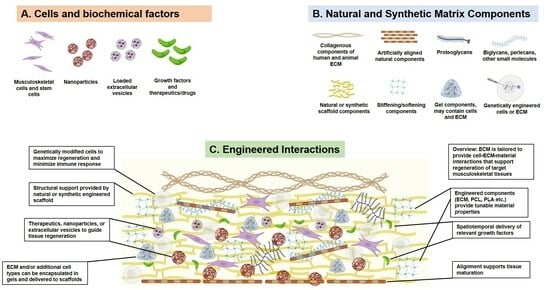
Engineering Cell–ECM–Material Interactions for Musculoskeletal Regeneration
Abstract
:1. Introduction
2. Survey of Engineered Cell–ECM–Material Interactions in Musculoskeletal Tissues
2.1. Skeletal Muscle
2.1.1. ECM-Based Approaches for Skeletal Muscle Regeneration
2.1.2. ECM-Based Personalized Disease Models
| Tissue | Model | Material | Main Findings |
|---|---|---|---|
| Skeletal Muscle | ECM combined with extracellular vesicles (EVs) and mesenchymal stem cells (MSCs) in a murine VML model. | Decellularized ECM and extracellular vesicles (EVs). | Muscle regeneration was enhanced after 30 days in mice treated with ECM and EVs. Higher MHC and gains in muscle function compared to control groups [34]. |
| ECM scaffolds with parallel microchannels (ECM-C) by subcutaneous implantation of sacrificial templates followed by template removal and decellularization. | ECM scaffolds with parallel microchannels (ECM-C). | Compared to controls, rats that received the scaffolds had extensive neo-tissue formation in the grafting area, as well as cell infiltration, blood vessel formation, and new ECM deposition, which were not observed in the controls. Neo-muscle tissue had acetylcholine receptors and nerve fiber contacts, resembling early neuromuscular junction formation [33]. | |
| Unilateral resection of the distal third of the vastus lateralis and medial half of the distal third of the vastus medialis in dogs; defects replaced with scaffolds composed of small intestinal submucosa extracellular matrix (SIS-ECM). | Scaffolds composed of small intestinal submucosa extracellular matrix (SIS-ECM). | SIS-ECM promoted integration of soft and bony tissues, suggesting it may be a useful tool in engineering the ECM after injury to promote an integrative response in the cells [35]. | |
| Xenogeneic porcine urinary bladder ECM scaffolds used as a surgical treatment for volumetric muscle loss in both a preclinical rodent model and human male patients. | Xenogeneic porcine urinary bladder ECM Scaffolds. | Porcine bladder ECM supported the formation of stimulus-responsive skeletal muscle cells and tissues in mice, and functional improvement was observed in three implanted human patients. ECM-treated mice showed muscle activation [23]. | |
| Preclinical model of collagen VI- related dystrophies (COL6-RDs) using cell-derived matrices (CDMs) developed using the forearm skin fibroblasts of both patients with (COL6-RD), as well as from healthy donors without neuromuscular disease. | Cell-derived matrices (CDMs) developed using the forearm skin fibroblasts of both patients with (COL6-RD), and from healthy donors without neuromuscular disease. | Disease markers were significantly increased in CDMs from COL6-RD patients compared to controls (CDMs derived from healthy patients). Higher collagen VI and fibronectin alignment, length, width, and straightness were observed in control CDMs compared to patient-derived CDMs [36]. | |
| Decellularized canine placentas and murine skeletal muscle ECM placed in male Wistar rats with pockets at the posterior limbs. | Decellularized canine placentas and murine skeletal muscles. | Higher percentage of proliferative PCNA+ cells three days after implantation in placenta-derived matrices, compared to muscle derived matrices. Higher percentage of CD163+high macrophages in muscle-derived ECM; higher percentage of CD163+low macrophages found in placenta-derived ECM 3- and 15-days post-implantation [39]. |
2.1.3. Interactions between ECM and the Immune System in Skeletal Muscle
2.1.4. Other ECM-Based Approaches in Skeletal Muscle
2.1.5. ECM-Cell Interactions and Muscle Fibrosis
2.2. Cartilage
2.2.1. Cartilage ECM as an Engineered Material
2.2.2. Cartilage ECM to Modulate Fibrosis
2.3. Tendon
2.3.1. Engineered Tendon ECM to Understand Development and Disease
| Tissue | Model | Material | Main Findings |
|---|---|---|---|
| Cartilage | Rat bone marrow-derived mesenchymal stem cells (rBMSCs) cultured with cryo-ground decellularized cartilage ECM. | Cryo-ground decellularized cartilage ECM. | Chemically decellularized cartilage (DCC) particles significantly outperformed TGF-β in chondroinduction of the rBMSCs. Collagen II gene expression was more than an order of magnitude greater compared to controls [74]. |
| Porcine methacryl-modified solubilized and devitalized cartilage (MeSDVC) hydrogels. | Cryo-ground decellularized cartilage ECM methacrylated with glycidyl methacrylate (GM) and methacrylic anhydride (MA). | Methacrylation of the ECM increased printability of the MeSDVC hydrogels by creating paste-like consistency. Hydrogel stiffness increased to physiologically useful ranges [98]. | |
| BMSCs grown in dual-stage crosslinked hyaluronic acid-based bioink that was covalently linked to transforming growth factor-beta 1 (TGF-β1). | Hyaluronic acid (HA) bioink with covalently bonded TGF-β1. | Tethered TGF-β1 maintained functionality post three-dimensional printing and generated high quality cartilaginous tissues without exogenous growth factors [99]. | |
| BMSCs grown in porcine photocrosslinkable methacrylated cartilage ECM-based hydrogel bioink (cECM-MA). | Decellularized MA- methacrylated cartilage ECM bioink. | BMSCs were viable post-printing and underwent chondrogenesis in vitro, generating tissue rich in sulphated glycosaminoglycans and collagens [100]. | |
| Rat chondrocytes grown in genipin-crosslinked gelatin scaffolds with varying porosity. | Genipin-crosslinked gelatin scaffolds. | Chondrocytes proliferated and readily generated ECM with pore sizes of 250 and 500 μm [101]. | |
| hMSCs grown in tunicate exoskeleton-derived dECM. | Tunicate dECM. | Tunicate ECM was decellularized while retaining the honeycombed-shaped microstructure that improved metabolic activity, cell proliferation, and chondrogenic differentiation in hMSCs [79]. | |
| Rat chondrocytes grown in high concentration collagen bioprinted hydrogel scaffolds. | An amount of 4% collagen hydrogel bioink. | Subcutaneous implantation of the bioprinted scaffold resulted in cartilage-like tissue formation in rats as early as one week post implantation [78]. | |
| BMSCs grown in polyethylene glycol diacrylate (PEGDA) and ECM electro-written hydrogel. | High porosity PEDGA and porcine-derived ECM electro-written scaffold. | Electro-written PEDGA and ECM scaffold induced chondrogenesis and had anti-inflammatory effects [79]. | |
