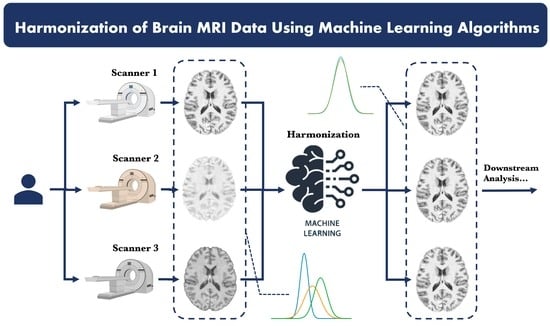
Machine Learning for Brain MRI Data Harmonisation: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria and Search Terms
2.2. Screening and Selection Process
2.3. Exclusion Criteria
2.4. Data Extraction
2.5. Quality Assessment
3. Results
3.1. Article Distribution among Journals and Conference Proceedings
3.2. Application of ML Methods
3.3. MRI Modality and Harmonised Features
3.4. Evaluation of Methodology
3.5. Data Characteristics and Reproducibility
4. Discussion
4.1. ML-Based Methods for Harmonisation
4.2. Dataset Used for Harmonisation
4.3. Data Representation for Model Training
4.4. Performance Evaluation for Harmonisation Models
4.5. Comparative Analysis
4.6. Toward Clinical Application of AI
4.7. Recommendation for Future Studies
- Understanding the purpose of harmonisation. Use explicit approaches for harmonising intensity values and image-derived metrics or correcting known sources, whereas using implicit approaches to improve the performance of a downstream task or to correct unknown sources of variability.
- Consider the nature and properties of the dataset, including the size, dimensionality, and variability in the data. Generally, the more extensive and diverse the dataset, the more complex the ML model can be. For example, when using a travelling-subject dataset for training, researchers may consider using simpler ML models to reduce the risks of overfitting.
- When defining the target and reference domains, ensure that there is enough training data in each domain so that the model can learn the relevant information.
- Conduct experiments using different ML approaches, varying feature extraction techniques, and adjusting hyperparameters. Evaluate the performance using the consistent metrics and interpret the results of image analysis carefully.
- Consider the trade-off between accuracy and interpretability when choosing an ML method. ML methods, such as GANs, are more complex, so although they may produce highly accurate results, they can be difficult to interpret. In contrast, other methods, such as regression models, may be more interpretable despite giving less accurate results.
4.8. Limitation of Systematic Review
5. Conclusions
- -
- There is a large amount of diverse imaging data (sMRI, dMRI, fMRI, DTI, longitudinal, static etc.) related to the same problem, but collected at different sites under different conditions, that need to be harmonised.
- -
- New methods for neuroimage data harmonisation are needed for better results.
- -
- Following harmonisation, methods for the integration of the multimodal harmonised data are needed for the development of better (1) personalised, (2) predictive and (3) explainable computational models.
- -
- The use of harmonisation as a strategy for improving downstream tasks is recommended; however, consistent evaluation methods are needed.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oba, H.; Yagishita, A.; Terada, H.; Barkovich, A.J.; Kutomi, K.; Yamauchi, T.; Furui, S.; Shimizu, T.; Uchigata, M.; Matsumura, K.; et al. New and reliable MRI diagnosis for progressive supranuclear palsy. Neurology 2005, 64, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Traboulsee, A.L.; Li, D.K.B. The role of MRI in the diagnosis of multiple sclerosis. Adv. Neurol. 2006, 98, 125–146. [Google Scholar] [PubMed]
- Yu-Feng, Z.; Yong, H.; Chao-Zhe, Z.; Qing-Jiu, C.; Man-Qiu, S.; Meng, L.; Li-Xia, T.; Tian-Zi, J. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007, 29, 83–91. [Google Scholar] [CrossRef]
- Swanton, J.K.; Rovira, A.; Tintore, M.; Altmann, D.R.; Barkhof, F.; Filippi, M.; Huerga, E.; Miszkiel, K.A.; Plant, G.T.; Polman, C.; et al. MRI criteria for multiple sclerosis in patients presenting with clinically isolated syndromes: A multicentre retrospective study. Lancet Neurol. 2007, 6, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.A.; Zielinski, B.A.; Fletcher, P.T.; Alexander, A.L.; Lange, N.; Bigler, E.D.; Lainhart, J.E.; Anderson, J.S. Multisite functional connectivity MRI classification of autism: ABIDE results. Front. Hum. Neurosci. 2013, 7, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hargreaves, B. Rapid gradient-echo imaging. J. Magn. Reson. Imaging 2012, 36, 1300–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, R.; Sakaie, K.; Lee, J.-C.; Debbins, J.; Liu, Y.; Arnold, D.; Melhem, E.; Smith, C.; Philips, M.; Lowe, M.; et al. A Validation Study of Multicenter Diffusion Tensor Imaging: Reliability of Fractional Anisotropy and Diffusivity Values. Am. J. Neuroradiol. 2011, 33, 695–700. [Google Scholar] [CrossRef] [Green Version]
- Seo, Y.; Wang, Z.J.; Morriss, M.C.; Rollins, N.K. Minimum SNR and acquisition for bias-free estimation of fractional anisotropy in diffusion tensor imaging—A comparison of two analytical techniques and field strengths. Magn. Reson. Imaging 2012, 30, 1123–1133. [Google Scholar] [CrossRef]
- Milidonis, X.; Lennen, R.J.; Jansen, M.A.; Mueller, S.; Boehm-Sturm, P.; Holmes, W.M.; Sena, E.S.; Macleod, M.R.; Marshall, I. Multicenter Evaluation of Geometric Accuracy of MRI Protocols Used in Experimental Stroke. PLoS ONE 2016, 11, e0162545. [Google Scholar] [CrossRef] [Green Version]
