 2014, 4, 3674. [Google Scholar] [CrossRef] [Green Version]
- Giggins, O.; Fullen, B.; Coughlan, G. Neuromuscular electrical stimulation in the treatment of knee osteoarthritis: A systematic review and meta-analysis. Clin. Rehabil. 2012, 26, 867–881. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Li, H.; Yang, T.; Deng, Z.H.; Yang, Y.; Zhang, Y.; Lei, G.H. Electrical stimulation for pain relief in knee osteoarthritis: Systematic review and network meta-analysis. Osteoarthr. Cartil. 2015, 23, 189–202. [Google Scholar] [CrossRef] [Green Version]
- Mobasheri, A.; Matta, C.; Uzieliene, I.; Budd, E.; Martin-Vasallo, P.; Bernotiene, E. The chondrocyte channelome: A narrative review. Jt. Bone Spine 2019, 86, 29–35. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bernotas, P.; Mobasheri, A.; Bernotiene, E. The Role of Physical Stimuli on Calcium Channels in Chondrogenic Differentiation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 2998. [Google Scholar] [CrossRef] [Green Version]
- Mahaut-Smith, M.P.; Hussain, J.F.; Mason, M.J. Depolarization-evoked Ca2+ release in a non-excitable cell, the rat megakaryocyte. J. Physiol. 1999, 515, 385–390. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sport Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Gomoll, A.H. Microfracture and augments. J. Knee Surg. 2012, 25, 9–15. [Google Scholar] [CrossRef]
- Carey, J.L. Fibrocartilage following microfracture is not as robust as native articular cartilage: Commentary on an article by Aaron, J.; Krych, M.D.; et al. Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee. A retrospective comparative study. J. Bone Joint. Surg. Am. 2012, 94, e80. [Google Scholar] [CrossRef]
- Devitt, B.M.; Bell, S.W.; Webster, K.E.; Feller, J.A.; Whitehead, T.S. Surgical treatments of cartilage defects of the knee: Systematic review of randomised controlled trials. Knee 2017, 24, 508–517. [Google Scholar] [CrossRef]
- Urlić, I.; Ivković, A. Cell Sources for Cartilage Repair-Biological and Clinical Perspective. Cells 2021, 10, 2496. [Google Scholar] [CrossRef]
- Kwon, H.J.; Lee, G.S.; Chun, H. Electrical stimulation drives chondrogenesis of mesenchymal stem cells in the absence of exogenous growth factors. Sci. Rep. 2016, 6, 39302. [Google Scholar] [CrossRef]
- Csaki, C.; Schneider, P.R.; Shakibaei, M. Mesenchymal stem cells as a potential pool for cartilage tissue engineering. Ann. Anat. 2008, 190, 395–412. [Google Scholar] [CrossRef]
- Ciobanu, F.; Golzio, M.; Kovacs, E.; Teissié, J. Control by Low Levels of Calcium of Mammalian Cell Membrane Electropermeabilization. J. Membr. Biol. 2018, 251, 221–228. [Google Scholar] [CrossRef]
- Brighton, C.T.; Wang, W.; Clark, C.C. The effect of electrical fields on gene and protein expression in human osteoarthritic cartilage explants. J. Bone Jt. Surg. Am. 2008, 90, 833–848. [Google Scholar] [CrossRef]
- Chang, C.; Park, J.; Noh, J. Electrical stimulation confers pre-chondrogenic differentiation by modulating TGF- β1 levels in canine adipose-derived mesenchymal stem cells. Cytotherapy 2020, 22, S72. [Google Scholar] [CrossRef]
- Park, H.J.; Rouabhia, M.; Lavertu, D.; Zhang, Z. Electrical Stimulation Modulates the Expression of Multiple Wound Healing Genes in Primary Human Dermal Fibroblasts. Tissue Eng. Part A 2015, 21, 1982–1990. [Google Scholar] [CrossRef]
- Lee, G.S.; Kim, M.G.; Kwon, H.J. Electrical stimulation induces direct reprogramming of human dermal fibroblasts into hyaline chondrogenic cells. Biochem. Biophys. Res. Commun. 2019, 513, 990–996. [Google Scholar] [CrossRef]
- Atsuta, Y.; Tomizawa, R.R.; Levin, M.; Tabin, C.J. L-type voltage-gated Ca2+ channel CaV1.2 regulates chondrogenesis during limb development. Proc. Natl. Acad. Sci. USA 2019, 116, 21592–21601. [Google Scholar] [CrossRef] [Green Version]
- Marszalek, P.; Liu, D.S.; Tsong, T.Y. Schwan equation and transmembrane potential induced by alternating electric field. Biophys. J. 1990, 58, 1053–1058. [Google Scholar] [CrossRef] [Green Version]
- Arena, C.B.; Sano, M.B.; Rossmeisl, J.H., Jr.; Caldwell, J.L.; Garcia, P.A.; Rylander, M.N.; Davalos, R.V. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomed. Eng. Online 2011, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Aycock, K.N.; Zhao, Y.; Lorenzo, M.F.; Davalos, R.V. A Theoretical Argument for Extended Interpulse Delays in Therapeutic High-Frequency Irreversible Electroporation Treatments. IEEE Trans. Biomed. Eng. 2021, 68, 1999–2010. [Google Scholar] [CrossRef]