| Adipose-derived stem cells (ADSCs) grown in cartilage dECM and waterborne polyurethane (WPU) scaffolds, using low-temperature deposition manufacturing (LDM). | Cartilage dECM and WPU. | Hierarchical macro-microporous dECM- WPU scaffolds regenerated hyaline cartilage in a rabbit articular cartilage microfracture model [69]. | |
| Mouse chondrocytes in human bone marrow-derived MSC-ECM (hBMSC). | hBMSC-ECM. | In vivo subcutaneous implantation of hBMSC-ECM scaffold in mice improved chondrocyte proliferation and development of a bioactive matrix [68]. | |
| Decellularized allogeneic hyaline cartilage graft (dLhCG) for porcine knee repair. | Decellularized pure hyaline-like cartilaginous ECM. | dLhCG resulted in superior efficacy in articular cartilage repair, surpassing living autologous chondrocyte-based cartilaginous engraftment repair methods [65]. | |
| Self-assembled articular cartilage constructs grown in bovine femoral condyle superficial zone cartilage ECM. | Bovine femoral condyle superficial zone cartilage ECM. | Extracted cartilage ECM reduced friction coefficients of the self-assembled articular cartilage constructs [63]. |
2.3.2. Engineered Tendon ECM as a Repair Material
| Tissue | Model | Material | Main Findings |
|---|---|---|---|
| Tendon | Acellular dermal matrix (ADM) tendon scaffold affixed to hand flexor tendon post-operation. | Decellularized dermal ECM. | Addition of ADM post operation reduced tendon adhesion and improved long term functionality of the flexor tendon [100]. |
| Decellularized bovine tendon ECM used as an anti-adhesion membrane. | Decellularized tendon matrix (DTM). | DTM improved tendon repair in rabbits by reducing adhesion and cellular proliferation, as well as improving healed tendon quality [98]. | |
| Human adipose-derived stem cells (hASCs) grown in urea-extracted bovine decellularized tendon matrix (DTM). | Urea-extracted decellularized tendon matrix (DTM). | Urea-extracted DTM increased hASC proliferation and tenogenic differentiation, and it also induced unique tenogenic gene expression profiles [96]. | |
| Rat tendon self-repair with implanted decellularized autologous extracellular matrix (aECM) scaffolds with highly aligned microchannels. | aECM scaffolds with aligned microchannels created through poly (ε-caprolactone) (PCL) microfiber bundle templates. | Subcutaneously implanted aECM scaffolds with aligned microchannels increased cellular infiltration and proliferation in the damaged tendon, resulting in improved restoration of rat tendon post-injury [84]. | |
| Human acellular dermal matrix graft for canine tendon repair. | Decellularized dermal ECM. | Within 12 weeks of implantation, the graft restored tendon functionality and mimicked autologous tendon both histologically and mechanically [24]. |
2.3.3. Engineering Cell Interactions with the Non-Collagenous Components of the Tendon ECM
2.4. Bone
2.4.1. Engineered Bone ECM-Mimicking Scaffolds
2.4.2. Bone Scaffold Functionalization with ECM
2.5. Tuning ECM Mechanical Signals for Bone Tissue Engineering
| Tissue | Model | Material | Main Findings |
|---|---|---|---|
| Bone | Polycaprolactone (PCL) scaffold integrated with decellularized bone ECM seeded with mouse mesenchymal stem cells (MSCs). | Polycaprolactone (PCL) scaffold integrated with decellularized bone ECM. | The addition of bone ECM to the PCL increased the mechanical properties of the resulting scaffold, increased cellular attachment, and enhanced osteogenesis of mouse mesenchymal stem cells (MSCs) [112]. |
| Growth plate injury was induced in rabbits and treated with engineered oriented ECM scaffolds and autogenous BMSCs, ECM scaffolds only, or injured but not treated with a scaffold or cells. | Engineered oriented ECM scaffolds. | BMSCs successfully adhered to and distributed within the oriented scaffold in the group treated with both the ECM scaffold and the cells. The ECM scaffold and BMSCs generated functional tissue-engineered cartilage superior to the other groups, and the scaffold and cell treatment decreased angular deformities and length discrepancy of the tibia when compared to other groups. Addition of BMSCs within the ECM scaffolds promoted regeneration of neogenetic chondrocytes during the repair of the injured growth plates and prevented the formation of bone bridges [113]. | |
| Critical-sized calvarial defect in a rat model; porous polycaprolactone (PCL)/decellularized small interesting submucosa (SIS) scaffolds injected into defect. Scaffolds were fabricated using cryogenic free-form extrusion and surface modification with aptamer and PlGF-2123-144peptide-fused bone morphogenetic protein 2 (pBMP2). | Porous polycaprolactone (PCL)/decellularized small interesting submucosa (SIS) scaffolds. | Four- and eight-weeks post-op, defects implanted with the PCL/SIS-BMP2-Apt and PCL /SIS-pBMP2-APT scaffolds had substantial mineralized tissue not seen in defects implanted with PCL/SIS and PCL/SIS-Apt groups. Significantly higher bone volume/tissue volume percentage and bone mineral density for defects implanted with PCL/SIS-Apt compared to controls. Eight weeks post-op, the bones in the injury site with PCL/SIS-pBMP2-Apt scaffold had completely bridged the defect, and angiogenesis occurred in rats implanted with the PCL/SIS-pBMP2-Apt scaffolds [116]. | |
| Porous PLGA (P) scaffold combined with magnesium hydroxide (MH, M), bone-extracellular matrix (bECM, E), and polydeoxyribonucleotide (PDRN, P). | Polylactic glycolic acid, magnesium hydroxide, and bone ECM. | PME and PMEP groups displayed significantly increased biocompatibility compared to the PLGA group, and both scaffolds had an increased population of calcein-AM positive human bone-marrow mesenchymal stem cells (hBMSCs), i.e., live cells at one, three, and seven days post-implantation [117]. | |
| ECM functionalized onto the surface of multi-channel biphasic calcium phosphate granules (MCG), seeded with MC3T3-E1 cells, and implanted into a rabbit femoral head defect model. | ECM functionalized onto the surface of multi-channel biphasic calcium phosphate granules (MCG). | Protein adsorption and osteogenic properties were improved on ECM functionalized MCG scaffolds compared to controls. ECM functionalized scaffolds enhanced bone regeneration in a rabbit model of a femoral head defect [118]. | |