- Palacios, E.; Martin, A.; Boss, M.; Ezekiel, F.; Chang, Y.; Yuh, E.; Vassar, M.; Schnyer, D.; MacDonald, C.; Crawford, K.; et al. Toward Precision and Reproducibility of Diffusion Tensor Imaging: A Multicenter Diffusion Phantom and Traveling Volunteer Study. Am. J. Neuroradiol. 2016, 38, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, D.L.; Tagge, I.; Powers, K.; Ahn, S.; Bakshi, R.; Calabresi, P.A.; Constable, R.T.; Grinstead, J.; Henry, R.G.; Nair, G.; et al. Multisite reliability and repeatability of an advanced brain MRI protocol. J. Magn. Reson. Imaging 2019, 50, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Mårtensson, G.; Ferreira, D.; Granberg, T.; Cavallin, L.; Oppedal, K.; Padovani, A.; Rektorova, I.; Bonanni, L.; Pardini, M.; Kramberger, M.G.; et al. The reliability of a deep learning model in clinical out-of-distribution MRI data: A multicohort study. Med. Image Anal. 2020, 66, 101714. [Google Scholar] [CrossRef] [PubMed]
- Knoll, F.; Hammernik, K.; Kobler, E.; Pock, T.; Recht, M.P.; Sodickson, D. Assessment of the generalization of learned image reconstruction and the potential for transfer learning. Magn. Reson. Med. 2019, 81, 116–128. [Google Scholar] [CrossRef]
- Clarke, W.; Mougin, O.; Driver, I.D.; Rua, C.; Morgan, A.; Asghar, M.; Clare, S.; Francis, S.; Wise, R.G.; Rodgers, C.T.; et al. Multi-site harmonization of 7 tesla MRI neuroimaging protocols. Neuroimage 2019, 206, 116335. [Google Scholar] [CrossRef] [PubMed]
- Jovicich, J.; Marizzoni, M.; Bosch, B.; Bartrés-Faz, D.; Arnold, J.; Benninghoff, J.; Wiltfang, J.; Roccatagliata, L.; Picco, A.; Nobili, F.; et al. Multisite longitudinal reliability of tract-based spatial statistics in diffusion tensor imaging of healthy elderly subjects. Neuroimage 2014, 101, 390–403. [Google Scholar] [CrossRef]
- Shinohara, R.; Oh, J.; Nair, G.; Calabresi, P.; Davatzikos, C.; Doshi, J.; Henry, R.; Kim, G.; Linn, K.; Papinutto, N.; et al. Volumetric Analysis from a Harmonized Multisite Brain MRI Study of a Single Subject with Multiple Sclerosis. Am. J. Neuroradiol. 2017, 38, 1501–1509. [Google Scholar] [CrossRef] [Green Version]
- Biberacher, V.; Schmidt, P.; Keshavan, A.; Boucard, C.C.; Righart, R.; Sämann, P.; Preibisch, C.; Fröbel, D.; Aly, L.; Hemmer, B.; et al. Intra-and interscanner variability of magnetic resonance imaging based volumetry in multiple sclerosis. Neuroimage 2016, 142, 188–197. [Google Scholar] [CrossRef]
- Pinto, M.S.; Paolella, R.; Billiet, T.; Van Dyck, P.; Guns, P.-J.; Jeurissen, B.; Ribbens, A.; Dekker, A.J.D.; Sijbers, J. Harmonization of Brain Diffusion MRI: Concepts and Methods. Front. Neurosci. 2020, 14, 396. [Google Scholar] [CrossRef]
- Mali, S.A.; Ibrahim, A.; Woodruff, H.C.; Andrearczyk, V.; Müller, H.; Primakov, S.; Salahuddin, Z.; Chatterjee, A.; Lambin, P. Making Radiomics More Reproducible across Scanner and Imaging Protocol Variations: A Review of Harmonization Methods. J. Pers. Med. 2021, 11, 842. [Google Scholar] [CrossRef]
- Stamoulou, E.; Spanakis, C.; Manikis, G.C.; Karanasiou, G.; Grigoriadis, G.; Foukakis, T.; Tsiknakis, M.; Fotiadis, D.I.; Marias, K. Harmonization Strategies in Multicenter MRI-Based Radiomics. J. Imaging 2022, 8, 303. [Google Scholar] [CrossRef]
- Kmet, L.M.; Cook, L.S.; Lee, R.C. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields; Alberta Heritage Foundation for Medical Research: Edmonton, AB, Canada, 2004. [Google Scholar] [CrossRef]
- Wen, J.; Li, S.; Lin, Z.; Hu, Y.; Huang, C. Systematic literature review of machine learning based software development effort estimation models. Inf. Softw. Technol. 2012, 54, 41–59. [Google Scholar] [CrossRef]
- Ho, T.K. Random Decision Forests. In Proceedings of the 3rd International Conference on Document Analysis and Recognition, Montreal, QC, Canada, 14–16 August 1995. [Google Scholar] [CrossRef]
- Fortin, J.-P.; Cullen, N.; Sheline, Y.I.; Taylor, W.D.; Aselcioglu, T.; Cook, P.A.; Adams, P.; Cooper, C.; Fava, M.; McGrath, P.J.; et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage 2018, 167, pp. 104–120. [Google Scholar] [CrossRef]
- Garcia-Dias, R.; Scarpazza, C.; Baecker, L.; Vieira, S.; Pinaya, W.H.; Corvin, A.; Redolfi, A.; Nelson, B.; Crespo-Facorro, B.; McDonald, C.; et al. Neuroharmony: A new tool for harmonizing volumetric MRI data from unseen scanners. Neuroimage 2020, 220, 117127. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, L.T.; Zhang, Q.; Armstrong, D.; Deen, M.J. Convolutional neural networks for medical image analysis: State-of-the-art, comparisons, improvement and perspectives. Neurocomputing 2021, 444, 92–110. [Google Scholar] [CrossRef]
- Dewey, B.E.; Zhao, C.; Reinhold, J.C.; Carass, A.; Fitzgerald, K.C.; Sotirchos, E.S.; Saidha, S.; Oh, J.; Pham, D.L.; Calabresi, P.A.; et al. DeepHarmony: A deep learning approach to contrast harmonization across scanner changes. Magn. Reson. Imaging 2019, 64, 160–170. [Google Scholar] [CrossRef]
- Tong, Q.; Gong, T.; He, H.; Wang, Z.; Yu, W.; Zhang, J.; Zhai, L.; Cui, H.; Meng, X.; Tax, C.W.; et al. A deep learning–based method for improving reliability of multicenter diffusion kurtosis imaging with varied acquisition protocols. Magn. Reson. Imaging 2020, 73, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Koppers, S.; Bloy, L.; Berman, J.I.; Tax, C.M.W.; Edgar, J.C.; Merhof, D. Spherical Harmonic Residual Network for Diffusion Signal Harmonization. In Proceedings of the International MICCAI Workshop, Granada, Spain, 20 September 2018; pp. 173–182. [Google Scholar] [CrossRef] [Green Version]