- Kotnik, T.; Rems, L.; Tarek, M.; Miklavčič, D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 2019, 48, 63–91. [Google Scholar] [CrossRef]
- Tekle, E.; Astumian, R.D.; Chock, P.B. Electro-permeabilization of cell membranes: Effect of the resting membrane potential. Biochem. Biophys. Res. Commun. 1990, 172, 282–287. [Google Scholar] [CrossRef]
- Kotnik, T.; Kramar, P.; Pucihar, G.; Miklavcic, D.; Tarek, M. Cell membrane electroporation- Part 1: The phenomenon. IEEE Electr. Insul. Mag. 2012, 28, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Geboers, B.; Scheffer, H.J.; Graybill, P.M.; Ruarus, A.H.; Nieuwenhuizen, S.; Puijk, R.S.; van den Tol, P.M.; Davalos, R.V.; Rubinsky, B.; de Gruijl, T.D.; et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiology 2020, 295, 254–272. [Google Scholar] [CrossRef]
- Wells, J.M.; Li, L.H.; Sen, A.; Jahreis, G.P.; Hui, S.W. Electroporation-enhanced gene delivery in mammary tumors. Gene Ther. 2000, 7, 541–547. [Google Scholar] [CrossRef] [Green Version]
- Antov, Y.; Barbul, A.; Mantsur, H.; Korenstein, R. Electroendocytosis: Exposure of cells to pulsed low electric fields enhances adsorption and uptake of macromolecules. Biophys. J. 2005, 88, 2206–2223. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Chang, D.C.; Lee, Y.K. Single-cell electroendocytosis on a micro chip using in situ fluorescence microscopy. Biomed. Microdevices 2011, 13, 1063–1073. [Google Scholar] [CrossRef]
- Cho, I.; Jackson, M.R.; Swift, J. Roles of Cross-Membrane Transport and Signaling in the Maintenance of Cellular Homeostasis. Cell. Mol. Bioeng. 2016, 9, 234–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, T.; Guo, J.; Zhang, K.; Li, K.; Zhang, J.; Yang, Z.; Ge, Z. Nanosecond pulsed electric fields enhanced chondrogenic potential of mesenchymal stem cells via JNK/CREB-STAT3 signaling pathway. Stem Cell Res. Ther. 2019, 10, 45. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Guo, J.; Ge, Z.; Zhang, J. Nanosecond pulsed electric fields (nsPEFs) regulate phenotypes of chondrocytes through Wnt/β-catenin signaling pathway. Sci. Rep. 2014, 4, 5836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thrivikraman, G.; Boda, S.K.; Basu, B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials 2018, 150, 60–86. [Google Scholar] [CrossRef] [PubMed]
- Miguel, F.; Barbosa, F.; Ferreira, F.C.; Silva, J.C. Electrically Conductive Hydrogels for Articular Cartilage Tissue Engineering. Gels 2022, 8, 710. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.; Zákány, R.; Mobasheri, A. Voltage-dependent calcium channels in chondrocytes: Roles in health and disease. Curr. Rheumatol. Rep. 2015, 17, 43. [Google Scholar] [CrossRef] [Green Version]
- Wen, L.; Wang, Y.; Wang, H.; Kong, L.; Zhang, L.; Chen, X.; Ding, Y. L-type calcium channels play a crucial role in the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2012, 424, 439–445. [Google Scholar] [CrossRef]
- Uzieliene, I.; Bironaite, D.; Bernotas, P.; Sobolev, A.; Bernotiene, E. Mechanotransducive Biomimetic Systems for Chondrogenic Differentiation In Vitro. Int. J. Mol. Sci. 2021, 22, 9690. [Google Scholar] [CrossRef]
- Mobini, S.; Talts, U.-L.; Xue, R.; Cassidy, N.; Cartmell, S. Electrical Stimulation Changes Human Mesenchymal Stem Cells Orientation and Cytoskeleton Organization. J. Biomater. Tissue Eng. 2017, 7, 829–833. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.J. Extracellular ATP signaling via P2X(4) receptor and cAMP/PKA signaling mediate ATP oscillations essential for prechondrogenic condensation. J. Endocrinol. 2012, 214, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wang, W.; Clark, C.C.; Brighton, C.T. Signal transduction in electrically stimulated articular chondrocytes involves translocation of extracellular calcium through voltage-gated channels. Osteoarthr. Cartil. 2009, 17, 397–405. [Google Scholar] [CrossRef] [Green Version]
- Krueger, S.; Achilles, S.; Zimmermann, J.; Tischer, T.; Bader, R.; Jonitz-Heincke, A. Re-Differentiation Capacity of Human Chondrocytes in Vitro Following Electrical Stimulation with Capacitively Coupled Fields. J. Clin. Med. 2019, 8, 1771. [Google Scholar] [CrossRef] [Green Version]
