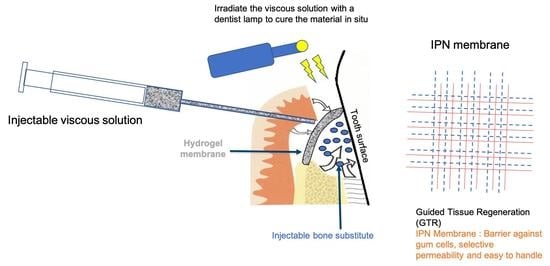
Injectable Hydrogel Membrane for Guided Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dextran Methacrylate Synthesis
2.3. Synthesis of Si-HPMC and Hydrogel Preparation
2.4. Dextran Methacrylate Hydrogel Formation
2.5. DexMA Filter Sterilization
2.6. IPN Hydrogel Preparation
2.7. Rheological Analysis
2.8. Injectability
2.9. Hydrogel Apposition on BCP
2.10. Cytocompatibility
2.11. In Vivo Experiments: Animal Model and Study Design
2.12. Surgical Protocol
2.13. Clinical Observation
2.14. Micro-Computed Tomography Analysis
2.15. Statistical Analysis
2.16. Histological Processing
3. Results
3.1. Polymer Synthesis
3.2. Dextran Methacrylate Hydrogel Formation
3.3. DexMA Filter Sterilization
3.4. DexMA Polymer Solution Stability
3.5. Rheological Analysis
3.6. Injectability
3.7. Hydrogel Apposition on BCP
3.8. Cytocompatibility
3.9. Surgical Procedure and Clinical Observation
3.10. Macroscopic Examination of Implants after Sacrifice
3.11. Micro-Computed Tomography Analysis
3.12. Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragan, E. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, E.L.; Cha, C.; Camci-Unal, G.; Dokmeci, R.M.; Peppas, A.N.; Khademhosseini, A. 25th Anniversary Article: Rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef] [PubMed]
- Edalat, F.; Bae, H.; Manoucheri, S.; Cha, J.M.; Khademhosseini, A. Engineering Approaches Toward Deconstructing and Controlling the Stem Cell Environment. Ann. Biomed. Eng. 2012, 40, 1301–1315. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Zhang, Y.; Hu, L.; Peng, Y.; Deng, Y.; He, W.; Ge, Y.; Tang, B. Strontium Laminarin polysaccharide modulates osteogenesis-angiogenesis for bone regenera-tion. Int. J. Biol. Macromol. 2021, 181, 452–461. [Google Scholar] [CrossRef]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Hennink, W.; Alhaique, F. Interpenetrating polymer networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Razak, S.I.A.; Rehman, S.; Hasan, A.; Qureshi, S.; Stojanović, G.M. Bioactive scaffold (sodium alginate)-g-(nHAp@SiO2@GO) for bone tissue engineering. Int. J. Biol. Macromol. 2022, 222, 462–472. [Google Scholar] [CrossRef]
- Hached, F.; Vinatier, C.; Pinta, P.; Hulin, P.; Le Visage, C.; Weiss, P.; Guicheux, J.; Billon-Chabaud, A.; Grimandi, G. Polysaccharide hydrogels support the long-Term viability of encapsulated human mesenchymal stem cells and their ability to secrete immunomodulatory factors. Stem Cells Int. 2017, 6–8. [Google Scholar] [CrossRef] [Green Version]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implants Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [Green Version]
- Milella, E.; Ramires, P.; Brescia, E.; La Sala, G.; Di Paola, L.; Bruno, V. Physicochemical, mechanical, and biological properties of commercial membranes for GTR. J. Biomed. Mater. Res. 2001, 58, 427–435. [Google Scholar] [CrossRef]
- Bottino, M.; Thomas, V.; Schmidt, G.; Vohra, Y.; Chu, T.; Kowolik, M.; Janowski, G.; Lafayette, W. Recent advances in the development of GTR/GBR membranes for periodontal regeneration — A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Nasser, N.; Friedman, A.; Friedman, M.; Moor, E.; Mosheiff, R. Guided bone regeneration in the treatment of segmental diaphyseal defects: A comparison between resorbable and non-resorbable membranes. Injury 2005, 36, 1460–1466. [Google Scholar] [CrossRef]