- Kreutz-Delgado, K.; Murray, J.F.; Rao, B.D.; Engan, K.; Lee, T.-W.; Sejnowski, T.J. Dictionary Learning Algorithms for Sparse Representation. Neural Comput. 2003, 15, 349–396. [Google Scholar] [CrossRef] [Green Version]
- St-Jean, S.; Viergever, M.A.; Leemans, A. Harmonization of diffusion MRI data sets with adaptive dictionary learning. Hum. Brain Mapp. 2020, 41, 4478–4499. [Google Scholar] [CrossRef]
- He, K.; Gan, C.; Li, Z.; Rekik, I.; Yin, Z.; Ji, W.; Gao, Y.; Wang, Q.; Zhang, J.; Shen, D. Transformers in medical image analysis. Intell. Med. 2022, 3, 59–78. [Google Scholar] [CrossRef]
- Robinson, R.; Dou, Q.; de Castro, D.C.; Kamnitsas, K.; de Groot, M.; Summers, R.M.; Rueckert, D.; Glocker, B. Image-Level Harmonization of Multi-site Data Using Image-and-Spatial Transformer Networks. In Proceedings of the Medical Image Computing and Computer Assisted Intervention—MICCAI 2020 23rd International Conference, Lima, Peru, 4–8 October 2020; pp. 710–719. [Google Scholar] [CrossRef]
- Goodfellow, I.; Pouget-Abadie, J.; Mirza, M.; Xu, B.; Warde-Farley, D.; Ozair, S.; Courville, A.; Bengio, Y. Generative Adversarial Networks. Commun. ACM 2014, 63, 139–144. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Wang, Y.; Shi, Z.; Yu, J. A Universal Intensity Standardization Method Based on a Many-to-One Weak-Paired Cycle Generative Adversarial Network for Magnetic Resonance Images. IEEE Trans. Med. Imaging 2019, 38, 2059–2069. [Google Scholar] [CrossRef]
- Zhao, F.; Wu, Z.; Wang, L.; Lin, W.; Xia, S.; Shen, D.; Li, G.; The UNC/UMN Baby Connectome Project Consortium. Harmonization of Infant Cortical Thickness Using Surface-to-Surface Cycle-Consistent Adversarial Networks. In Proceedings of the Medical Image Computing and Computer Assisted Intervention—MICCAI 2019 22nd International Conference, Shenzhen, China, 13–17 October 2019; Volume 11767, pp. 475–483. [Google Scholar] [CrossRef]
- Ren, M.; Dey, N.; Fishbaugh, J.; Gerig, G. Segmentation-Renormalized Deep Feature Modulation for Unpaired Image Harmonization. IEEE Trans. Med. Imaging 2021, 40, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Maiti, P.; Thomopoulos, S.; Zhu, A.; Chai, Y.; Kim, H.; Jahanshad, N. Style Transfer Using Generative Adversarial Networks for Multi-site MRI Harmonization. In Proceedings of the Medical Image Computing and Computer Assisted Intervention—MICCAI 2021 24th International Conference, Strasbourg, France, 27 September–1 October 2021; pp. 313–322. [Google Scholar] [CrossRef]
- Weninger, L.; Ahmad, M.; Merhof, D. From Supervised to Unsupervised Harmonization of Diffusion Mri Acquisitions. In Proceedings of the 2022 IEEE 19th International Symposium on Biomedical Imaging (ISBI), Kolkata, India, 28–31 March 2022; pp. 1–5. [Google Scholar] [CrossRef]
- Arai, H.; Onga, Y.; Ikuta, K.; Chayama, Y.; Iyatomi, H.; Oishi, K. Disease-Oriented Image Embedding With Pseudo-Scanner Standardization for Content-Based Image Retrieval on 3D Brain MRI. IEEE Access 2021, 9, 165326–165340. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, Y.; Li, J.; Xue, X.; Liu, S.; Wang, M.; Gao, X.; Wang, Q.; Yang, J.; Li, X. Inter-site harmonization based on dual generative adversarial networks for diffusion tensor imaging: Application to neonatal white matter development. Biomed. Eng. Online 2020, 19, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingma, D.P.; Welling, M. Auto-Encoding Variational Bayes. In Proceedings of the 2nd International Conference on Learning Representations, ICLR 2014, Banff, AB, Canada, 14–16 April 2014. [Google Scholar] [CrossRef]
- Dewey, B.E.; Zuo, L.; Carass, A.; He, Y.; Liu, Y.; Mowry, E.M.; Newsome, S.; Oh, J.; Calabresi, P.A.; Prince, J.L. A Disentangled Latent Space for Cross-Site MRI Harmonization. In Proceedings of the Medical Image Computing and Computer Assisted Intervention—MICCAI 2020 23rd International Conference, Lima, Peru, 4–8 October 2020; pp. 720–729. [Google Scholar] [CrossRef]
- Zuo, L.; Dewey, B.E.; Liu, Y.; He, Y.; Newsome, S.D.; Mowry, E.M.; Resnick, S.M.; Prince, J.L.; Carass, A. Unsupervised MR harmonization by learning disentangled representations using information bottleneck theory. Neuroimage 2021, 243, 118569. [Google Scholar] [CrossRef] [PubMed]
- Torbati, M.E.; Tudorascu, D.L.; Minhas, D.S.; Maillard, P.; DeCarli, C.S.; Hwang, S.J. Multi-scanner Harmonization of Paired Neuroimaging Data via Structure Preserving Embedding Learning. In Proceedings of the 2021 IEEE/CVF International Conference on Computer Vision Workshops (ICCVW), Montreal, BC, Canada, 11–17 October 2021; pp. 3277–3286. [Google Scholar] [CrossRef]
- Tian, D.; Zeng, Z.; Sun, X.; Tong, Q.; Li, H.; He, H.; Gao, J.-H.; He, Y.; Xia, M. A deep learning-based multisite neuroimage harmonization framework established with a traveling-subject dataset. Neuroimage 2022, 257, 119297. [Google Scholar] [CrossRef] [PubMed]