- Farooqi, A.R.; Bader, R.; van Rienen, U. Numerical Study on Electromechanics in Cartilage Tissue with Respect to Its Electrical Properties. Tissue Eng. Part B Rev. 2019, 25, 152–166. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhang, K.; Dong, H.; Wang, Y.; Yan, Y.; Yu, J.; Wu, X.; Zhang, M.; Wang, Y.; Chen, W. Layered mechanical and electrical properties of porcine articular cartilage. Med. Biol. Eng. Comput. 2022, 60, 3019–3028. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, Y.C.; Kim, H.S.; Lee, J.; Kim, E.; Findeklee, C.; Katscher, U. In vivo electrical conductivity measurement of muscle, cartilage, and peripheral nerve around knee joint using MR-electrical properties tomography. Sci. Rep. 2022, 12, 73. [Google Scholar] [CrossRef]
- Binette, J.S.; Garon, M.; Savard, P.; McKee, M.D.; Buschmann, M.D. Tetrapolar Measurement of Electrical Conductivity and Thickness of Articular Cartilage. J. Biomech. Eng. 2004, 126, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Rim, Y.A.; Nam, Y.; Ju, J.H. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2358. [Google Scholar] [CrossRef] [Green Version]
- van der Kraan, P.M.; van den Berg, W.B. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012, 20, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Heinegård, D.; Saxne, T. The role of the cartilage matrix in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 50–56. [Google Scholar] [CrossRef]
- Mow, V.C.; Guo, X.E. Mechano-electrochemical properties of articular cartilage: Their inhomogeneities and anisotropies. Annu. Rev. Biomed. Eng. 2002, 4, 175–209. [Google Scholar] [CrossRef]
- Brady, M.A.; Waldman, S.D.; Ethier, C.R. The application of multiple biophysical cues to engineer functional neocartilage for treatment of osteoarthritis. Part I: Cellular response. Tissue Eng. Part B Rev. 2015, 21, 1–19. [Google Scholar] [CrossRef]
- Mow, V.C.; Gibbs, M.C.; Lai, W.M.; Zhu, W.B.; Athanasiou, K.A. Biphasic indentation of articular cartilage—II. A numerical algorithm and an experimental study. J. Biomech. 1989, 22, 853–861. [Google Scholar] [CrossRef]
- Lai, W.M.; Mow, V.C. Drag-induced compression of articular cartilage during a permeation experiment. Biorheology 1980, 17, 111–123. [Google Scholar] [CrossRef]
- Mellor, L.F.; Mohiti-Asli, M.; Williams, J.; Kannan, A.; Dent, M.R.; Guilak, F.; Loboa, E.G. Extracellular Calcium Modulates Chondrogenic and Osteogenic Differentiation of Human Adipose-Derived Stem Cells: A Novel Approach for Osteochondral Tissue Engineering Using a Single Stem Cell Source. Tissue Eng. Part A 2015, 21, 2323–2333. [Google Scholar] [CrossRef] [Green Version]
- Matta, C.; Zakany, R. Calcium signalling in chondrogenesis: Implications for cartilage repair. Front. Biosci. 2013, 5, 305–324. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.F.; Lee, Y.S.; Tang, T.K.; Cheng, J.Y. Pulsed DC Electric Field-Induced Differentiation of Cortical Neural Precursor Cells. PLoS ONE 2016, 11, e0158133. [Google Scholar] [CrossRef] [Green Version]
- Enayati, S.; Chang, K.; Achour, H.; Cho, K.-S.; Xu, F.; Guo, S.; Enayati, K.Z.; Xie, J.; Zhao, E.; Turunen, T.; et al. Electrical Stimulation Induces Retinal Müller Cell Proliferation and Their Progenitor Cell Potential. Cells 2020, 9, 781. [Google Scholar] [CrossRef] [Green Version]
- Hernández, D.; Millard, R.; Sivakumaran, P.; Wong, R.C.B.; Crombie, D.E.; Hewitt, A.W.; Liang, H.; Hung, S.S.C.; Pébay, A.; Shepherd, R.K.; et al. Electrical Stimulation Promotes Cardiac Differentiation of Human Induced Pluripotent Stem Cells. Stem Cells Int. 2016, 2016, 1718041. [Google Scholar] [CrossRef] [Green Version]
- Szlasa, W.; Michel, O.; Sauer, N.; Novickij, V.; Lewandowski, D.; Kasperkiewicz, P.; Tarek, M.; Saczko, J.; Kulbacka, J. Nanosecond pulsed electric field suppresses growth and reduces multi-drug resistance effect in pancreatic cancer. Sci. Rep. 2023, 13, 351. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Rouabhia, M.; Zhang, Z. Electrical stimulation modulates osteoblast proliferation and bone protein production through heparin-bioactivated conductive scaffolds. Bioelectromagnetics 2013, 34, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Rouabhia, M.; Semlali, A.; Zhang, Z. Effects of electrical stimulation on human skin keratinocyte growth and the secretion of cytokines and growth factors. Biomed. Mater. 2021, 16, 065021. [Google Scholar] [CrossRef] [PubMed]