| Bone ECM was configured into demineralized bone paper (DBP) as a material to direct osteoblasts to deposit structural mineralized bone tissue, and it was seeded with osteoblasts from DsRed reporter mice. | ECM configured into demineralized bone paper (DBP). | DBP effectively stimulated the trabecular osteoid, directed rapid and structural mineralization by osteoblasts, and contained the microenvironment necessary to support bone remodeling. Compared to control cells cultured on TCP, cells grown in the DBP displayed significantly higher mineralization, collagen alignment, and elongated morphology that was aligned with the underlying lamellar structure of the demineralized bone [119]. | |
| Osteoporotic mice models (established by ovariectomy) with three-dimensional complexes of encapsulated bone marrow derived mesenchymal stem cells (BMMSCs) in methacrylate gelatin (GelMA) hydrogels, and they inserted it into surgically induced femoral defects. | Methacrylate gelatin (GelMA) hydrogels. | Mitochondrial phosphoenolpyruvate carboxykinase (PCK2) promoted osteogenesis in three-dimensional ECM with tunable stiffness in vitro and in vivo. PCK2 enhanced the rate-limiting metabolic enzyme pallet isoform phosphofructokinase (PFKP) in three-dimensional ECM, and it further activated AKT/extracellular signal-regulated kinase 1/2 (ERK1/2) cascades to regulate osteogenic differentiation of MSCs [121]. | |
| ECM-loaded three-dimensional printed gelatin (Gel), sodium alginate (SA), and 58s bioglass (58sBG) gels were seeded with either rat aortic endothelial cells (RAOECs) or rat bone mesenchymal stem cells (RBMSCs), and they were implanted into rat mandibular defects. | ECM-loaded three-dimensional printed gelatin (Gel), sodium alginate (SA), and 58s bioglass (58sBG) gels. | Scaffolds coated with ECM significantly increased the expression of osteogenic and angiogenic genes. ECM-scaffolds promoted bone defect healing in vivo compared to the pure scaffold. RAOECs–ECM scaffolds and RBMSCs–ECM scaffolds enhanced bone formation, likely via increased expression of RUNX2, OCN, BMP2, CD31, and VEGF [125]. |
3. Future Directions
3.1. Genetically Engineered ECM and ECM-Based Bioinks
3.2. Engineered ECM for Understanding and Treating Musculoskeletal Fibrosis
3.3. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AAOS. United States Bone and Joint Decade: The Burden of Musculoskeletal Diseases in the United States; American Academy of Orthopaedic Surgeons: Rosemont, IL, USA, 2008. [Google Scholar]
- Assunção, M.; Dehghan-Baniani, D.; Yiu, C.H.K.; Später, T.; Beyer, S.; Blocki, A. Cell-Derived Extracellular Matrix for Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 602009. Available online: https://www.frontiersin.org/articles/10.3389/fbioe.2020.602009 (accessed on 15 December 2022). [CrossRef] [PubMed]
- Benders, K.E.M.; van Weeren, P.R.; Badylak, S.F.; Saris, D.B.F.; Dhert, W.J.A.; Malda, J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013, 31, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D.L. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Bueno, E.M.; Ruberti, J.W. Optimizing Collagen Transport through Track-Etched Nanopores. J. Memb. Sci. 2008, 321, 250–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, J.-O.; Rafat, M.; Almeda, D.; Maldonado, N.; Guo, P.; Nabzdyk, C.S.; Chun, M.; LoGerfo, F.W.; Hutchinson, J.W.; Pradhan-Nabzdyk, L.K.; et al. pH-responsive scaffolds generate a pro-healing response. Biomaterials 2015, 57, 22–32. [Google Scholar] [CrossRef]
- Cicuéndez, M.; Doadrio, J.C.; Hernández, A.; Portolés, M.T.; Izquierdo-Barba, I.; Vallet-Regí, M. Multifunctional pH sensitive 3D scaffolds for treatment and prevention of bone infection. Acta Biomater. 2018, 65, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Basurto, I.M.; Passipieri, J.A.; Gardner, G.M.; Smith, K.K.; Amacher, A.R.; Hansrisuk, A.I.; Christ, G.J.; Caliari, S.R. Photoreactive Hydrogel Stiffness Influences Volumetric Muscle Loss Repair. Tissue Eng. Part A 2022, 28, 312–329. [Google Scholar] [CrossRef]
- Guvendiren, M.; Burdick, J.A. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat. Commun. 2012, 3, 792. [Google Scholar] [CrossRef] [Green Version]
- Stoppel, W.L.; Gao, A.E.; Greaney, A.M.; Partlow, B.P.; Bretherton, R.C.; Kaplan, D.L.; Black, L.D. Elastic, silk-cardiac extracellular matrix hydrogels exhibit time-dependent stiffening that modulates cardiac fibroblast response. J. Biomed. Mater. Res. Part A 2016, 104, 3058–3072. [Google Scholar] [CrossRef] [Green Version]
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, L. Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable T gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials 2020, 232, 119720. [Google Scholar] [CrossRef]
- Cheng, W.; Ding, Z.; Zheng, X.; Lu, Q.; Kong, X.; Zhou, X.; Lu, G.; Kaplan, D.L. Injectable hydrogel systems with multiple biophysical and biochemical cues for bone regeneration. Biomater. Sci. 2020, 8, 2537. [Google Scholar] [CrossRef]
- Dixon, T.A.; Cohen, E.; Cairns, D.M.; Rodriguez, M.; Mathews, J.; Jose, R.R.; Kaplan, D.L. Bioinspired Three-Dimensional Human Neuromuscular Junction Development in Suspended Hydrogel Arrays. Tissue Eng. Part C Methods 2018, 24, 346–359. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Badu-Mensah, A.; Thomas, M.C.; McAleer, C.W.; Hickman, J.J. Characterization of Functional Human Skeletal Myotubes and Neuromuscular Junction Derived—From the Same Induced Pluripotent Stem Cell Source. Bioengineering 2020, 7, 133. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, K.J.; Park, S.Y.; Huh, J.E.; Kim, H.J.; Yu, W.R. 3D braid scaffolds for regeneration of articular cartilage. J. Mech. Behav. Biomed. Mater. 2014, 34, 37–46. [Google Scholar] [CrossRef]
- Hao, D.; Lopez, J.M.; Chen, J.; Iavorovschi, A.M.; Lelivelt, N.M.; Wang, A. Engineering Extracellular Microenvironment for Tissue Regeneration. Bioengineering 2022, 9, 202. [Google Scholar] [CrossRef]
- Brown, M.; Li, J.; Moraes, C.; Tabrizian, M.; Li-Jessen, N.Y.K. Decellularized extracellular matrix: New promising and challenging biomaterials for regenerative medicine. Biomaterials 2022, 289, 121786. [Google Scholar] [CrossRef]
- Kaukonen, R.; Jacquemet, G.; Hamidi, H.; Ivaska, J. Cell-derived matrices for studying cell proliferation and directional migration in a complex 3D microenvironment. Nat. Protoc. 2017, 12, 2376–2390. [Google Scholar] [CrossRef]
