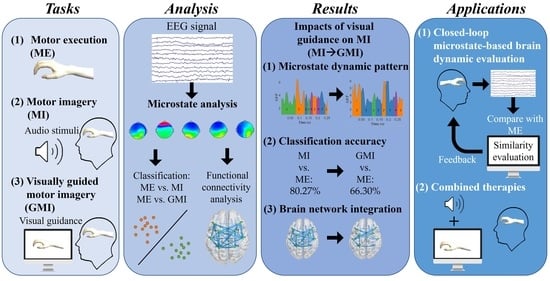
Exploring the Role of Visual Guidance in Motor Imagery-Based Brain-Computer Interface: An EEG Microstate-Specific Functional Connectivity Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiments and Data Acquisition
2.2. Preprocessing
2.3. EEG Microstate Analysis
2.4. Microstate Parameters Calculation
2.5. Microstate-Specific Functional Connectivity Analysis
2.6. Support Vector Machine Classifier
2.7. Statistical and Visualization Tools
3. Results
3.1. Microstate Maps
3.2. Microstate Parameters Analysis
3.3. Classification between Motor Conditions
3.4. Microstate-Specific Functional Connectivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Larraz, E.; Sarasola-Sanz, A.; Irastorza-Landa, N.; Birbaumer, N.; Ramos-Murguialday, A. Brain-Machine Interfaces for Rehabilitation in Stroke: A Review. NeuroRehabilitation 2018, 43, 77–97. [Google Scholar] [CrossRef] [Green Version]
- Orban, M.; Elsamanty, M.; Guo, K.; Zhang, S.; Yang, H. A Review of Brain Activity and EEG-Based Brain–Computer Interfaces for Rehabilitation Application. Bioengineering 2022, 9, 768. [Google Scholar] [CrossRef]
- Rungsirisilp, N.; Wongsawat, Y. Applying Combined Action Observation and Motor Imagery to Enhance Classification Performance in a Brain–Computer Interface System for Stroke Patients. IEEE Access 2022, 10, 73145–73155. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, Q.; Wu, B.; Qin, B.; Hu, H.; Qian, Z. A Comparison of Directed Functional Connectivity among Fist-Related Brain Activities during Movement Imagery, Movement Execution, and Movement Observation. Brain Res. 2022, 1777, 147769. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.-H.; Yang, J.; Sawan, M. Intelligent Classification Technique of Hand Motor Imagery Using EEG Beta Rebound Follow-Up Pattern. Biosensors 2022, 12, 384. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-L.; Chen, S.-H.; Chang, T.-C.; Lee, W.-K.; Hsu, H.-T.; Chang, H.-H. Continual Learning of a Transformer-Based Deep Learning Classifier Using an Initial Model from Action Observation EEG Data to Online Motor Imagery Classification. Bioengineering 2023, 10, 186. [Google Scholar] [CrossRef]
- Khare, S.K.; Gaikwad, N.; Bokde, N.D. An Intelligent Motor Imagery Detection System Using Electroencephalography with Adaptive Wavelets. Sensors 2022, 22, 8128. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Zhai, X.; Cheng, D.; Pan, Y.; Dou, W. EEG Microstate-Specific Functional Connectivity and Stroke-Related Alterations in Brain Dynamics. Front. Neurosci. 2022, 16, 848737. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.M.; Koenig, T. EEG Microstates as a Tool for Studying the Temporal Dynamics of Whole-Brain Neuronal Networks: A Review. NeuroImage 2018, 180, 577–593. [Google Scholar] [CrossRef]
- Lehmann, D.; Ozaki, H.; Pal, I. EEG Alpha Map Series: Brain Micro-States by Space-Oriented Adaptive Segmentation. Electroencephalogr. Clin. Neurophysiol. 1987, 67, 271–288. [Google Scholar] [CrossRef]
- Milz, P.; Pascual-Marqui, R.D.; Achermann, P.; Kochi, K.; Faber, P.L. The EEG Microstate Topography Is Predominantly Determined by Intracortical Sources in the Alpha Band. NeuroImage 2017, 162, 353–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, G.C.; Tewarie, P.; Vidaurre, D.; Liuzzi, L.; Woolrich, M.W.; Brookes, M.J. Dynamics of Large-Scale Electrophysiological Networks: A Technical Review. NeuroImage 2018, 180, 559–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duc, N.T.; Lee, B. Microstate Functional Connectivity in EEG Cognitive Tasks Revealed by a Multivariate Gaussian Hidden Markov Model with Phase Locking Value. J. Neural Eng. 2019, 16, 026033. [Google Scholar] [CrossRef]