- Fatania, K.; Clark, A.; Frood, R.; Scarsbrook, A.; Al-Qaisieh, B.; Currie, S.; Nix, M. Harmonisation of scanner-dependent contrast variations in magnetic resonance imaging for radiation oncology, using style-blind auto-encoders. Phys. Imaging Radiat. Oncol. 2022, 22, 115–122. [Google Scholar] [CrossRef]
- Moyer, D.; Steeg, G.V.; Tax, C.M.W.; Thompson, P.M. Scanner invariant representations for diffusion MRI harmonization. Magn. Reson. Med. 2020, 84, 2174–2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, L.; Dewey, B.E.; Carass, A.; Liu, Y.; He, Y.; Calabresi, P.A.; Prince, J.L. Information-Based Disentangled Representation Learning for Unsupervised MR Harmonization. In Proceedings of the Information Processing in Medical Imaging 27th International Conference, IPMI 2021, Virtual, 28–30 June 2021; pp. 346–359. [Google Scholar] [CrossRef]
- Tzeng, E.; Hoffman, J.; Saenko, K.; Darrell, T. Adversarial Discriminative Domain Adaptation. In Proceedings of the 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017; pp. 2962–2971. [Google Scholar]
- Guan, H.; Liu, Y.; Yang, E.; Yap, P.-T.; Shen, D.; Liu, M. Multi-site MRI harmonization via attention-guided deep domain adaptation for brain disorder identification. Med. Image Anal. 2021, 71, 102076. [Google Scholar] [CrossRef]
- Dinsdale, N.K.; Jenkinson, M.; Namburete, A.I. Deep learning-based unlearning of dataset bias for MRI harmonisation and confound removal. Neuroimage 2020, 228, 117689. [Google Scholar] [CrossRef] [PubMed]
- Orbes-Arteaga, M.; Varsavsky, T.; Sudre, C.H.; Eaton-Rosen, Z.; Haddow, L.J.; Sørensen, L.; Nielsen, M.; Pai, A.; Ourselin, S.; Modat, M.; et al. Multi-domain Adaptation in Brain MRI Through Paired Consistency and Adversarial Learning. In Domain Adaptation and Representation Transfer and Medical Image Learning with Less Labels and Imperfect Data; Springer: Cham, Switzerland, 2019; pp. 54–62. [Google Scholar] [CrossRef] [Green Version]
- Ackaouy, A.; Courty, N.; Vallée, E.; Commowick, O.; Barillot, C.; Galassi, F. Unsupervised Domain Adaptation With Optimal Transport in Multi-Site Segmentation of Multiple Sclerosis Lesions From MRI Data. Front. Comput. Neurosci. 2020, 14, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Liu, M.; Pan, Y.; Shen, D. Unsupervised Conditional Consensus Adversarial Network for Brain Disease Identification with Structural MRI. In Proceedings of the 10th International Workshop, MLMI 2019, Shenzhen, China, 13 October 2019; pp. 391–399. [Google Scholar] [CrossRef]
- Delisle, P.-L.; Anctil-Robitaille, B.; Desrosiers, C.; Lombaert, H. Realistic image normalization for multi-domain segmentation. Med. Image Anal. 2021, 74, 102191. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Hsieh, W.-T.; Yang, H.-C.; Lee, C.-C. Conditional Domain Adversarial Transfer for Robust Cross-Site ADHD Classification Using Functional MRI. In Proceedings of the ICASSP 2020—2020 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Barcelona, Spain, 4–8 May 2020; pp. 1190–1194. [Google Scholar] [CrossRef]
- Delisle, P.-L.; Anctil-Robitaille, B.; Desrosiers, C.; Lombaert, H. Adversarial Normalization for Multi Domain Image Segmentation. In Proceedings of the 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI), Iowa City, IA, USA, 3–7 April 2020; pp. 849–853. [Google Scholar] [CrossRef]
- Murez, Z.; Kolouri, S.; Kriegman, D.; Ramamoorthi, R.; Kim, K. Image to Image Translation for Domain Adaptation. arXiv 2017. [Google Scholar] [CrossRef]
- Wang, N.; Yao, D.; Ma, L.; Liu, M. Multi-site clustering and nested feature extraction for identifying autism spectrum disorder with resting-state fMRI. Med. Image Anal. 2022, 75, 102279. [Google Scholar] [CrossRef] [PubMed]
- Yousefnezhad, M.; Selvitella, A.; Zhang, D.; Greenshaw, A.J.; Greiner, R. Shared space transfer learning for analyzing multi-site fMRI data. In Proceedings of the 34th International Conference on Neural Information Processing Systems, Red Hook, NY, USA, 6–12 December 2020; pp. 15990–16000. [Google Scholar]
- Wang, M.; Zhang, D.; Huang, J.; Yap, P.-T.; Shen, D.; Liu, M. Identifying Autism Spectrum Disorder With Multi-Site fMRI via Low-Rank Domain Adaptation. IEEE Trans. Med. Imaging 2019, 39, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, J.; Hong, Q.-Q.; Teku, R.; Wang, S.-H.; Zhang, Y.-D. Transfer learning for medical images analyses: A survey. Neurocomputing 2022, 489, 230–254. [Google Scholar] [CrossRef]
- Monte-Rubio, G.C.; Segura, B.; Strafella, A.P.; van Eimeren, T.; Ibarretxe-Bilbao, N.; Diez-Cirarda, M.; Eggers, C.; Lucas-Jiménez, O.; Ojeda, N.; Peña, J.; et al. Parameters from site classification to harmonize MRI clinical studies: Application to a multi-site Parkinson’s disease dataset. Hum. Brain Mapp. 2022, 43, 3130–3142. [Google Scholar] [CrossRef]
- Chen, C.-L.; Hsu, Y.-C.; Yang, L.-Y.; Tung, Y.-H.; Luo, W.-B.; Liu, C.-M.; Hwang, T.-J.; Hwu, H.-G.; Tseng, W.-Y.I. Generalization of diffusion magnetic resonance imaging–based brain age prediction model through transfer learning. Neuroimage 2020, 217, 116831. [Google Scholar] [CrossRef]
- Wachinger, C.; Reuter, M. Domain adaptation for Alzheimer’s disease diagnostics. Neuroimage 2016, 139, 470–479. [Google Scholar] [CrossRef] [Green Version]