- Schneider, K.H.; Aigner, P.; Holnthoner, W.; Monforte, X.; Nürnberger, S.; Rünzler, D.; Redl, H.; Teuschl, A.H. Decellularized human placenta chorion matrix as a favorable source of small-diameter vascular grafts. Acta Biomater. 2016, 29, 125–134. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, T.W.; Stewart-Akers, A.M.; Simmons-Byrd, A.; Badylak, S.F. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J. Bone Jt. Surg. Am. 2007, 89, 621–630. [Google Scholar] [CrossRef]
- Woods, T.; Gratzer, P.F. Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciate ligament-bone graft. Biomaterials 2005, 26, 7339–7349. [Google Scholar] [CrossRef] [PubMed]
- Sicari, B.M.; Rubin, J.P.; Dearth, C.L.; Wolf, M.T.; Ambrosio, F.; Boninger, M.; Turner, N.J.; Weber, D.J.; Simpson, T.W.; Wyse, A.; et al. An Acellular Biologic Scaffold Promotes Skeletal Muscle Formation in Mice and Humans with Volumetric Muscle Loss. Sci. Transl. Med. 2014, 6, 234ra58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, J.E.; Zobitz, M.E.; Reach, J.S.; An, K.N.; Steinmann, S.P. Rotator Cuff Repair Using an Acellular Dermal Matrix Graft: An In Vivo Study in a Canine Model. Arthrosc. J. Arthrosc. Relat. Surg. 2006, 22, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Bandzerewicz, A.; Gadomska-Gajadhur, A. Into the Tissues: Extracellular Matrix and Its Artificial Substitutes: Cell Signalling Mechanisms. Cells 2022, 11, 914. [Google Scholar] [CrossRef]
- Xing, H.; Lee, H.; Luo, L.; Kyriakides, T.R. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol. Adv. 2020, 42, 107421. [Google Scholar] [CrossRef]
- Kim, K.M.; Jang, H.C.; Lim, S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J. Intern. Med. 2016, 31, 643. [Google Scholar] [CrossRef] [Green Version]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal Muscle Extracellular Matrix—What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front. Physiol. 2020, 11, 253. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2020.00253 (accessed on 21 January 2023). [CrossRef] [Green Version]
- Gillies, A.R.; Lieber, R.L. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef] [Green Version]
- Fuoco, C.; Petrilli, L.L.; Cannata, S.; Gargioli, C. Matrix scaffolding for stem cell guidance toward skeletal muscle tissue engineering. J. Orthop. Surg. 2016, 11, 86. [Google Scholar] [CrossRef] [Green Version]
- Grogan, B.F.; Hsu, J.R. Volumetric muscle loss. J. Am. Acad. Orthop. Surg. 2011, 19 (Suppl. S1), S35–S37. [Google Scholar] [CrossRef]
- Stantzou, A.; Relizani, K.; Morales-Gonzalez, S.; Gallen, C.; Grassin, A.; Ferry, A.; Schuelke, M.; Amthor, H. Extracellular matrix remodelling is associated with muscle force increase in overloaded mouse plantaris muscle. Neuropathol. Appl. Neurobiol. 2021, 47, 218–235. [Google Scholar] [CrossRef]
- Zhu, M.; Li, W.; Dong, X.; Yuan, X.; Midgley, A.C.; Chang, H.; Wang, Y.; Wang, H.; Wang, K.; Ma, P.X.; et al. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun. 2019, 10, 4620. [Google Scholar] [CrossRef] [Green Version]
- Magarotto, F.; Sgrò, A.; Dorigo Hochuli, A.H.; Andreetta, M.; Grassi, M.; Saggioro, M.; Nogara, L.; Tolomeo, A.M.; Francescato, R.; Collino, F.; et al. Muscle functional recovery is driven by extracellular vesicles combined with muscle extracellular matrix in a volumetric muscle loss murine model. Biomaterials 2021, 269, 120653. [Google Scholar] [CrossRef]
- Turner, N.J.; Badylak, J.S.; Weber, D.J.; Badylak, S.F. Biologic Scaffold Remodeling in a Dog Model of Complex Musculoskeletal Injury. J. Surg. Res. 2012, 176, 490–502. [Google Scholar] [CrossRef]
- Almici, E.; Chiappini, V.; López-Márquez, A.; Badosa, C.; Blázquez, B.; Caballero, D.; Montero, J.; Natera-de Benito, D.; Nascimento, A.; Roldán, M.; et al. Personalized in vitro Extracellular Matrix Models of Collagen VI-Related Muscular Dystrophies. Front. Bioeng. Biotechnol. 2022, 10, 851825. [Google Scholar] [CrossRef]
- Hu, M.; Ling, Z.; Ren, X. Extracellular matrix dynamics: Tracking in biological systems and their implications. J. Biol. Eng. 2022, 16, 13. [Google Scholar] [CrossRef]
- Petrosino, J.M.; Longenecker, J.Z.; Angell, C.D.; Hinger, S.A.; Martens, C.R.; Accornero, F. CCN2 participates in overload-induced skeletal muscle hypertrophy. Matrix Biol. 2022, 106, 1–11. [Google Scholar] [CrossRef]
- Carvalho, C.M.F.; Leonel, L.C.P.C.; Cañada, R.R.; Barreto, R.S.N.; Maria, D.A.; Del Sol, M.; Miglino, M.A.; Lobo, S.E. Comparison between placental and skeletal muscle ECM: In vivo implantation. Connect. Tissue Res. 2021, 62, 629–642. [Google Scholar] [CrossRef]
- Chen, H.; Qian, Z.; Zhang, S.; Tang, J.; Fang, L.; Jiang, F.; Ge, D.; Chang, J.; Cao, J.; Yang, L.; et al. Silencing COX-2 blocks PDK1/TRAF4-induced AKT activation to inhibit fibrogenesis during skeletal muscle atrophy. Redox Biol. 2021, 38, 101774. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Yan, Y.; Li, S.; Tong, H. SPARCL1 promotes C2C12 cell differentiation via BMP7-mediated BMP/TGF-β cell signaling pathway. Cell Death Dis. 2019, 10, 852. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, J.; Gonzalez, D.; Escalona-Rivano, R.; Takahashi, C.; Brandan, E. Reduced RECK levels accelerate skeletal muscle differentiation, improve muscle regeneration, and decrease fibrosis. FASEB J. 2021, 35, e21503. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.J.; Lin, C.H.; Li, M.; Nishtala, K.; Alaei, S.; Rossello, F.; Sonntag, C.; Hersey, L.; Miles, L.B.; Krisp, C.; et al. FKRP-dependent glycosylation of fibronectin regulates muscle pathology in muscular dystrophy. Nat. Commun. 2021, 12, 2951. [Google Scholar] [CrossRef] [PubMed]
- Sahani, R.; Wallace, C.H.; Jones, B.K.; Blemker, S.S. Diaphragm Muscle Fibrosis Involves Changes in Collagen Organization with Mechanical Implications in Duchenne Muscular Dystrophy. J. Appl. Physiol. 2022, 132, 653–672. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo, D.L.; González, D.; Faundez-Contreras, J.; Contreras, O.; Vio, C.P.; Murphy-Ullrich, J.E.; Lipson, K.E.; Brandan, E. Denervation-Induced Skeletal Muscle Fibrosis Is Mediated by CTGF/CCN2 Independently of TGF-β. Matrix Biol. 2019, 82, 20–37. [Google Scholar] [CrossRef]
- Piasecki, A.; Leiva, O.; Ravid, K. Lysyl Oxidase Inhibition in Primary Myelofibrosis: A Renewed Strategy. Arch. Stem Cell Ther. 2020, 1, 23–27. [Google Scholar] [CrossRef]
- CDC. Arthritis. Centers for Disease Control and Prevention. 2021. Available online: https://www.cdc.gov/chronicdisease/resources/publications/factsheets/arthritis.htm (accessed on 2 January 2023).