- Tait, L.; Zhang, J. MEG Cortical Microstates: Spatiotemporal Characteristics, Dynamic Functional Connectivity and Stimulus-Evoked Responses. Neuroimage 2022, 251, 119006. [Google Scholar] [CrossRef] [PubMed]
- Guan, K.; Zhang, Z.; Chai, X.; Tian, Z.; Liu, T.; Niu, H. EEG Based Dynamic Functional Connectivity Analysis in Mental Workload Tasks with Different Types of Information. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Wang, S.; Peng, W.; Yi, W.; Zhang, C.; Zhang, J.; He, H. Continuous Theta-Burst Stimulation Modulates Resting-State EEG Microstates in Healthy Subjects. Cogn. Neurodyn. 2022, 16, 621–631. [Google Scholar] [CrossRef]
- Heremans, E.; Helsen, W.F.; De Poel, H.J.; Alaerts, K.; Meyns, P.; Feys, P. Facilitation of Motor Imagery through Movement-Related Cueing. Brain Res. 2009, 1278, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Almulla, L.; Al-Naib, I.; Ateeq, I.S.; Althobaiti, M. Observation and Motor Imagery Balance Tasks Evaluation: An FNIRS Feasibility Study. PLoS ONE 2022, 17, e0265898. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kim, L. Identifying Error Features in a MI-BCI System Using Microstates. In Proceedings of the 2020 8th International Winter Conference on Brain-Computer Interface (BCI), Gangwon, Republic of Korea, 26–28 February 2020; IEEE: Gangwon, Republic of Korea, 2020; pp. 1–3. [Google Scholar]
- Li, Y.; Chen, M.; Sun, S.; Huang, Z. Exploring Differences for Motor Imagery Using Teager Energy Operator-Based EEG Microstate Analyses. J. Integr. Neurosci. 2021, 20, 411–417. [Google Scholar] [CrossRef]
- Liu, W.; Liu, X.; Dai, R.; Tang, X. Exploring Differences between Left and Right Hand Motor Imagery via Spatio-Temporal EEG Microstate. Comput. Assist. Surg. 2017, 22, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Chen, J.; Xiong, X. Calculation and Analysis of Microstate Related to Variation in Executed and Imagined Movement of Force of Hand Clenching. Comput. Intell. Neurosci. 2018, 2018, 9270685. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Zhao, L.; Zhang, Y.; Yu, X.; Li, F.; Li, J.; Lai, Y.; Liu, T.; Yao, D. Reference Electrode Standardization Interpolation Technique (RESIT): A Novel Interpolation Method for Scalp EEG. Brain Topogr. 2021, 34, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tait, L.; Zhang, J. +microstate: A MATLAB Toolbox for Brain Microstate Analysis in Sensor and Cortical EEG/MEG. NeuroImage 2022, 258, 119346. [Google Scholar] [CrossRef] [PubMed]
- Brusini, L.; Stival, F.; Setti, F.; Menegatti, E.; Menegaz, G.; Storti, S.F. A Systematic Review on Motor-Imagery Brain-Connectivity-Based Computer Interfaces. IEEE Trans. Hum.-Mach. Syst. 2021, 51, 725–733. [Google Scholar] [CrossRef]
- Skrandies, W. Global Field Power and Topographic Similarity. Brain Topogr. 1990, 3, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Koenig, T.; Prichep, L.; Lehmann, D.; Sosa, P.V.; Braeker, E.; Kleinlogel, H.; Isenhart, R.; John, E.R. Millisecond by Millisecond, Year by Year: Normative EEG Microstates and Developmental Stages. NeuroImage 2002, 16, 41–48. [Google Scholar] [CrossRef]