- Ghafoorian, M.; Mehrtash, A.; Kapur, T.; Karssemeijer, N.; Marchiori, E.; Pesteie, M.; Guttmann, C.R.; de Leeuw, F.-E.; Tempany, C.M.; Van Ginneken, B. Transfer learning for domain adaptation in mri: Application in brain lesion segmentation. In Proceedings of the Medical Image Computing and Computer Assisted Intervention—MICCAI 2017, 20th International Conference, Quebec City, QC, Canada, 11–13 September 2017; pp. 516–524. [Google Scholar]
- Balboni, E.; Nocetti, L.; Carbone, C.; Dinsdale, N.; Genovese, M.; Guidi, G.; Malagoli, M.; Chiari, A.; Namburete, A.I.L.; Jenkinson, M.; et al. The impact of transfer learning on 3D deep learning convolutional neural network segmentation of the hippocampus in mild cognitive impairment and Alzheimer disease subjects. Hum. Brain Mapp. 2022, 43, 3427–3438. [Google Scholar] [CrossRef]
- Shi, C.; Xin, X.; Zhang, J. Domain Adaptation Using a Three-Way Decision Improves the Identification of Autism Patients from Multisite fMRI Data. Brain Sci. 2021, 11, 603. [Google Scholar] [CrossRef]
- Van Opbroek, A.; Ikram, M.A.; Vernooij, M.W.; de Bruijne, M. Transfer Learning Improves Supervised Image Segmentation Across Imaging Protocols. IEEE Trans. Med. Imaging 2014, 34, 1018–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Chaudhari, P.; Davatzikos, C. Embracing the disharmony in medical imaging: A Simple and effective framework for domain adaptation. Med. Image Anal. 2021, 76, 102309. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.-L.; Xin, X.-W.; Zhang, J.-C. Domain adaptation based on rough adjoint inconsistency and optimal transport for identifying autistic patients. Comput. Methods Programs Biomed. 2022, 215, 106615. [Google Scholar] [CrossRef] [PubMed]
- Van Opbroek, A.; Vernooij, M.W.; Ikram, M.A.; de Bruijne, M. Weighting training images by maximizing distribution similarity for supervised segmentation across scanners. Med. Image Anal. 2015, 24, 245–254. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Q. An overview of multi-task learning. Natl. Sci. Rev. 2017, 5, 30–43. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Zhang, T.; Zanetti, M.V.; Shen, H.; Satterthwaite, T.D.; Wolf, D.H.; Gur, R.E.; Fan, Y.; Hu, D.; Busatto, G.F.; et al. Classification of multi-site MR images in the presence of heterogeneity using multi-task learning. NeuroImage Clin. 2018, 19, 476–486. [Google Scholar] [CrossRef]
- Ning, L.; Bonet-Carne, E.; Grussu, F.; Sepehrband, F.; Kaden, E.; Veraart, J.; Blumberg, S.B.; Khoo, C.S.; Palombo, M.; Coll-Font, J.; et al. Muti-shell Diffusion MRI Harmonisation and Enhancement Challenge (MUSHAC): Progress and Results. In Proceedings of the Computational Diffusion MRI International MICCAI Workshop, Granada, Spain, 20 September 2018; pp. 217–224. [Google Scholar] [CrossRef]
- Van Essen, D.C.; Ugurbil, K.; Auerbach, E.; Barch, D.; Behrens, T.E.J.; Bucholz, R.; Chang, A.; Chen, L.; Corbetta, M.; Curtiss, S.W.; et al. The Human Connectome Project: A data acquisition perspective. NeuroImage 2012, 62, 2222–2231. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.C.; Yamashita, A.; Yahata, N.; Itahashi, T.; Lisi, G.; Yamada, T.; Ichikawa, N.; Takamura, M.; Yoshihara, Y.; Kunimatsu, A.; et al. A multi-site, multi-disorder resting-state magnetic resonance image database. Sci. Data 2021, 8, 1–15. [Google Scholar] [CrossRef]
- Marcus, D.S.; Fotenos, A.F.; Csernansky, J.G.; Morris, J.C.; Buckner, R.L. Open Access Series of Imaging Studies: Longitudinal MRI Data in Nondemented and Demented Older Adults. J. Cogn. Neurosci. 2010, 22, 2677–2684. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Park, T.; Isola, P.; Efros, A.A. Unpaired image-to-image translation using cycle-consistent adversarial networks. In Proceedings of the IEEE International Conference on Computer Vision, Venice, Italy, 22–29 October 2017; pp. 2223–2232. [Google Scholar]
- Sundar, L.K.S.; Muzik, O.; Buvat, I.; Bidaut, L.; Beyer, T. Potentials and caveats of AI in hybrid imaging. Methods 2020, 188, 4–19. [Google Scholar] [CrossRef]
- Sundar, L.K.S.; Iommi, D.; Muzik, O.; Chalampalakis, Z.; Klebermass, E.-M.; Hienert, M.; Rischka, L.; Lanzenberger, R.; Hahn, A.; Pataraia, E.; et al. Conditional Generative Adversarial Networks Aided Motion Correction of Dynamic 18F-FDG PET Brain Studies. J. Nucl. Med. 2020, 62, 871–879. [Google Scholar] [CrossRef]
- Mathieu, E.; Rainforth, T.; Siddharth, N.; Teh, Y.W. Disentangling Disentanglement in Variational Autoencoders. ICML 2019, 97, 4402–4412. [Google Scholar]
- Tajbakhsh, N.; Shin, J.Y.; Gurudu, S.R.; Hurst, R.T.; Kendall, C.B.; Gotway, M.B.; Liang, J. Convolutional Neural Networks for Medical Image Analysis: Full Training or Fine Tuning? IEEE Trans. Med. Imaging 2016, 35, 1299–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Shin, J.; Zhang, L.; Gurudu, S.; Gotway, M.; Liang, J. Fine-tuning Convolutional Neural Networks for Biomedical Image Analysis: Actively and Incrementally. In Proceedings of the 30th IEEE Conference on Computer Vision and Pattern Recognition (CVPR 2017), Las Vegas, NV, USA, 27–30 June 2017; pp. 4761–4772. [Google Scholar] [CrossRef]
- Maikusa, N.; Zhu, Y.; Uematsu, A.; Yamashita, A.; Saotome, K.; Okada, N.; Kasai, K.; Okanoya, K.; Yamashita, O.; Tanaka, S.C.; et al. Comparison of traveling-subject and ComBat harmonization methods for assessing structural brain characteristics. Hum. Brain Mapp. 2021, 42, 5278–5287. [Google Scholar] [CrossRef] [PubMed]