- Donahue, R.P.; Link, J.M.; Meli, V.S.; Hu, J.C.; Liu, W.F.; Athanasiou, K.A. Stiffness- and Bioactive Factor-Mediated Protection of Self-Assembled Cartilage against Macrophage Challenge in a Novel Co-Culture System. Cartilage 2022, 13, 19476035221081466. [Google Scholar] [CrossRef]
- Friedemann, M.; Kalbitzer, L.; Franz, S.; Moeller, S.; Schnabelrauch, M.; Simon, J.C.; Pompe, T.; Franke, K. Instructing Human Macrophage Polarization by Stiffness and Glycosaminoglycan Functionalization in 3D Collagen Networks. Adv. Healthc. Mater. 2017, 6, 1600967. [Google Scholar] [CrossRef] [Green Version]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Previtera, M.L.; Sengupta, A. Substrate Stiffness Regulates Proinflammatory Mediator Production through TLR4 Activity in Macrophages. PLoS ONE 2015, 10, e0145813. [Google Scholar] [CrossRef]
- Blakney, A.K.; Swartzlander, M.D.; Bryant, S.J. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J. Biomed. Mater. Res. A 2012, 100, 1375–1386. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, M.; Nam, J. The Role of Changes in Extracellular Matrix of Cartilage in the Presence of Inflammation on the Pathology of Osteoarthritis. BioMed Res. Int. 2013, 2013, 284873. [Google Scholar] [CrossRef] [Green Version]
- Guilak, F.; Nims, R.J.; Dicks, A.; Wu, C.L.; Meulenbelt, I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 2018, 71–72, 40–50. [Google Scholar] [CrossRef]
- Verzijl, N.; DeGroot, J.; Zaken, C.B.; Braun-Benjamin, O.; Maroudas, A.; Bank, R.A.; Mizrahi, J.; Schalkwijk, C.G.; Thorpe, S.R.; Baynes, J.W.; et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: A possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002, 46, 114–123. [Google Scholar] [CrossRef]
- Nasiri, N.; Hosseini, S.; Alini, M.; Khademhosseini, A.; Baghaban Eslaminejad, M. Targeted cell delivery for articular cartilage regeneration and osteoarthritis treatment. Drug Discov. Today 2019, 24, 2212–2224. [Google Scholar] [CrossRef]
- Han, L.; Grodzinsky, A.J.; Ortiz, C. Nanomechanics of the Cartilage Extracellular Matrix. Annu. Rev. Mater. Res. 2011, 41, 133–168. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Peng, Z. Investigation of the nano-mechanical properties and surface topographies of wear particles and human knee cartilages. Wear 2015, 324–325, 74–79. [Google Scholar] [CrossRef]
- Okamoto, T.; Takagi, Y.; Kawamoto, E.; Park, E.J.; Usuda, H.; Wada, K.; Shimaoka, M. Reduced Substrate Stiffness Promotes M2-like Macrophage Activation and Enhances Peroxisome Proliferator-Activated Receptor γ Expression. Exp. Cell Res. 2018, 367, 264–273. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [Green Version]
- Akkiraju, H.; Nohe, A. Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Sun, H.; Bunpetch, V.; Koh, Y.; Wen, Y.; Wu, D.; Ouyang, H. The Regulation of Cartilage Extracellular Matrix Homeostasis in Joint Cartilage Degeneration and Regeneration. Biomaterials 2021, 268, 120555. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Wang, M.; Zhao, S.; Zhao, Z.; Fang, J. Mechanotransduction pathways in the regulation of cartilage chondrocyte homoeostasis. J. Cell. Mol. Med. 2020, 24, 5408–5419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.L.; Duan, L.; Zhu, W.; Xiong, J.; Wang, D. Extracellular matrix production in vitro in cartilage tissue engineering. J. Transl. Med. 2014, 12, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plainfossé, M.; Hatton, P.V.; Crawford, A.; Jin, Z.M.; Fisher, J. Influence of the extracellular matrix on the frictional properties of tissue-engineered cartilage. Biochem. Soc. Trans. 2007, 35, 677–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, G.; McNary, S.M.; Athanasiou, K.A.; Reddi, A.H. Superficial Zone Extracellular Matrix Extracts Enhance Boundary Lubrication of Self-Assembled Articular Cartilage. Cartilage 2016, 7, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmood, H.; Eckold, D.; Stead, I.; Shepherd, D.E.T.; Espino, D.M.; Dearn, K.D. A method for the assessment of the coefficient of friction of articular cartilage and a replacement biomaterial. J. Mech. Behav. Biomed. Mater. 2020, 103, 103580. [Google Scholar] [CrossRef]
- Nie, X.; Chuah, Y.J.; Zhu, W.; He, P.; Peck, Y.; Wang, D.A. Decellularized tissue engineered hyaline cartilage graft for articular cartilage repair. Biomaterials 2020, 235, 119821. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, J.; Guo, Q.; Huang, J.; Zhang, L.; Yao, J.; Yang, F.; Wang, S.; Xu, W.; Wang, A.; et al. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials 2008, 29, 2378–2387. [Google Scholar] [CrossRef]
- Hanai, H.; Jacob, G.; Nakagawa, S.; Tuan, R.S.; Nakamura, N.; Shimomura, K. Potential of Soluble Decellularized Extracellular Matrix for Musculoskeletal Tissue Engineering—Comparison of Various Mesenchymal Tissues. Front. Cell Dev. Biol. 2020, 8, 581972. Available online: https://www.frontiersin.org/articles/10.3389/fcell.2020.581972 (accessed on 21 January 2023). [CrossRef]
- Yang, Y.; Lin, H.; Shen, H.; Wang, B.; Lei, G.; Tuan, R.S. Mesenchymal stem cell-derived extracellular matrix enhances chondrogenic phenotype of and cartilage formation by encapsulated chondrocytes in vitro and in vivo. Acta Biomater. 2018, 69, 71–82. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Liu, S.; Feng, Z.; Wang, H.; Yang, D.; Guo, W.; Yuan, Z.; Gao, S.; Zhang, Y.; et al. Hierarchical macro-microporous WPU-ECM scaffolds combined with Microfracture Promote in Situ Articular Cartilage Regeneration in Rabbits. Bioact. Mater. 2021, 6, 1932–1944. [Google Scholar] [CrossRef]