- Khanna, A.; Pascual-Leone, A.; Farzan, F. Reliability of Resting-State Microstate Features in Electroencephalography. PLoS ONE 2014, 9, e114163. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, D.; Faber, P.L.; Galderisi, S.; Herrmann, W.M.; Kinoshita, T.; Koukkou, M.; Mucci, A.; Pascual-Marqui, R.D.; Saito, N.; Wackermann, J.; et al. EEG Microstate Duration and Syntax in Acute, Medication-Naïve, First-Episode Schizophrenia: A Multi-Center Study. Psychiatry Res. Neuroimaging 2005, 138, 141–156. [Google Scholar] [CrossRef]
- Lachaux, J.P.; Rodriguez, E.; Martinerie, J.; Varela, F.J. Measuring Phase Synchrony in Brain Signals. Hum. Brain Mapp. 1999, 8, 194–208. [Google Scholar] [CrossRef]
- Basti, A.; Chella, F.; Guidotti, R.; Ermolova, M.; D’Andrea, A.; Stenroos, M.; Romani, G.L.; Pizzella, V.; Marzetti, L. Looking through the Windows: A Study about the Dependency of Phase-Coupling Estimates on the Data Length. J. Neural Eng. 2022, 19, 016039. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Astolfi, L.; Valdes-Sosa, P.A.; Marinazzo, D.; Palva, S.; Benar, C.G.; Michel, C.M.; Koenig, T. Electrophysiological Brain Connectivity: Theory and Implementation. IEEE Trans. Biomed. Eng. 2019, 66, 2115–2137. [Google Scholar] [CrossRef] [Green Version]
- Freeman, L.C. A Set of Measures of Centrality Based on Betweenness. Sociometry 1977, 40, 35. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.; Wang, L.; Chen, Z.J.; Yan, C.; Yang, H.; Tang, H.; Zhu, C.; Gong, Q.; Zang, Y.; et al. Uncovering Intrinsic Modular Organization of Spontaneous Brain Activity in Humans. PLoS ONE 2009, 4, e5226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, X.; Xia, M.; Liao, X.; Evans, A.; He, Y. GRETNA: A Graph Theoretical Network Analysis Toolbox for Imaging Connectomics. Front. Hum. Neurosci. 2015, 9, 386. [Google Scholar] [CrossRef] [Green Version]
- Kaul, A.; Raina, S. Support Vector Machine versus Convolutional Neural Network for Hyperspectral Image Classification: A Systematic Review. Concurr. Comput. Pract. Exp. 2022, 34, e6945. [Google Scholar] [CrossRef]
- Paul, J.K.; Iype, T.; Dileep, R.; Hagiwara, Y.; Koh, J.W.; Acharya, U.R. Characterization of Fibromyalgia Using Sleep EEG Signals with Nonlinear Dynamical Features. Comput. Biol. Med. 2019, 111, 103331. [Google Scholar] [CrossRef]
- Sheykhmousa, M.; Mahdianpari, M.; Ghanbari, H.; Mohammadimanesh, F.; Ghamisi, P.; Homayouni, S. Support Vector Machine Versus Random Forest for Remote Sensing Image Classification: A Meta-Analysis and Systematic Review. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 6308–6325. [Google Scholar] [CrossRef]
- Abdullah; Faye, I.; Islam, M.R. EEG Channel Selection Techniques in Motor Imagery Applications: A Review and New Perspectives. Bioengineering 2022, 9, 726. [Google Scholar] [CrossRef]
- Jenke, R.; Peer, A.; Buss, M. Feature Extraction and Selection for Emotion Recognition from EEG. IEEE Trans. Affect. Comput. 2014, 5, 327–339. [Google Scholar] [CrossRef]
- Kincses, Z.T.; Johansen-Berg, H.; Tomassini, V.; Bosnell, R.; Matthews, P.M.; Beckmann, C.F. Model-Free Characterization of Brain Functional Networks for Motor Sequence Learning Using FMRI. NeuroImage 2008, 39, 1950–1958. [Google Scholar] [CrossRef]