- Jose, R.; Rodriguez, M.; Dixon, T.; Omenetto, F.; Kaplan, D. Evolution of Bioinks and Additive Manufacturing Technologies for 3D Bioprinting. Biomater. Sci. Eng. 2016, 2, 1662–1678. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.; Calori, G.; Giannoudis, P. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef] [Green Version]
- Jung, R.E.; Hälg, G.A.; Thoma, D.S.; Hämmerle, C.H.F. A randomized, controlled clinical trial to evaluate a new membrane for guided bone regeneration around dental implants. Clin. Oral Implants Res. 2009, 20, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Coonts, B.A.; Whitman, S.L.; Donnell, M.O.; Polson, A.M.; Bogle, G.; Garrett, S.; Swanbom, D.D.; Fulfs, J.C.; Rodgers, P.W.; Southard, G.L.; et al. Biodegradation and biocompatibility of a guided tissue regeneration barrier membrane formed from a liquid polymer material. J. Biomed. Mater. Res. 1998, 42, 303–311. [Google Scholar] [CrossRef]
- Fatimi, A.; Tassin, J.F.; Quillard, S.; Axelos, M.A.V.; Weiss, P. The rheological properties of silated hydroxypropylmethylcellulose tissue engineering matrices. Biomaterials 2008, 29, 533–543. [Google Scholar] [CrossRef]
- Fatimi, A.; Tassin, J.F.; Turczyn, R.; Axelos, M.A.V.; Weiss, P. Gelation studies of a cellulose-based biohydrogel: The influence of pH, temperature and sterilization. Acta Biomater. 2009, 5, 3423–3432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourges, X.; Weiss, P.; Daculsi, G.; Legeay, G. Synthesis and general properties of silated-hydroxypropyl methylcellulose in prospect of biomedical use. Adv. Colloid Interface Sci. 2002, 99, 215–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, N.; Clouet, J.; Fragale, A.; Griveau, L.; Véziers, J.; Weiss, P.; Le Bideau, J.; Guicheux, J.; Henry, N.; Clouet, J.; et al. Pullulan microbeads/Si-HPMC hydrogel injectable system for the sustained delivery of GDF-5 and TGF- β 1: New insight into intervertebral disc regenerative medicine. Drug Deliv. 2017, 24, 999–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merceron, C.; Portron, S.; Masson, M.; Lesoeur, J.; Fellah, B.; Gauthier, O.; Geffroy, O.; Weiss, P. The Effect of Two- and Three-Dimensional Cell Culture on the Chondrogenic Potential of Human Adipose-Derived Mesenchymal Stem Cells After Subcutaneous Transplantation with an Injectable Hydrogel. Cell Transpl. 2011, 20, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Struillou, X.; Boutigny, H.; Badran, Z.; Fellah, B.; Gauthier, O.; Sourice, S.; Pilet, P.; Rouillon, T.; Layrolle, P.; Weiss, P.; et al. Treatment of periodontal defects in dogs using an injectable composite hydrogel/biphasic calcium phosphate. J. Mater. Sci. Mater. Med. 2011, 22, 1707–1717. [Google Scholar] [CrossRef]
- Struillou, X.; Fruchet, A.; Rakic, M.; Badran, Z.; Rethore, G.; Sourice, S.; Fellah, B.; Le Guehennec, L.; Gauthier, O.; Weiss, P.; et al. Evaluation of a hydrogel membrane on bone regeneration in furcation periodontal defects in dogs. Dent. Mater. J. 2018, 37, 825–834. [Google Scholar] [CrossRef] [Green Version]
- Davis, K.; Burdick, J.; Anseth, K. New directions in photopolymerizable biomaterials. Biomaterials 2003, 24, 2485–2495. [Google Scholar] [CrossRef]
- Burkoth, A.; Anseth, K. A review of photocrosslinked polyanhydrides: In situ forming degradable networks. Biomaterials 2000, 21, 2395–2404. [Google Scholar] [CrossRef]