- Rim, Y.A.; Ju, J.H. The Role of Fibrosis in Osteoarthritis Progression. Life 2020, 11, 3. [Google Scholar] [CrossRef]
- Islam, M.S.; Afrin, S.; Singh, B.; Jayes, F.L.; Brennan, J.T.; Borahay, M.A.; Leppert, P.C.; Segars, J.H. Extracellular matrix and Hippo signaling as therapeutic targets of antifibrotic compounds for uterine fibroids. Clin. Transl. Med. 2021, 11, e475. [Google Scholar] [CrossRef]
- Wang, C.; Qu, L. The anti-fibrotic agent nintedanib protects chondrocytes against tumor necrosis factor-α (TNF-α)-induced extracellular matrix degradation. Bioengineered 2022, 13, 5318–5329. [Google Scholar] [CrossRef]
- Liu, Y.; Shah, K.M.; Luo, J. Strategies for Articular Cartilage Repair and Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 770655. [Google Scholar] [CrossRef]
- Sutherland, A.J.; Beck, E.C.; Dennis, S.C.; Converse, G.L.; Hopkins, R.A.; Berkland, C.J.; Detamore, M.S. Decellularized Cartilage May Be a Chondroinductive Material for Osteochondral Tissue Engineering. PLoS ONE 2015, 10, e0121966. [Google Scholar] [CrossRef]
- Mohan, N.; Gupta, V.; Sridharan, B.; Sutherland, A.; Detamore, M.S. The potential of encapsulating “raw materials” in 3D osteochondral gradient scaffolds: Chondroitin Sulfate and Bioactive Glass as Raw Materials. Biotechnol. Bioeng. 2014, 111, 829–841. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Chen, S.; Pei, Y.A.; Pei, M. Impact of perlecan, a core component of basement membrane, on regeneration of cartilaginous tissues. Acta Biomater. 2021, 135, 13–26. [Google Scholar] [CrossRef]
- Han, Y.; Lian, M.; Zhang, C.; Jia, B.; Wu, Q.; Sun, B.; Qiao, Z.; Sun, B.; Dai, K. Study on bioactive PEGDA/ECM hybrid bi-layered hydrogel scaffolds fabricated by electro-writing for cartilage regeneration. Appl. Mater. Today 2022, 28, 101547. [Google Scholar] [CrossRef]
- Beketov, E.E.; Isaeva, E.V.; Yakovleva, N.D.; Demyashkin, G.A.; Arguchinskaya, N.V.; Kisel, A.A.; Lagoda, T.S.; Malakhov, E.P.; Kharlov, V.I.; Osidak, E.O.; et al. Bioprinting of Cartilage with Bioink Based on High-Concentration Collagen and Chondrocytes. Int. J. Mol. Sci. 2021, 22, 11351. [Google Scholar] [CrossRef]
- Govindharaj, M.; Hashimi, N.A.; Soman, S.S.; Kanwar, S.; Vijayavenkataraman, S. 3D Bioprinting of human Mesenchymal Stem Cells in a novel tunic decellularized ECM bioink for Cartilage Tissue Engineering. Materialia 2022, 23, 101457. [Google Scholar] [CrossRef]
- Screen, H.R.C.; Birk, D.E.; Kadler, K.E.; Ramirez, F.; Young, M.F. Tendon Functional Extracellular Matrix. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2015, 33, 793–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Murrell, G.A.C. The Basic Science of Tendinopathy. Clin. Orthop. 2008, 466, 1528–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorpe, C.T.; Birch, H.L.; Clegg, P.D.; Screen, H.R.C. The role of the non-collagenous matrix in tendon function. Int. J. Exp. Pathol. 2013, 94, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Screen, H.R.C.; Chhaya, V.H.; Greenwald, S.E.; Bader, D.L.; Lee, D.A.; Shelton, J.C. The influence of swelling and matrix degradation on the microstructural integrity of tendon. Acta Biomater. 2006, 2, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Midgley, A.C.; Bai, Y.; Zhu, M.; Chang, H.; Zhu, W.; Wang, L.; Wang, Y.; Wang, H.; Kong, D. Subcutaneously engineered autologous extracellular matrix scaffolds with aligned microchannels for enhanced tendon regeneration. Biomaterials 2019, 224, 119488. [Google Scholar] [CrossRef]
- Magnusson, S.P.; Langberg, H.; Kjaer, M. The pathogenesis of tendinopathy: Balancing the response to loading. Nat. Rev. Rheumatol. 2010, 6, 262–268. [Google Scholar] [CrossRef]
- Theodossiou, S.K.; Schiele, N.R. Models of tendon development and injury. BMC Biomed. Eng. 2019, 1, 1–24. [Google Scholar] [CrossRef]
- Theodossiou, S.K.; Pancheri, N.M.; Bozeman, A.L.; Brumley, M.R.; Raveling, A.R.; Schiele, N.R. Spinal Cord Transection Disrupts Neonatal Locomotion and Tendon Mechanical Properties. In Proceedings of the Biomedical Engineering Society (BMES) Annual Meeting (2019), Philadelphia, PA, USA, 11–14 October 2019. [Google Scholar]
- Chatterjee, M.; Muljadi, P.M.; Andarawis-Puri, N. The role of the tendon ECM in mechanotransduction: Disruption and repair following overuse. Connect. Tissue Res. 2022, 63, 28–42. [Google Scholar] [CrossRef]
- Subramanian, A.; Kanzaki, L.F.; Galloway, J.L.; Schilling, T.F. Mechanical force regulates tendon extracellular matrix organization and tenocyte morphogenesis through TGFbeta signaling. eLife 2018, 7, e38069. [Google Scholar] [CrossRef]
- Birk, D.E.; Trelstad, R.L. Extracellular compartments in tendon morphogenesis: Collagen fibril, bundle, and macroaggregate formation. J. Cell Biol. 1986, 103, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.E. Elasticity in extracellular matrix “shape modules” of tendon, cartilage, etc. A sliding proteoglycan-filament model. J. Physiol. 2003, 553 Pt 2, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Samiric, T.; Ilic, M.Z.; Handley, C.J. Characterisation of proteoglycans and their catabolic products in tendon and explant cultures of tendon. Matrix Biol. 2004, 23, 127–140. [Google Scholar] [CrossRef]
- Fessel, G.; Snedeker, J.G. Evidence against proteoglycan mediated collagen fibril load transmission and dynamic viscoelasticity in tendon. Matrix Biol. 2009, 28, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Birch, H.L.; Thorpe, C.T.; Rumian, A.P. Specialisation of extracellular matrix for function in tendons and ligaments. Muscles Ligaments Tendons J. 2013, 3, 12–22. [Google Scholar] [CrossRef]