- Hardwick, R.M.; Caspers, S.; Eickhoff, S.B.; Swinnen, S.P. Neural Correlates of Action: Comparing Meta-Analyses of Imagery, Observation, and Execution. Neurosci. Biobehav. Rev. 2018, 94, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.; Bird, G.; Catmur, C.; Press, C.; Heyes, C. Mirror Neurons: From Origin to Function. Behav. Brain Sci. 2014, 37, 177–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, A.; Pascual-Leone, A.; Michel, C.M.; Farzan, F. Microstates in Resting-State EEG: Current Status and Future Directions. Neurosci. Biobehav. Rev. 2015, 49, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britz, J.; Van De Ville, D.; Michel, C.M. BOLD Correlates of EEG Topography Reveal Rapid Resting-State Network Dynamics. NeuroImage 2010, 52, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W. The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J. Neurosci. 2019, 39, 9878–9882. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Shimobayashi, H.; Hirayama, J.-I.; Kawanabe, M. Asymmetric Directed Functional Connectivity within the Frontoparietal Motor Network during Motor Imagery and Execution. NeuroImage 2022, 247, 118794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, M.; Zhang, L.; Biswal, B.; Yao, D.; Xu, P. The Time-Varying Network Patterns in Motor Imagery Revealed by Adaptive Directed Transfer Function Analysis for FMRI. IEEE Access 2018, 6, 60339–60352. [Google Scholar] [CrossRef]
- Zhan, G.; Chen, S.; Ji, Y.; Xu, Y.; Song, Z.; Wang, J.; Niu, L.; Bin, J.; Kang, X.; Jia, J. EEG-Based Brain Network Analysis of Chronic Stroke Patients After BCI Rehabilitation Training. Front. Hum. Neurosci. 2022, 16, 909610. [Google Scholar] [CrossRef]
- He, S.-F.; Zhang, N.-N.; Zhang, C.-M.; Fu, Z.-H.; Chen, X.-L.; Xie, P. Enhancement of Motor Cortex EEG during Motor Imagery: A Visual Feedback Training Study. In Proceedings of the 2019 IEEE International Conference on Computational Intelligence and Virtual Environments for Measurement Systems and Applications (CIVEMSA), Tianjin, China, 14–16 June 2019; pp. 1–5. [Google Scholar]
- Smith, S.R.; Wood, G.; Coyles, G.; Roberts, J.W.; Wakefield, C.J. The Effect of Action Observation and Motor Imagery Combinations on Upper Limb Kinematics and EMG during Dart-Throwing. Scand. J. Med. Sci. Sports 2019, 29, 1917–1929. [Google Scholar] [CrossRef]





| Motion | Microstate Parameters | |||
|---|---|---|---|---|
| Coverage | Duration | Occurrence | Mean Duration | |
| Left-hand finger tapping | M2, M4, M5 | M1, M3, M4, M5 | M1, M2, M3, M5 | √ |
| Right-hand finger tapping | M1, M2, M4, M5 | M1, M3, M4, M5 | M2, M3, M5 | √ |
| Motion | Microstate Parameters | |||
|---|---|---|---|---|
| Coverage | Duration | Occurrence | Mean Duration | |
| Left-hand finger tapping | M2, M4, M5 | M1, M2, M3, M4, M5 | M1, M2, M5 | √ |
| Right-hand finger tapping | M2, M4 | M1, M3, M4, M5 | M1, M3, M5 | √ |
| Motion | Microstate Parameters | |||
|---|---|---|---|---|
| Coverage | Duration | Occurrence | Mean Duration | |
| Left-hand finger tapping | M2, M3, M4 | √ | ||
| Right-hand finger tapping | M5 | M4 | √ | |
| Right-Finger Tap | Left-Finger Tap | Hold a Pen | Open a Pen | Cross Fingers | Arm Movement | Mean ± STD | |
|---|---|---|---|---|---|---|---|
| ME vs. MI | 72.98 | 80.91 | 77.31 | 75.56 | 83.96 | 90.91 | 80.27 ± 6.50 |
| ME vs. GMI | 70.54 | 57.95 | 73.33 | 62.22 | 67.08 | 66.67 | 66.30 ± 5.56 |
| Accuracy difference | 2.44 | 22.96 | 3.98 | 13.34 | 16.88 | 24.24 | 13.97 ± 9.25 |
| Authors | Main Results | Classification Study | FC Analysis | Motor Conditions | Specific Biomarkers | Motion Number | ||
|---|---|---|---|---|---|---|---|---|