- Bartzokis, G.; Mintz, J.; Marx, P.; Osborn, D.; Gutkind, D.; Chiang, F.; Phelan, C.; Marder, S.R. Reliability of in vivo volume measures of hippocampus and other brain structures using MRI. Magn. Reson. Imaging 1993, 11, 993–1006. [Google Scholar] [CrossRef]
- Morey, R.A.; Selgrade, E.S.; Ii, H.R.W.; Huettel, S.A.; Wang, L.; McCarthy, G. Scan-rescan reliability of subcortical brain volumes derived from automated segmentation. Hum. Brain Mapp. 2010, 31, 1751–1762. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Chen, N.-X.; Shen, Y.-Q.; Li, H.-X.; Li, L.; Lu, B.; Zhu, Z.-C.; Fan, Z.; Yan, C.-G. The subsystem mechanism of default mode network underlying rumination: A reproducible neuroimaging study. Neuroimage 2020, 221, 117185. [Google Scholar] [CrossRef]
- Singh, S.P.; Wang, L.; Gupta, S.; Goli, H.; Padmanabhan, P.; Gulyás, B. 3D Deep Learning on Medical Images: A Review. Sensors 2020, 20, 5097. [Google Scholar] [CrossRef]
- Kao, P.-Y.; Shailja, S.; Jiang, J.; Zhang, A.; Khan, A.; Chen, J.W.; Manjunath, B.S. Improving Patch-Based Convolutional Neural Networks for MRI Brain Tumor Segmentation by Leveraging Location Information. Front. Neurosci. 2020, 13, 1449. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-C.; Wu, T.-H.; Lai, S.-H. Multi-Scale Patch-Based Representation Learning for Image Anomaly Detection and Segmentation. In Proceedings of the 2022 IEEE/CVF Winter Conference on Applications of Computer Vision (WACV), Waikoloa, HI, USA, 3–8 January 2022; pp. 3065–3073. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Huang, H.; Zhang, W.; Zhao, S.; Makkie, M.; Zhang, M.; Li, Q.; Liu, T. Four-Dimensional Modeling of fMRI Data via Spatio–Temporal Convolutional Neural Networks (ST-CNNs). IEEE Trans. Cogn. Dev. Syst. 2019, 12, 451–460. [Google Scholar] [CrossRef]
- Mittal, A.; Moorthy, A.K.; Bovik, A.C. Blind/Referenceless Image Spatial Quality Evaluator. In Proceedings of the 2011 Conference Record of the Forty Fifth Asilomar Conference on Signals, Systems and Computers (ASILOMAR), Pacific Grove, CA, USA, 6–9 November 2011; pp. 723–727. [Google Scholar] [CrossRef] [Green Version]
- Obuchowicz, R.; Oszust, M.; Bielecka, M.; Bielecki, A.; Piórkowski, A. Magnetic Resonance Image Quality Assessment by Using Non-Maximum Suppression and Entropy Analysis. Entropy 2020, 22, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Küstner, T.; Gatidis, S.; Liebgott, A.; Schwartz, M.; Mauch, L.; Martirosian, P.; Schmidt, H.; Schwenzer, N.; Nikolaou, K.; Bamberg, F.; et al. A machine-learning framework for automatic reference-free quality assessment in MRI. Magn. Reson. Imaging 2018, 53, 134–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Fu, J.; Li, X.; Zhou, W.; Liu, S.; Zhang, X.; Wu, W.; Jia, C.; Liu, Y.; Chen, Z. RTN: Reinforced Transformer Network for Coronary CT Angiography Vessel-level Image Quality Assessment. In Proceedings of the Medical Image Computing and Computer Assisted Intervention—MICCAI 2022 25th International Conference, Singapore, 18–22 September 2022; pp. 644–653. [Google Scholar] [CrossRef]
- Liu, S.; Thung, K.-H.; Lin, W.; Yap, P.-T.; Shen, D.; The UNC/UMN Baby Connectome Project Consortium. Multi-stage Image Quality Assessment of Diffusion MRI via Semi-supervised Nonlocal Residual Networks. In Proceedings of the Medical Image Computing and Computer Assisted Intervention—MICCAI 2019 22nd International Conference, Shenzhen, China, 13–17 October 2019; pp. 521–528. [Google Scholar] [CrossRef]
- Tax, C.M.; Grussu, F.; Kaden, E.; Ning, L.; Rudrapatna, U.; Evans, C.J.; St-Jean, S.; Leemans, A.; Koppers, S.; Merhof, D.; et al. Cross-scanner and cross-protocol diffusion MRI data harmonisation: A benchmark database and evaluation of algorithms. Neuroimage 2019, 195, 285–299. [Google Scholar] [CrossRef]
- Ning, L.; Bonet-Carne, E.; Grussu, F.; Sepehrband, F.; Kaden, E.; Veraart, J.; Blumberg, S.B.; Khoo, C.S.; Palombo, M.; Kokkinos, I.; et al. Cross-scanner and cross-protocol multi-shell diffusion MRI data harmonization: Algorithms and results. Neuroimage 2020, 221, 117128. [Google Scholar] [CrossRef]
- Kasabov, N.K. Time-Space, Spiking Neural Networks and Brain-Inspired Artificial Intelligence; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Doborjeh, M.; Doborjeh, Z.; Merkin, A.; Bahrami, H.; Sumich, A.; Krishnamurthi, R.; Medvedev, O.N.; Crook-Rumsey, M.; Morgan, C.; Kirk, I.; et al. Personalised predictive modelling with brain-inspired spiking neural networks of longitudinal MRI neuroimaging data and the case study of dementia. Neural Netw. 2021, 144, 522–539. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, N.; McNabb, C.B.; Kasabov, N.; Russell, B.R. Integrating Space, Time, and Orientation in Spiking Neural Networks: A Case Study on Multimodal Brain Data Modeling. IEEE Trans. Neural Netw. Learn. Syst. 2018, 29, 5249–5263. [Google Scholar] [CrossRef]
- Kasabov, N.K. NeuCube: A spiking neural network architecture for mapping, learning and understanding of spatio-temporal brain data. Neural Netw. 2014, 52, 62–76. [Google Scholar] [CrossRef]