- Novickij, V.; Rembiałkowska, N.; Kasperkiewicz-Wasilewska, P.; Baczyńska, D.; Rzechonek, A.; Błasiak, P.; Kulbacka, J. Pulsed electric fields with calcium ions stimulate oxidative alternations and lipid peroxidation in human non-small cell lung cancer. Biochim. Biophys. Acta Biomembr. 2022, 1864, 184055. [Google Scholar] [CrossRef] [PubMed]
- Taghian, T.; Narmoneva, D.A.; Kogan, A.B. Modulation of cell function by electric field: A high-resolution analysis. J. R. Soc. Interface 2015, 12, 20150153. [Google Scholar] [CrossRef]
- Xiang, X.-W.; Liu, H.-T.; Liu, W.; Yan, Z.-Y.; Zeng, Y.-L.; Wang, Y.-J.; Liu, J.; Chen, Y.-C.; Yu, S.-X.; Zhu, C.-H.; et al. Microsecond pulse electrical stimulation modulates cell migration. bioRxiv 2022. [Google Scholar] [CrossRef]
- Schoenbach, K.H.; Baum, C.E.; Joshi, R.P.; Beebe, S.J. A Scaling Law for Bioelectric Effects of Nanosecond Pulses. IEEE Trans. Diel. Electr. Insul. 2009, 16, 1224–1235. [Google Scholar] [CrossRef]
- Kranjc, M.; Miklavčič, D. Electric Field Distribution and Electroporation Threshold. In Handbook of Electroporation; Miklavčič, D., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1043–1058. [Google Scholar]
- Avazzadeh, S.; O’Brien, B.; Coffey, K.; O’Halloran, M.; Keane, D.; Quinlan, L.R. Establishing Irreversible Electroporation Electric Field Potential Threshold in A Suspension In Vitro Model for Cardiac and Neuronal Cells. J. Clin. Med. 2021, 10, 5443. [Google Scholar] [CrossRef]
- Weaver, J.C.; Smith, K.C.; Esser, A.T.; Son, R.S.; Gowrishankar, T.R. A brief overview of electroporation pulse strength-duration space: A region where additional intracellular effects are expected. Bioelectrochemistry 2012, 87, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Beebe, S.J.; Blackmore, P.F.; White, J.; Joshi, R.P.; Schoenbach, K.H. Nanosecond pulsed electric fields modulate cell function through intracellular signal transduction mechanisms. Physiol. Meas. 2004, 25, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zou, Y.; Sun, Y.; Chen, X.; Chen, X.; Ren, Z. Effects of Nanosecond Pulsed Electric Fields in Cell Vitality, Apoptosis, and Proliferation of TPC-1 Cells. Anal. Cell. Pathol. 2021, 2021, 9913716. [Google Scholar] [CrossRef]
- Hanna, H.; Andre, F.M.; Mir, L.M. Electrical control of calcium oscillations in mesenchymal stem cells using microsecond pulsed electric fields. Stem Cell Res. Ther. 2017, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Rossi, A.; Pakhomova, O.N.; Mollica, P.A.; Casciola, M.; Mangalanathan, U.; Pakhomov, A.G.; Muratori, C. Nanosecond Pulsed Electric Fields Induce Endoplasmic Reticulum Stress Accompanied by Immunogenic Cell Death in Murine Models of Lymphoma and Colorectal Cancer. Cancers 2019, 11, 2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, H.; Denzi, A.; Liberti, M.; André, F.M.; Mir, L.M. Electropermeabilization of Inner and Outer Cell Membranes with Microsecond Pulsed Electric Fields: Quantitative Study with Calcium Ions. Sci. Rep. 2017, 7, 13079. [Google Scholar] [CrossRef] [Green Version]
- Cemazar, M.; Sersa, G.; Frey, W.; Miklavcic, D.; Teissié, J. Recommendations and requirements for reporting on applications of electric pulse delivery for electroporation of biological samples. Bioelectrochemistry 2018, 122, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.H.; Roy, R.; Mauck, R.L.; Liu, W.; Valhmu, W.B.; Hung, C.T. Chondrocyte translocation response to direct current electric fields. J. Biomech. Eng. 2000, 122, 261–267. [Google Scholar] [CrossRef]
- Akkiraju, H.; Nohe, A. Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Hiemer, B.; Krogull, M.; Bender, T.; Ziebart, J.; Krueger, S.; Bader, R.; Jonitz-Heincke, A. Effect of electric stimulation on human chondrocytes and mesenchymal stem cells under normoxia and hypoxia. Mol. Med. Rep. 2018, 18, 2133–2141. [Google Scholar] [CrossRef] [Green Version]
- Khatib, L.; Golan, D.E.; Cho, M. Physiologic electrical stimulation provokes intracellular calcium increase mediated by phospholipase C activation in human osteoblasts. FASEB J. 2004, 18, 1903–1905. [Google Scholar] [CrossRef]
- Berridge, M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Laha, M.; Mondal, S.; Sengupta, S.; Kaplan, D.L. In vitro model of mesenchymal condensation during chondrogenic development. Biomaterials 2009, 30, 6530–6540. [Google Scholar] [CrossRef] [Green Version]