- Theodossiou, S.K.; Tokle, J.; Schiele, N.R. TGFβ2-induced tenogenesis impacts cadherin and connexin cell-cell junction proteins in mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2019, 508, 889–893. [Google Scholar] [CrossRef]
- Rao, Y.; Zhu, C.; Suen, H.C.; Huang, S.; Liao, J.; Ker, D.F.E.; Tuan, R.S.; Wang, D. Tenogenic induction of human adipose-derived stem cells by soluble tendon extracellular matrix: Composition and transcriptomic analyses. Stem Cell Res. Ther. 2022, 13, 380. [Google Scholar] [CrossRef]
- Kjaer, M.; Magnusson, P.; Krogsgaard, M.; Boysen Møller, J.; Olesen, J.; Heinemeier, K.; Hansen, M.; Haraldsson, B.; Koskinen, S.; Esmarck, B.; et al. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J. Anat. 2006, 208, 445–450. [Google Scholar] [CrossRef]
- Kiyotake, E.A.; Cheng, M.E.; Thomas, E.E.; Detamore, M.S. The Rheology and Printability of Cartilage Matrix-Only Biomaterials. Biomolecules 2022, 12, 846. [Google Scholar] [CrossRef]
- Hauptstein, J.; Forster, L.; Nadernezhad, A.; Groll, J.; Teßmar, J.; Blunk, T. Tethered TGF-Β1 in a Hyaluronic Acid-Based Bioink for Bioprinting Cartilaginous Tissues. Int. J. Mol. Sci. 2022, 23, 924. [Google Scholar] [CrossRef]
- Behan, K.; Dufour, A.; Garcia, O.; Kelly, D. Methacrylated Cartilage ECM-Based Hydrogels as Injectables and Bioinks for Cartilage Tissue Engineering. Biomolecules 2022, 12, 216. [Google Scholar] [CrossRef]
- Lien, S.-M.; Ko, L.-Y.; Huang, T.-J. Effect of Pore Size on ECM Secretion and Cell Growth in Gelatin Scaffold for Articular Cartilage Tissue Engineering. Acta Biomater. 2009, 5, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Liang, F.; He, J.; Ye, W.; Javed, R.; Wang, W.; Yu, T.; Fan, J.; Tian, X.; Wang, X.; et al. Decellularized tendon matrix membranes prevent post-surgical tendon adhesion and promote functional repair. Acta Biomater. 2021, 134, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bai, J.; Yu, K.; Liu, G.; Tian, S.; Tian, D. Biological Amnion Prevents Flexor Tendon Adhesion in Zone II: A Controlled, Multicentre Clinical Trial. BioMed Res. Int. 2019, 2019, 2354325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.J.; Ryoo, H.J.; Shim, H.S. Prevention of postoperative adhesions after flexor tendon repair with acellular dermal matrix in Zones III, IV, and V of the hand: A randomized controlled (CONSORT-compliant) trial. Medicine 2022, 101, e28630. [Google Scholar] [CrossRef]
- Shim, H.S.; Park, K.S.; Kim, S.W. Preventing postoperative adhesions after hand tendon repair using acellular dermal matrix. J. Wound Care 2021, 30, 890–895. [Google Scholar] [CrossRef]
- Eisner, L.E.; Rosario, R.; Andarawis-Puri, N.; Arruda, E.M. The Role of the Non-Collagenous Extracellular Matrix in Tendon and Ligament Mechanical Behavior: A Review. J. Biomech. Eng. 2022, 144, 050801. [Google Scholar] [CrossRef]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, J.; Zhou, Y.; Thampatty, B.P.; Wang, J.H.C. Tendon Stem/Progenitor Cells and Their Interactions with Extracellular Matrix and Mechanical Loading. Stem Cells Int. 2019, 2019, 3674647. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Yang, X.; He, C.; Chen, Y.; Li, C.; Li, S.; Chen, Y.; Wu, Y.; Xiang, Z.; Kang, J.; et al. Rejuvenation of tendon stem/progenitor cells for functional tendon regeneration through platelet-derived exosomes loaded with recombinant Yap1. Acta Biomater. 2023, 161, 80–99. [Google Scholar] [CrossRef]
- Regard, J.B.; Zhong, Z.; Williams, B.O.; Yang, Y. Wnt Signaling in Bone Development and Disease: Making Stronger Bone with Wnts. Cold Spring Harb. Perspect. Biol. 2012, 4, a007997. [Google Scholar] [CrossRef] [Green Version]
- Schaffler, M.B.; Kennedy, O.D. Osteocyte Signaling in Bone. Curr. Osteoporos Rep. 2012, 10, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.h.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. 2021, 16, 609. [Google Scholar] [CrossRef]
- Conti, V.; Russomanno, G.; Corbi, G.; Toro, G.; Simeon, V.; Filippelli, W.; Ferrara, N.; Grimaldi, M.; D’Argenio, V.; Maffulli, N.; et al. A Polymorphism at the Translation Start Site of the Vitamin D Receptor Gene Is Associated with the Response to Anti-Osteoporotic Therapy in Postmenopausal Women from Southern Italy. Int. J. Mol. Sci. 2015, 16, 5452–5466. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Zhang, Y.; Lu, D.; Dong, F.; Lian, Y. ECM-receptor interaction as a prognostic indicator for clinical outcome of primary osteoporosis. Int. J. Clin. Exp. Med. 2016, 9, 9–20. [Google Scholar]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef]
- Freeman, F.E.; Browe, D.C.; Nulty, J.; Von Euw, S.; Grayson, W.L.; Kelly, D.J. Biofabrication of multiscale bone extracellular matrix scaffolds for bone tissue engineering. Eur. Cell Mater. 2019, 38, 168–187. [Google Scholar] [CrossRef]
- Li, W.; Xu, R.; Huang, J.; Bao, X.; Zhao, B. Treatment of rabbit growth plate injuries with oriented ECM scaffold and autologous BMSCs. Sci Rep. 2017, 7, 44140. [Google Scholar] [CrossRef] [Green Version]
- Research Progress Related to Growth Plate Injuries. Growth Plate Injuries; National Institute of Arthritis and Musculoskeletal and Skin Diseases: Bethesda, MD, USA, 2017. Available online: https://www.niams.nih.gov/health-topics/growth-plate-injuries (accessed on 20 January 2023).