| MI | ME | GMI | ||||||
| Liu et al., 2017 [21] | Mean accuracy of 89.17% was achieved for two motion classification using microstate-based features. | Used SVM to classify motions. | × | √ | × | × | × | 2 |
| Li et al., 2021 [20] | Mean accuracy of 93.93% was achieved for two motion classification using microstate and Teager energy operator features. | Used SVM to classify motions. | × | √ | × | × | × | 2 |
| Fu et al., 2018 [22] | Discussed the change of microstate parameters between ME and MI of grip tasks; proved alpha wave has the highest correlation with microstates. | × | × | √ | √ | × | × | 3 |
| Kim et al., 2020 [19] | Topography of M5 in their study can be used as a biomarker for errors in MI-BCI. | × | × | √ | × | × | Topography of M5. | 2 |
| This work | Duration of M4 and mean duration can be biomarkers to evaluate motor condition; SVM classifier can be used to quantitatively evaluate motor condition difference; GMI could induce similar brain activation pattern with ME, but may reduce the functional integration of the brain network. | Used SVM to classify motor conditions. | √ | √ | √ | √ | Duration of M4 and mean duration. | 6 |
| Authors | Main Results | Classification Study | FC Analysis | Motor Condition | Specific Biomarkers | Motion Number | |||
|---|---|---|---|---|---|---|---|---|---|
| MI | ME | GMI | MO | ||||||
| Romano-Smith et al., 2019 [52] | After GMI training, task performance was significantly increased compared to MI and MO interventions. | × | × | √ | × | √ | √ | × | 1 |
| He et al., 2019 [51] | After GMI training, EEG signals during MI tasks show enhancement in various features, Common Spatial Pattern is most significant, indicating improved spatial resolution. | × | × | √ | × | √ | × | Characteristics of Common Spatial Pattern | 1 |
| Almulla et al., 2022 [18] | Analyzed with functional near-infrared spectroscopy signals, GMI activated greater HbO responses compared MI or MO alone. | × | × | √ | × | √ | √ | HbO response | 2 |
| Rungsirisilp et al., 2022 [3] | GMI can induce higher ERD values in sensorimotor area and achieve better classification performance than MI. | Used SVM to classify motions. | × | √ | × | √ | × | ERD/ERS values of channel C3 or C4 | 2 |
| This work | GMI induces similar brain activation pattern with ME than MI; Dominant hand motions are less benefit from visual guidance than nondominant hand, two hand, and arm motions; the brain network is less integrated during GMI than MI. | Used SVM to classify motor conditions. | √ | √ | √ | √ | × | Duration of M4 and mean duration. | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Chen, Y.-H.; Sawan, M. Exploring the Role of Visual Guidance in Motor Imagery-Based Brain-Computer Interface: An EEG Microstate-Specific Functional Connectivity Study. Bioengineering 2023, 10, 281. https://doi.org/10.3390/bioengineering10030281
Wang T, Chen Y-H, Sawan M. Exploring the Role of Visual Guidance in Motor Imagery-Based Brain-Computer Interface: An EEG Microstate-Specific Functional Connectivity Study. Bioengineering. 2023; 10(3):281. https://doi.org/10.3390/bioengineering10030281
Chicago/Turabian StyleWang, Tianjun, Yun-Hsuan Chen, and Mohamad Sawan. 2023. "Exploring the Role of Visual Guidance in Motor Imagery-Based Brain-Computer Interface: An EEG Microstate-Specific Functional Connectivity Study" Bioengineering 10, no. 3: 281. https://doi.org/10.3390/bioengineering10030281