- Ifkovits, J.L.; Burdick, J.A. Review: Photopolymerizable and degradable biomaterials for tissue engineering applications. J. Tissue Eng. 2007, 13, 2369–2385. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, Y.; Wang, Y. Photo-polymerization efficiency of self-etch dental adhesives composed of camphorquinone or trimethylbenzoyl-diphenyl-phosphine oxide. Int. J. Adhes. Adhes. 2013, 45, 53–58. [Google Scholar] [CrossRef]
- Steenbergenj, V.; Den Bosch, K.; Henninkt, W. Synthesis, Characterization, and Polymerization of Glycidyl Methacrylate Derivatized Dextran. Macromolecules 1995, 28, 6317–6322. [Google Scholar]
- Van Dijk-Wolthuis, W.; Kettenes-van den Bosch, J.J.; van der Kerk-van Hoof, A.; Hennink, W. Reaction of Dextran with Glycidyl Methacrylate: An Unexpected Transesterification. Macromolecules 1997, 30, 3411–3413. [Google Scholar] [CrossRef]
- D’Arrigo, G.; Di Meo, C.; Pescosolido, L.; Coviello, T.; Alhaique, F.; Matricardi, P. Calcium alginate/dextran methacrylate IPN beads as protecting carriers for protein delivery. J. Mater. Sci. Mater. Med. 2012, 23, 1715–1722. [Google Scholar] [CrossRef]
- Pescosolido, L.; Schuurman, W.; Malda, J.; Matricardi, P.; Alhaique, F.; Coviello, T.; Van Weeren, P.; Dhert, W.; Hennink, W.; Vermonden, T. Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting. Biomacromolecules 2011, 12, 1831–1838. [Google Scholar] [CrossRef]
- Matricardi, P.; Pontoriero, M.; Coviello, T.; Casadei, M.; Alhaique, F. In situ cross-linkable novel alginate-dextran methacrylate IPN hydrogels for biomedical applications: Mechanical characterization and drug delivery properties. Biomacromolecules 2008, 9, 2014–2020. [Google Scholar] [CrossRef]
- Pescosolido, L.; Vermonden, T.; Malda, J.; Censi, R.; Dhert, W.; Alhaique, F.; Hennink, W.; Matricardi, P. In situ forming IPN hydrogels of calcium alginate and dextran-HEMA for biomedical applications. Acta Biomater. 2011, 7, 1627–1633. [Google Scholar] [CrossRef] [Green Version]
- Mirahmadi, F.; Tafazzoli-Shadpour, M.; Shokrgozar, M.; Bonakdar, S. Enhanced mechanical properties of thermosensitive chitosan hydrogel by silk fibers for cartilage tissue engineering. Mater. Sci. Eng. C 2013, 33, 4786–4794. [Google Scholar] [CrossRef]
- Jin, R.; Teixeira, L.M.; Dijkstra, P.; Karperien, M.; van Blitterswijk, C.; Zhong, Z.; Feijen, J. Injectable chitosan-based hydrogels for cartilage tissue engineering. J. Biomater. 2009, 30, 2544–2551. [Google Scholar] [CrossRef]
- Van Dijk-Wolthuis, W.; Van Steenbergen, M.; Underberg, W.; Hennink, W. Degradation kinetics of methacrylated dextrans in aqueous solution. J. Pharm. Sci. 1997, 86, 413–417. [Google Scholar] [CrossRef]
- Flégeau, K.; Pace, R.; Gautier, H.; Rethore, G.; Guicheux, J.; Le, C.; Weiss, P. Toward the development of biomimetic injectable and macroporous biohydrogels for regenerative medicine. Adv. Colloid Interface Sci. 2017, 247, 589–609. [Google Scholar] [CrossRef]
- Encinas, M.; Rufs, A.; Bertolotti, S.; Previtali, C. Free Radical Polymerization Photoinitiated by Riboflavin/Amines. Effect of the Amine Structure. Excit. States 2001, 2845–2847. [Google Scholar] [CrossRef]
- Bertolotti, S.; Previtali, C.; Rufs, A.; Encinas, M.V. Riboflavin/triethanolamine as photoinitiator system of vinyl polymerization. A mechanistic study by laser flash photolysis. Macromolecules 1999, 32, 2920–2924. [Google Scholar] [CrossRef]
- Williams, C.; Malik, A.; Kim, T.; Manson, P.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. J. Biomater. 2005, 26, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Elbadawy, H.; Kamoun, A. Crosslinking Behavior of Dextran Modified with Hydroxyethyl Methacrylate upon Irradiation with Visible Light—Effect of Concentration, Coinitiator Type, and Solvent. InterScience 2010, 21, 449–456. [Google Scholar]