- Kasabov, N.K.; Doborjeh, M.G.; Doborjeh, Z.G. Mapping, Learning, Visualization, Classification, and Understanding of fMRI Data in the NeuCube Evolving Spatiotemporal Data Machine of Spiking Neural Networks. IEEE Trans. Neural Netw. Learn. Syst. 2016, 28, 887–899. [Google Scholar] [CrossRef]
- Whitney, H.M.; Li, H.; Ji, Y.; Liu, P.; Giger, M.L. Harmonization of radiomic features of breast lesions across international DCE-MRI datasets. J. Med. Imaging 2020, 7, 012707. [Google Scholar] [CrossRef]
- Crombé, A.; Kind, M.; Fadli, D.; Le Loarer, F.; Italiano, A.; Buy, X.; Saut, O. Intensity harmonization techniques influence radiomics features and radiomics-based predictions in sarcoma patients. Sci. Rep. 2020, 10, 15496. [Google Scholar] [CrossRef]
- Mirzaalian, H.; Ning, L.; Savadjiev, P.; Pasternak, O.; Bouix, S.; Michailovich, O.; Karmacharya, S.; Grant, G.; Marx, C.E.; Morey, R.A.; et al. Multi-site harmonization of diffusion MRI data in a registration framework. Brain Imaging Behav. 2018, 12, 284–295. [Google Scholar] [CrossRef] [PubMed]





| Categories | Keywords |
|---|---|
| ML methods | machine learning, Artificial Intelligence, Naive Bayes, Bayesian learning, neural network, neural networks, support vector, random forest, boosting, deep learning, machine intelligence, computational intelligence, ComBat, reinforcement learning, decision tree, linear discriminant analysis, regression, supervised learning, unsupervised learning, independent component analysis, dictionary learning, domain adaptation, generative model |
| Harmonisation | harmonisation, normalisation, normalisation, multi-site, multicentre, multi-scanner |
| Brain MRI | MRI, magnetic resonance imaging, imaging, neuroimaging, diffusion MRI, DWI, DTI, structural MRI, T1, T2, proton density-weighted (PD), susceptibility-weighted imaging, fluid-attenuated inversion recovery (FLAIR), double inversion recovery (DIR), functional MRI perfusion MRI |
| Section | Item | Definitions | Values (Counts If Applicable) |
|---|---|---|---|
| Data | Imaging modality | What is the modality of the input data? | Structural MRI (n = 28), diffusion MRI (n = 7), functional MRI (n = 6) |
| Harmonised features | What feature of MRI data is harmonised? | Raw signals (n = 29), derived features (n = 12) | |
| Property | Whether the dataset(s) is (are) in-house or public; whether travelling subject dataset(s) (datasets that contain multiple scans of each participant) include(s)? | In-house (n = 8), public (n = 30), in-house and public (n = 3); travelling subjects included (n = 13), otherwise (n = 28) | |
| Model Selection | Study design | Whether the study aims to produce harmonised data or not? | Implicit approach (n = 21), explicit approach (n = 20) |
| Algorithm selection | What ML algorithm(s) or model(s) is (are) adapted to the proposed methods? | Explicit: random forest regression (n = 1), convolutional neural networks (n = 3), transformer networks (n = 1), generative adversarial networks (n = 7), dictionary learning (n = 1), autoencoders (n = 7). Implicit: adversarial learning (n = 7), feature extraction (n = 3), fine-tuning (n = 10), multi-task learning (n = 1) | |
| Model training | Input dimension | What is the dimension of the input data? | 1D (n = 6), 2D (including 2.5D, n = 22), 3D (n = 13) |
| Input size | What is the input size of the model? | 256 × 256, 16 × 16 × 16 etc. | |
| validation | What validation method(s) is (are) used? | Cross validation (n = 23), split dataset (n = 18) | |
| Model evaluation | Evaluation approaches | What approaches are used to evaluate the proposed method? Indirect evaluation is when a downstream prediction model is used; otherwise, is direct evaluation? | direct evaluation (n = 20), indirect evaluation (n = 25) |
| Evaluation metrics | What metrics are used to quantitatively evaluate the proposed method? | Structural similarity index, signal-to-noise ratio, etc. | |
| Implementation | Code and reproducibility | Does the author provide the source code? | Yes (n = 11), No (n = 30) |
| Implementation | What programming library is used to build the model? | Keras and/or TensorFlow (n = 6), PyTorch (n = 11), others (n = 6), unknown (n = 18) |
| Q# | Quality Questions | Yes | Partly | No |
|---|---|---|---|---|
| Q1 | Are the research aims clearly defined? | |||
| Q2 | Is the data collection procedure clearly described? | |||
| Q3 | Is the data pre-processing procedure clearly defined? | |||
| Q4 | Are the characteristics of the input data clearly described? | |||
| Q5 | Are the ML techniques well-defined? | |||
| Q6 | Is the training procedure clearly defined? | |||
| Q7 | Are the results and findings clearly stated? | |||
| Q8 | Is the proposed method compared to any statistical method? | |||
| Q9 | Is the proposed method compared to any other state-of-art ML method? | |||
| Q10 | Are the limitations of the study specified? |
| Categories | Specific Methods | Description | Studies, n | References |
|---|---|---|---|---|
| Explicit approach | Random Forest Regression | A random forest is an ensemble learning approach built from many decision trees [23]. Ground truth is estimated through statistical harmonisation methods such as Combat [24]. | 1 | [25] |
| Convolutional neural networks (CNNs) | CNNs directly learn the mapping between intra-subject paired scans from two domains. Multiple layers of convolutional layers are used to gather and learn data features [26]. | 3 | [27,28,29] | |