- Yoon, I.S.; Chung, C.W.; Sung, J.H.; Cho, H.J.; Kim, J.S.; Shim, W.S.; Shim, C.K.; Chung, S.J.; Kim, D.D. Proliferation and chondrogenic differentiation of human adipose-derived mesenchymal stem cells in porous hyaluronic acid scaffold. J. Biosci. Bioeng. 2011, 112, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Xia, X.; Yeh, J.; Kua, H.; Liu, H.; Mishina, Y.; Hao, A.; Li, B. PDGF-AA Promotes Osteogenic Differentiation and Migration of Mesenchymal Stem Cell by Down-Regulating PDGFRα and Derepressing BMP-Smad1/5/8 Signaling. PLoS ONE 2014, 9, e113785. [Google Scholar] [CrossRef] [Green Version]
- Ataliotis, P. Platelet-derived growth factor A modulates limb chondrogenesis both in vivo and in vitro. Mech. Dev. 2000, 94, 13–24. [Google Scholar] [CrossRef]
- Fisher, M.C.; Meyer, C.; Garber, G.; Dealy, C.N. Role of IGFBP2, IGF-I and IGF-II in regulating long bone growth. Bone 2005, 37, 741–750. [Google Scholar] [CrossRef]
- McQueeney, K.; Dealy, C.N. Roles of insulin-like growth factor-I (IGF-I) and IGF-I binding protein-2 (IGFBP2) and -5 (IGFBP5) in developing chick limbs. Growth Horm. IGF Res. 2001, 11, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, G.; De Gori, M.; Paonessa, F.; Chiefari, E.; Brunetti, A.; Galasso, O. Functional relationship between high mobility group A1 (HMGA1) protein and insulin-like growth factor-binding protein 3 (IGFBP-3) in human chondrocytes. Arthritis Res. Ther. 2012, 14, R207. [Google Scholar] [CrossRef] [Green Version]
- Eviatar, T.; Kauffman, H.; Maroudas, A. Synthesis of insulin-like growth factor binding protein 3 in vitro in human articular cartilage cultures. Arthritis Rheum. 2003, 48, 410–417. [Google Scholar] [CrossRef]
- Zhou, N.; Li, Q.; Lin, X.; Hu, N.; Liao, J.-Y.; Lin, L.-B.; Zhao, C.; Hu, Z.-M.; Liang, X.; Xu, W.; et al. BMP2 induces chondrogenic differentiation, osteogenic differentiation and endochondral ossification in stem cells. Cell Tissue Res. 2016, 366, 101–111. [Google Scholar] [CrossRef]
- Majumdar, M.K.; Wang, E.; Morris, E.A. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J. Cell. Physiol. 2001, 189, 275–284. [Google Scholar] [CrossRef]
- Choi, K.H.; Choi, B.H.; Park, S.R.; Kim, B.J.; Min, B.H. The chondrogenic differentiation of mesenchymal stem cells on an extracellular matrix scaffold derived from porcine chondrocytes. Biomaterials 2010, 31, 5355–5365. [Google Scholar] [CrossRef]
- Mardani, M.; Roshankhah, S.; Hashemibeni, B.; Salahshoor, M.; Naghsh, E.; Esfandiari, E. Induction of chondrogenic differentiation of human adipose-derived stem cells by low frequency electric field. Adv. Biomed. Res. 2016, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Z.; Zhang, G.; Clark, C.C.; Brighton, C.T. Up-regulation of chondrocyte matrix genes and products by electric fields. Clin. Orthop. Relat. Res. 2004, 427, S163–S173. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, E.; Roshankhah, S.; Mardani, M.; Hashemibeni, B.; Naghsh, E.; Kazemi, M.; Salahshoor, M. The effect of high frequency electric field on enhancement of chondrogenesis in human adipose-derived stem cells. Iran. J. Basic Med. Sci. 2014, 17, 571–576. [Google Scholar] [PubMed]
- Fitzsimmons, R.J.; Gordon, S.L.; Kronberg, J.; Ganey, T.; Pilla, A.A. A pulsing electric field (PEF) increases human chondrocyte proliferation through a transduction pathway involving nitric oxide signaling. J. Orthop. Res. 2008, 26, 854–859. [Google Scholar] [CrossRef]
- Li, K.; Fan, L.; Lin, J.; Heng, B.C.; Deng, Z.; Zheng, Q.; Zhang, J.; Jiang, Y.; Ge, Z. Nanosecond pulsed electric fields prime mesenchymal stem cells to peptide ghrelin and enhance chondrogenesis and osteochondral defect repair in vivo. Sci. China Life Sci. 2022, 65, 927–939. [Google Scholar] [CrossRef]
- Li, K.; Ning, T.; Wang, H.; Jiang, Y.; Zhang, J.; Ge, Z. Nanosecond pulsed electric fields enhance mesenchymal stem cells differentiation via DNMT1-regulated OCT4/NANOG gene expression. Stem Cell Res. Ther. 2020, 11, 308. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Yang, J.; Li, K.; Jiang, Y.; Heng, B.C.; Cai, Q.; Zhang, J.; Ge, Z. Multiple nanosecond pulsed electric fields stimulation with conductive poly(l-lactic acid)/carbon nanotubes films maintains the multipotency of mesenchymal stem cells during prolonged in vitro culture. J. Tissue Eng. Regen. Med. 2020, 14, 1136–1148. [Google Scholar] [CrossRef]
- Vaca-González, J.J.; Clara-Trujillo, S.; Guillot-Ferriols, M.; Ródenas-Rochina, J.; Sanchis, M.J.; Ribelles, J.L.G.; Garzón-Alvarado, D.A.; Ferrer, G.G. Effect of electrical stimulation on chondrogenic differentiation of mesenchymal stem cells cultured in hyaluronic acid—Gelatin injectable hydrogels. Bioelectrochemistry 2020, 134, 107536. [Google Scholar] [CrossRef]