- Sun, T.; Meng, C.; Ding, Q.; Yu, K.; Zhang, X.; Zhang, W.; Tian, W.; Zhang, Q.; Guo, X.; Wu, B.; et al. In situ bone regeneration with sequential delivery of aptamer and BMP2 from an ECM-based scaffold fabricated by cryogenic free-form extrusion. Bioact. Mater. 2021, 6, 4163–4175. [Google Scholar] [CrossRef]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lee, J.-K.; Jung, J.-W.; Baek, S.-W.; Kim, J.H.; Heo, Y.; Kim, T.-H.; Han, D.K. Promotion of Bone Regeneration Using Bioinspired PLGA/MH/ECM Scaffold Combined with Bioactive PDRN. Materials 2021, 14, 4149. [Google Scholar] [CrossRef]
- Ventura, R.D.; Padalhin, A.R.; Lee, B.T. Functionalization of extracellular matrix (ECM) on multichannel biphasic calcium phosphate (BCP) granules for improved bone regeneration. Mater. Des. 2020, 192, 108653. [Google Scholar] [CrossRef]
- Park, Y.; Cheong, E.; Kwak, J.G.; Carpenter, R.; Shim, J.H.; Lee, J. Trabecular bone organoid model for studying the regulation of localized bone remodeling. Sci. Adv. 2021, 7, eabd6495. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Berardinelli, S.J.; Cameron, D.C.; Grady, R.C.; Komatsu, D.E.; Percival, C.J.; Takeuchi, M.; Ito, A.; Liu, T.-W.; Nairn, A.V.; et al. O-fucosylation of thrombospondin type 1 repeats is essential for ECM remodeling and signaling during bone development. Matrix Biol. 2022, 107, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yue, M.; Liu, X.; Liu, Y.; Lv, L.; Zhang, P.; Zhou, Y. The PCK2-glycolysis axis assists three-dimensional-stiffness maintaining stem cell osteogenesis. Bioact. Mater. 2022, 18, 492–506. [Google Scholar] [CrossRef]
- Huber, A.K.; Patel, N.; Pagani, C.A.; Marini, S.; Padmanabhan, K.R.; Matera, D.L.; Said, M.; Hwang, C.; Hsu, G.C.-Y.; Poli, A.A.; et al. Immobilization after injury alters extracellular matrix and stem cell fate. J. Clin. Investig. 2020, 130, 5444–5460. [Google Scholar] [CrossRef]
- Barruet, E.; Garcia, S.M.; Wu, J.; Morales, B.M.; Tamaki, S.; Moody, T.; Pomerantz, J.H.; Hsiao, E.C. Modeling the ACVR1R206H mutation in human skeletal muscle stem cells. eLife 2021, 10, e66107. [Google Scholar] [CrossRef]
- Wang, B.; Ke, W.; Wang, K.; Li, G.; Ma, L.; Lu, S.; Xiang, Q.; Liao, Z.; Luo, R.; Song, Y.; et al. Mechanosensitive Ion Channel Piezo1 Activated by Matrix Stiffness Regulates Oxidative Stress-Induced Senescence and Apoptosis in Human Intervertebral Disc Degeneration. Oxidative Med. Cell. Longev. 2021, 2021, 8884922. [Google Scholar] [CrossRef]
- Tan, G.; Chen, R.; Tu, X.; Guo, L.; Guo, L.; Xu, J.; Zhang, C.; Zou, T.; Sun, S.; Jiang, Q. Research on the osteogenesis and biosafety of ECM–Loaded 3D–Printed Gel/SA/58sBG scaffolds. Front. Bioeng. Biotechnol. 2022, 10, 973886. [Google Scholar] [CrossRef]
- Padhi, A.; Nain, A.S. ECM in Differentiation: A Review of Matrix Structure, Composition and Mechanical Properties. Ann. Biomed. Eng. 2020, 48, 1071–1089. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Guo, S.S.; Fässler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Young, J.L.; Holle, A.W.; Spatz, J.P. Nanoscale and mechanical properties of the physiological cell–ECM microenvironment. Exp. Cell Res. 2016, 343, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhes. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Feltz, K.P.; Kalaf, E.A.G.; Chen, C.; Martin, R.S.; Sell, S.A. A review of electrospinning manipulation techniques to direct fiber deposition and maximize pore size. Electrospinning 2017, 1, 46–61. [Google Scholar] [CrossRef]
- Variola, F. Atomic force microscopy in biomaterials surface science. Phys. Chem. Chem. Phys. 2015, 17, 2950–2959. [Google Scholar] [CrossRef] [Green Version]
- Norman, M.D.A.; Ferreira, S.A.; Jowett, G.M.; Bozec, L.; Gentleman, E. Measuring the elastic modulus of soft culture surfaces and three-dimensional hydrogels using atomic force microscopy. Nat. Protoc. 2021, 16, 2418–2449. [Google Scholar] [CrossRef]
- Jowett, G.M.; Norman, M.D.A.; Yu, T.T.L.; Rosell Arévalo, P.; Hoogland, D.; Lust, S.T.; Read, E.; Hamrud, E.; Walters, N.J.; Niazi, U.; et al. ILC1 drive intestinal epithelial and matrix remodelling. Nat. Mater. 2021, 20, 250–259. [Google Scholar] [CrossRef]
- Grad, S.; Loparic, M.; Peter, R.; Stolz, M.; Aebi, U.; Alini, M. Sliding motion modulates stiffness and friction coefficient at the surface of tissue engineered cartilage. Osteoarthr. Cartil. 2012, 20, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Davis-Hall, D.; Thomas, E.E.; Peña, B.; Magin, C.M. 3D-bioprinted, phototunable hydrogel models for studying adventitial fibroblast activation in pulmonary arterial hypertension. Biofabrication 2022, 15, 015017. Available online: http://iopscience.iop.org/article/10.1088/1758-5090/aca8cf (accessed on 16 December 2022).
- Park, J.H.; Gillispie, G.J.; Copus, J.S.; Zhang, W.; Atala, A.; Yoo, J.J.; Yelick, P.C.; Lee, S.J. The Effect of BMP-Mimetic Peptide Tethering Bioinks on the Differentiation of Dental Pulp Stem Cells (DPSCs) in 3D Bioprinted Dental Constructs. Biofabrication 2020, 12, 035029. [Google Scholar] [CrossRef]
- Valentin, J.E.; Stewart-Akers, A.M.; Gilbert, T.W.; Badylak, S.F. Macrophage Participation in the Degradation and Remodeling of Extracellular Matrix Scaffolds. Tissue Eng. Part A 2009, 15, 1687–1694. [Google Scholar] [CrossRef] [Green Version]
- Freeberg, M.A.T.; Perelas, A.; Rebman, J.K.; Phipps, R.P.; Thatcher, T.H.; Sime, P.J. Mechanical Feed-Forward Loops Contribute to Idiopathic Pulmonary Fibrosis. Am. J. Pathol. 2021, 191, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.E.C.; Best, K.T.; Loiselle, A.E. The Cellular Basis of Fibrotic Tendon Healing: Challenges and Opportunities. Transl. Res. 2019, 209, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.J.; Horejs, C.-M.; Stevens, M.M. Scarring vs. Functional Repair: Matrix-Based Strategies to Regulate Tissue Healing. Adv. Drug Deliv. Rev. 2018, 129, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.C.; Bodnar, R.; Wells, A. Matrix Control of Scarring. Cell Mol. Life Sci. 2011, 68, 1871–1881. [Google Scholar] [CrossRef] [Green Version]
- Loomis, T.; Hu, L.-Y.; Wohlgemuth, R.P.; Chellakudam, R.R.; Muralidharan, P.D.; Smith, L.R. Matrix Stiffness and Architecture Drive Fibro-Adipogenic Progenitors’ Activation into Myofibroblasts. Sci. Rep. 2022, 12, 13582. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, C.L.; Penney, B.T.; Theodossiou, S.K. Engineering Cell–ECM–Material Interactions for Musculoskeletal Regeneration. Bioengineering 2023, 10, 453. https://doi.org/10.3390/bioengineering10040453
Jones CL, Penney BT, Theodossiou SK. Engineering Cell–ECM–Material Interactions for Musculoskeletal Regeneration. Bioengineering. 2023; 10(4):453. https://doi.org/10.3390/bioengineering10040453
Chicago/Turabian StyleJones, Calvin L., Brian T. Penney, and Sophia K. Theodossiou. 2023. "Engineering Cell–ECM–Material Interactions for Musculoskeletal Regeneration" Bioengineering 10, no. 4: 453. https://doi.org/10.3390/bioengineering10040453