| Sparse dictionary learning | Models learn to create an implicit linear mapping between scanners based on dictionary representation [30]. Scanner-specific dictionary information can be learnt from a set of data and used for harmonisation. | 1 | [31] | |
| Transformer networks | Neural networks using transformer modules, which have spatial and/or image-level transformation capabilities, can learn shape and/or appearance differences across MRI domains [32]. | 1 | [33] | |
| Generative adversarial networks (GANs) | GANs perform unsupervised learning to transform images [34]. Generators and discriminators work together and are against each other to convert MR images from source groups to a reference and vice versa. | 7 | [35,36,37,38,39,40,41] | |
| Autoencoders | It has an encoder-decoder architecture, where the MRI data is encoded into disentangled latent space structural and site-specific information and then decoded by harmonising the intensities with the embeddings without altering the structures [42]. | 7 | [43,44,45,46,47,48,49] | |
| Implicit approach | Adversarial Transfer Learning | This method indicates developing a learning system that composes of domain discriminators and feature extractors that focuses on the scanner invariant features while simultaneously maintaining performance on the main task of interest [50]. | 8 | [51,52,53,54,55,56,57,58] |
| Feature extraction-based transfer learning | The feature extraction modules network aims to extract a set of common features in each scanner/site and then map them to a gold-standard space to improve the performance of the final learning task [59]. | 3 | [60,61,62] | |
| Fine-tuning | This approach utilises a well-trained model on a source dataset as the base and uses a small subset from the target dataset to re-train the model by updating the weight of layers in the re-trained model during the re-training process [63]. | 10 | [64,65,66,67,68,69,70,71,72,73] | |
| Multi-task learning | Multi-task learning considers the site a task and learns the site-shared and site-specific features to generate more accurate models on multiple sites by assuming that the feature weights of different sites share similar sparse patterns [74]. | 1 | [75] |
| Data Repository | Related Study | Web Page if Applicable | Image Modality | Data Description |
|---|---|---|---|---|
| Zhejiang University Travelling Adults Dataset | [38] | https://brain.labsolver.org/test_retest.html, accessed on 15 February 2023 | T1-weighted (sMRI), dMRI | 3 travelling subjects were scanned in 10 scanners with different protocols within 13 months. |
| Multi-shell Diffusion MRI Harmonisation Challenge (MUSHAC) [76] | [31] | https://www.cardiff.ac.uk/cardiff-university-brain-research-imaging-centre/research/projects/cross-scanner-and-cross-protocol-diffusion-MRI-data-harmonisation, accessed on 15 February 2023 | dMRI | 14 healthy controls were scanned on three scanners with five acquisition protocols. |
| Human Connectome Project [77] | [39] | https://www.humanconnectome.org/study/hcp-young-adult/article/reprocessed-7t-fmri-data-released-other-updates, accessed on 15 February 2023 | dMRI, Resting-state fMRI, Task-based fMRI | 184 subjects which were scanned on a 3T and a 7T MRI scanner, separately. |
| SRPBS Travelling Subject MRI Dataset [78] | [46] | https://bicr-resource.atr.jp/srpbsts/, accessed on 15 February 2023 | T1-weighted (sMRI), Resting-state fMRI | 411 scans of the 3T MRI imaging data from 9 travelling subjects collected at 9 sites. |
| The Open Access Series of Imaging Studies (OASIS) 3 [79] | [44] | https://www.oasis-brains.org/, accessed on 15 February 2023 | T1-weighted, T2-weighted (sMRI), resting-state fMRI, dMRI, etc. | A large longitudinal neuroimaging dataset that contains longitudinal scans with small intervals between different visits. |
| Private Dataset | [45] | - | T1-weighted (sMRI) | 18 subjects were scanned on 4 different 3T scanners. The scans are at most four months apart. |
| Private dataset | [44] | - | T1-weighted FLAIR PD-/T2-weighted (sMRI) | 12 subjects were scanned twice within 30 days on two scanners |
| Private dataset | [28] | - | dMRI | 5 subjects were scanned using four scanners with different protocols. |
| Private dataset | [35] | - | T2, T2-FLAIR, T1-FLAIR | 10 subjects were scanned using two scanners with 6 different protocols. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, G.; Shim, V.; Holdsworth, S.J.; Fernandez, J.; Qiao, M.; Kasabov, N.; Wang, A. Machine Learning for Brain MRI Data Harmonisation: A Systematic Review. Bioengineering 2023, 10, 397. https://doi.org/10.3390/bioengineering10040397
Wen G, Shim V, Holdsworth SJ, Fernandez J, Qiao M, Kasabov N, Wang A. Machine Learning for Brain MRI Data Harmonisation: A Systematic Review. Bioengineering. 2023; 10(4):397. https://doi.org/10.3390/bioengineering10040397
Chicago/Turabian StyleWen, Grace, Vickie Shim, Samantha Jane Holdsworth, Justin Fernandez, Miao Qiao, Nikola Kasabov, and Alan Wang. 2023. "Machine Learning for Brain MRI Data Harmonisation: A Systematic Review" Bioengineering 10, no. 4: 397. https://doi.org/10.3390/bioengineering10040397