- Gavénis, K.; Andereya, S.; Schmidt-Rohlfing, B.; Mueller-Rath, R.; Silny, J.; Schneider, U. Millicurrent stimulation of human articular chondrocytes cultivated in a collagen type-I gel and of human osteochondral explants. BMC Complement. Altern. Med. 2010, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- Deliormanlı, A.M.; Atmaca, H. Biological Response of Osteoblastic and Chondrogenic Cells to Graphene-Containing PCL/Bioactive Glass Bilayered Scaffolds for Osteochondral Tissue Engineering Applications. Appl. Biochem. Biotechnol. 2018, 186, 972–989. [Google Scholar] [CrossRef] [PubMed]
- Prasopthum, A.; Deng, Z.; Khan, I.M.; Yin, Z.; Guo, B.; Yang, J. Three dimensional printed degradable and conductive polymer scaffolds promote chondrogenic differentiation of chondroprogenitor cells. Biomater. Sci. 2020, 8, 4287–4298. [Google Scholar] [CrossRef]
- Gupta, K.; Patel, R.; Dias, M.; Ishaque, H.; White, K.; Olabisi, R. Development of an Electroactive Hydrogel as a Scaffold for Excitable Tissues. Int. J. Biomater. 2021, 2021, 6669504. [Google Scholar] [CrossRef] [PubMed]
- Krueger, S.; Riess, A.; Jonitz-Heincke, A.; Weizel, A.; Seyfarth, A.; Seitz, H.; Bader, R. Establishment of a New Device for Electrical Stimulation of Non-Degenerative Cartilage Cells In Vitro. Int. J. Mol. Sci. 2021, 22, 394. [Google Scholar] [CrossRef] [PubMed]
- Raso, J.; Frey, W.; Ferrari, G.; Pataro, G.; Knorr, D.; Teissie, J.; Miklavčič, D. Recommendations guidelines on the key information to be reported in studies of application of PEF technology in food and biotechnological processes. Innov. Food Sci. Emerg. Technol. 2016, 37, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Cvetkoska, A.; Pirc, E.; Reberšek, M.; Magjarevic, R.; Miklavčič, D. Towards standardization of electroporation devices and protocols. IEEE Instrum. Meas. Mag. 2020, 23, 74–81. [Google Scholar] [CrossRef]
- Aragón, Á.; Cebro-Márquez, M.; Perez, E.; Pazos, A.; Lage, R.; González-Juanatey, J.R.; Moscoso, I.; Bao-Varela, C.; Nieto, D. Bioelectronics-on-a-chip for cardio myoblast proliferation enhancement using electric field stimulation. Biomater. Res. 2020, 24, 15. [Google Scholar] [CrossRef]
- Cao, J.; Cheng, P.; Hong, F. Applications of electrohydrodynamics and Joule heating effects in microfluidic chips: A review. Sci. China Ser. E Technol. Sci. 2009, 52, 3477–3490. [Google Scholar] [CrossRef]
- Hur, J.; Chung, A.J. Microfluidic and Nanofluidic Intracellular Delivery. Adv. Sci. 2021, 8, 2004595. [Google Scholar] [CrossRef]
- Hu, M.; Hong, L.; He, S.; Huang, G.; Cheng, Y.; Chen, Q. Effects of electrical stimulation on cell activity, cell cycle, cell apoptosis and β-catenin pathway in the injured dorsal root ganglion cell. Mol. Med. Rep. 2020, 21, 2385–2394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista Napotnik, T.; Miklavčič, D. In vitro electroporation detection methods—An overview. Bioelectrochemistry 2018, 120, 166–182. [Google Scholar] [CrossRef] [PubMed]
- Tas, J. The Alcian blue and combined Alcian blue—Safranin O staining of glycosaminoglycans studied in a model system and in mast cells. Histochem. J. 1977, 9, 205–230. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.Y.; Chin, K.Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 8293921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, G.; Lee, M.H. What are the roles of metalloproteinases in cartilage and bone damage? Ann. Rheum. Dis. 2005, 64, iv44. [Google Scholar] [CrossRef] [PubMed]





| Type of Cartilage | Conductivity | Reference |
|---|---|---|
| Baseline—post-exercise | [48] | |
| All cartilages, | 1.12–2.98 S/m; | |
| Patellar cartilage, | 1.11–2.80 S/m; | |
| Trochlear cartilage | 1.51–2.98 S/m | |
| Humeral head bovine articular cartilage * | 1.14 ± 0.11 S/m | [49] |
| Articular cartilage ** | 0.88 ± 0.08 S/m | [49] |
| Cell Type | ES Conditions | Result | Reference | |
|---|---|---|---|---|
| Nanosecond pulse | Cancer cell lines CT-26 and EL-4 | 300 and 100 pulses (200 ns, 7 kV/cm, 10 Hz) | Induced ER stress | [75] |
| Porcine bone marrow-derived stromal cells (pBM-MSCs) | 10 ns at 20 kV/cm, 100 ns at 10 kV/cm | Affects intracellular signaling pathways (JNK, P38, ERK, and Wnt signaling pathways) | [35] | |
| TPC-1 (papillary thyroid carcinoma cell line) | 900 ns | Reduced viability and proliferation, induced apoptosis | [73] | |
| Microsecond pulse | Tumor cell lines (DC3F, IGROV 1, SA-1, MCF7, B16F0, TBL.Cl2, TBL.Cl2 PT, HeLa, IGROV 1/DDP, B16F1, MM46T, EAT) | 400–600 V/cm, 1 HZ, 100 μs | Reversible plasmic membrane electroporation | [77] |
| HL1 cardiomyocytes, PC12, F11, and SH-S5Y5 neural cells | 1000–1250 V/cm, 100 μs 30–50 pulses | Irreversible plasmic membrane electroporation | [70] | |
| Human adipose mesenchymal stem cells (haMSC) | One single micropulse of 100 μs | Induced spontaneous Ca2+ oscillations | [74] | |
| haMSC | One single micropulse of 100 μs | Permeabilization of ER membrane | [76] |
| Growth Factor | Function in Chondrogenesis | Reference |
|---|---|---|
| Transforming growth factor (TGF)-β1 | Induces condensation of MSCs; Induces the production of fibronectin and N-cadherin | [16] |
| Platelet-derived growth factor (PDGF)-AA | Promotes MSC osteogenic differentiation and migration; Promotes chondrogenesis in the early stages of limb development | [85,86] |
| Insulin-like growth factor-binding protein (IGFPB)-2 | Reduces proliferation of chondrocytes; Stimulates expression of prehypertrophy marker Indian hedgehog; Inhibits chondrogenic differentiation and ECM synthesis of micromass cultures | [87,88] |
| IGFPB-3 | Reduces proliferation of chondrocytes; Might diminish the synthesis of matrix collagen and aggrecan | [89,90] |
| BMP-2 | Increases chondrogenic differentiation by increasing Sox9a and Runx2 proteins expression in vitro, Increases hypertrophy and expression of osteogenic markers: type I collagen, type X collagen | [91,92] |
| Cell Type | ES Conditions | Beneficial Effects | Adverse Effects | References |
|---|---|---|---|---|
| Continuous Stimulation | ||||
| Human dermal fibroblasts (HDFs) | 100–500 mV/mm electric field, bipolar square-wave pulse, 6–10 ms at 5 Hz for 3 days | 8 ms pulse: ↑ condensation; ↑ expression of chondrogenic genes (COL2A1, ACAN, SOX9;) ↓ expression of COL1A1, COL1A2 genes; ↑level of COL2 protein, GAGs; ↑ secretion of growth factors (TGF-β1, PDGF-AA, IGFBP-2 and 3) | Pulse duration 6 ms: No condensation Pulse duration 10 ms: Damaged cells, No compact condensation | [22] |
| Murine BMMSCs | 100–2500 mV/mm electric field, bipolar square-wave pulse, 8 ms at 5 Hz for 3 days | Electrical field of 500 mV/mm: ↑ condensation; ↑ expression of chondrogenic genes (COL2A1, ACAN, SOX9); ↓ expression of COL1 gene; ↑ level of COL2 protein, GAGs; ↑ expression of TGF-β1 and BMP2. | Electrical field of 100 and 2500 mV/mm: ↓ Ca2+/ATP oscillations; ↓ condensation | [16] |
| Human ADSCs are extracted from subcutaneous abdominal adipose tissue | 1 kHz, 20 mv/cm for 20 min. | ↑ aggrecan secretion ↑ expression of COLII and SOX9 genes ↓ expression of COLX gene | [94] | |
| Bovine chondrocytes | 0.02–4 mV/mm, sine-wave with a frequency of 60 kHz; stimulation time 0.5 h | 0.5-h of 2 mV/mm (1 min on (1′ ON), 7 off (7′ OFF)—30 cycles and 1′ ON/1′ OFF 30 cycles), harvest after 3.5 h of ES: ↑ expression of ACAN gene. 2 mV/mm, regimen—1′ ON/7′ OFF—30 cycles and continuous stimulation, harvest after 5.5 h: ↑ expression of COL2 gene. | 2–6 h of stimulation, 0.1, 0.5, 4 mV/mm amplitude: No change in expression of ACAN gene 0.02, 0.1, 0.5, 1, 4 mV/mm amplitude: No change in expression of COL2 gene | [95] |
| Explants from human osteoarthritic cartilage | 2 mV/mm, static for 30 min followed by 8.33 μs square wave pulse at 60 kHz for 1 h × 4 times a day (gap 5 h) for 7 or 14 days | ↑ proteoglycan and collagen content; ↑ expression of ACAN and COL2 genes; ↓ expression of IL-1β induced MMP-1, MMP-3, MMP-13, ADAM-TS4 genes. | - | [19] |
| Human ADSCs were extracted from subcutaneous abdominal adipose tissue | Capacitively electric fields (20 mV/cm, 60 KHz) pulsed wave applied for 20 min daily for 7 days. | ↑ expression of COLII, SOX9 genes; ↓ expression of COL X and COLI genes ↑ secretion of aggrecan No difference in cell viability | - | [96] |
| Articular chondrocytes were isolated from adult bovine patellae | 2 mV/mm, 8.33 μs square wave pulse at 60 kHz for 1–6 h 1′ ON/7′ OFF) for 1 h for aggrecan and 1′ ON/1′ OFF for 6 h for collagen II; for MMPs—30 min static stimulation at 2 mV/mm | ↑ expression of ACAN, COLII genes ↓ expression of IL-1β induced MMP-1, MMP-3, MMP-13, ADAMTS-4, ADAMTS-5 genes | - | [44] |
| Nanosecond pulsed electrical field | ||||
| Normal human chondrocytes (#CC2550, Lonza) | Asymmetrical biphasic rectangular pulses, 210/30 ms in each polarity, respectively, repeating at 4150 Hz, delivered in 10-ms bursts 15 times per second for 30 min. PEF generated peak changes in current of 2.7 mAmps and an electric field in culture media of 0.2 mV/cm | ↑ proliferation ↑ NO and cGMP | [97] | |
| Chondrocytes from porcine articular cartilage tissue | 1–2 × 106 mV/mm, square wave with transients; 5 × 100 ns, 1 Hz | ↑ proliferation of chondrocytes. | ↓ GAG production; ↓ expression of COLII, SOX9; ↑ expression COLI gene and COLX. | [36] |
| Porcine BMMSCs | 0.5–3 × 106 mV/mm, square wave with transients; 5 × 10−300 ns, 1 Hz | 10 ns at 2 × 106 mV/mm, 60 ns at 5 and 2 × 106 mV/mm, 100 ns at 1 × 106 mV/mm: ↑ expression of COLII, SOX9, and ACAN genes; 10 ns at × 106 mV/mm and 100 ns at 10 × 106 mV/mm: ↑ production of GAGs | 2 and 3 × 106 mV/mm with longer pulse duration (100, 300 ns): ↓ viability; 60 ns at 0.5–2 × 106 mV/mm: ↑ expression of COLI and COLX genes | [35] |
| Rat BMMSCs | 100 ns duration, 10 kV cm−1, 1 Hz) | ↑ OCT4 and NANOG expression Together with grelin ↑ expression of SOX9, COLII, ACAN genes ↑ de novo cartilage regeneration (smoother cartilage surface in defect area, ↑ ICRS histology score) | - | [98] |
| Porcine BMMSCs and human BMMSCs | 5 pulses of nsPEFs (10 ns at 20 kV/cm, 60 ns at 5 kV/cm, 60 ns at 10 kV/cm, 60 ns at 20 kV/cm, and 100 ns at 10 kV/cm, 1 Hz) with 1 s time interval between two pulses | 10 ns at 20 kV/cm, and 100 ns at 10 kV/cm in both types of cells: ↑ enhance trilineage differentiation potential; -No influence on proliferation; ↑ expression of OCT4 and NANOG genes; | - | [99] |
| Porcine BMMSCs | Stimulation was carried out with 5 pulses of nsPEFs (10~25 kV/cm, 10~100 ns), and the time interval between each pulse was 1 s. | Four 100 ns at 10 kV/cm pulse on cells cultured on PLLA/CNT films: ↑ tri-lineage differentiation 4 times of PES ↑ expression of pluripotency genes (OCT4, NANOG, SOX2) | Single 100 ns at 10 kV/cm pulse on cells cultured on PLLA/CNT films: ↑ expression of pluripotency genes (OCT4, NANOG, SOX2) 3 days after pulse | [100] |
| Scaffold Characteristics | Cell Type | Electrical Stimulation Parameters | Beneficial Effects | Adverse Effects | Reference |
|---|---|---|---|---|---|
| 3D collagen I -elastin scaffolds | Human chondrocytes (OA and control) | 5.2 × 10−6 and 5.2 × 10−5 mV/mm, sine wave 1 kHz. 3 times in a day for 45 min for 7 days | 5.2 × 10−6 mV/mm ↑ GAG, COLII protein synthesis | ↑ COLI protein synthesis | [45] |
| CNT (carbon nanotubes)/PCU polycarbonate urethane | Human chondrocytes (Cell Applications) | Alternating current (AC) stimulation of voltages was generated at 10 lA with 10 Hz throughout the entire cell experiment but with different stimulation times (specifically, either 3 or 6 h) | Both 3 and 6 h stimulation ↑ proliferation of chondrocytes | - | [38] |
| Collagen I-elastin scaffold | Chondrocytes (non-degraded) | 0.005–2.5 mV/mm bipolar wave delivered at 1 or 60 kHz for 45 min per day for seven days. | ↑ chondrogenic re-differentiation at the gene and protein level of human de-differentiated chondrocytes | - | [106] |
| Graphene-Containing PCL/Bioactive Glass Bilayer | Pre-osteoblastic MC3T3-E1 and chondrogenic ATDC5 | 2 mV/mm, sine wave at 60 kHz 30 min/day for 3 days | ↑ viability of ATDC5 cells | ↓ viability of MC3T3-E1 cells | [103] |
| Injectable hyaluronic acid—gelatin hydrogel | Porcine MSCs | 0.9–1.2 mV/mm sine wave at 60 kHz, 30 min 4 times a day, 21 days | ↑ SOX9 and aggrecan and COLII protein | ↓ production of GAG | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaiciuleviciute, R.; Uzieliene, I.; Bernotas, P.; Novickij, V.; Alaburda, A.; Bernotiene, E. Electrical Stimulation in Cartilage Tissue Engineering. Bioengineering 2023, 10, 454. https://doi.org/10.3390/bioengineering10040454
Vaiciuleviciute R, Uzieliene I, Bernotas P, Novickij V, Alaburda A, Bernotiene E. Electrical Stimulation in Cartilage Tissue Engineering. Bioengineering. 2023; 10(4):454. https://doi.org/10.3390/bioengineering10040454
Chicago/Turabian StyleVaiciuleviciute, Raminta, Ilona Uzieliene, Paulius Bernotas, Vitalij Novickij, Aidas Alaburda, and Eiva Bernotiene. 2023. "Electrical Stimulation in Cartilage Tissue Engineering" Bioengineering 10, no. 4: 454. https://doi.org/10.3390/bioengineering10040454