- Kim, S.; Chu, C. Visible light induced dextran-methacrylate hydrogel formation using (-)-riboflavin vitamin B2 as a photoinitiator and L-arginine as a co-initiator. Fibers Polym. 2009, 10, 14–20. [Google Scholar] [CrossRef]
- Sheikh, Z.; Qureshi, J.; Alshahrani, A.; Nassar, H.; Ikeda, Y.; Glogauer, M.; Ganss, B. Collagen based barrier membranes for periodontal guided bone regeneration applications. Odontology 2017, 105, 1–12. [Google Scholar] [CrossRef]
- SLi, T.; Chen, H.-C.; Lee, N.; Ringshia, R.; Yuen, D. A Comparative study of Zimmer BioMend® and BioMend® Extend{\texttrademark} membranes made at two different manufacturing facilities. Zimmer 2013, 1–5. [Google Scholar]
- Garcia, J.J.; Berghezan, S.; Caramês, J.; Dard, M.; Marques, D. Effect of cross-linked vs. non-cross-linked collagen membranes on bone: A systematic review. J. Periodontal Res. 2017, 52, 955–964. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, E.; Lamirault, G.; Toquet, C.; Lhommet, P.; Rederstorff, E.; Sourice, S.; Biteau, K.; Hulin, P.; Forest, V.; Weiss, P.; et al. Intramyocardial Delivery of Mesenchymal Stem Cell-Seeded Hydrogel Preserves Cardiac Function and Attenuates Ventricular Remodeling after Myocardial Infarction. PLoS ONE 2012, 7, e51991. [Google Scholar] [CrossRef]
- Nativel, F.; Renard, D.; Hached, F.; Pinta, P.; Arros, C.; Weiss, P.; Le Visage, C.; Billon-Chabaud, A. Application of Millifluidics to Encapsulate and Support Viable Human Mesenchymal Stem Cells in a Polysaccharide Hydrogel. Int. J. Mol. Sci. 2018, 19, 1952. [Google Scholar] [CrossRef] [Green Version]
- Moussa, L.; Pattappa, G.; Doix, B.; Benselama, S.; Demarquay, C.; Benderitter, M.; Alexandra, S.; Weiss, P.; Rethore, G. A biomaterial-assisted mesenchymal stromal cell therapy alleviates colonic radiation-induced damage. Biomaterials 2017, 115, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Viguier, A.; Boyer, C.; Chassenieux, C.; Benyahia, L.; Guicheux, J.; Weiss, P.; Rethore, G.; Nicolai, T. Interpenetrated Si-HPMC/alginate hydrogels as a potential scaffold for human tissue regeneration. J. Mater. Sci. Mater. Med. 2016, 27, 99. [Google Scholar] [CrossRef]
- Park, J.; Jung, I.; Kim, Y.; Lim, H.; Lee, J.; Jung, U.; Choi, S. Guided bone regeneration using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)-cross-linked type-I collagen membrane with biphasic calcium phosphate at rabbit calvarial defects. Biomater. Res. 2015, 19, 15. [Google Scholar] [CrossRef]
- Park, J.; Kang, D.; Hanawa, T. New bone formation induced by surface strontium-modified ceramic bone graft substitute. Oral Dis. 2016, 22, 53–61. [Google Scholar] [CrossRef]
- Al-Qutub, M.; Al-Omar, N.; Ramalingam, S.; Javed, F.; Al-Kindi, M.; Ar-Rejaie, A.; Aldahmash, A.; Nooh, N.; Wang, H.-L.; Al-Hezaimi, K. Guided Bone Regeneration Using Biphasic Calcium Phosphate with Adjunct Recombinant Human Bone Morphogenetic Protein-2 With and Without Collagen Membrane in Standardized Calvarial Defects in Rats: A Histologic and Biomechanical Analysis. Int. J. Periodontics Restor. Dent. 2016, 36, s1. [Google Scholar] [CrossRef]











| Abbreviation | Full Name |
|---|---|
| Si-HPMC | Silanized hydroxypropyl methylcellulose |
| Dex40MA20 | Dextran 40 kDa with a theoretical methacrylation degree of 20% * |
| Dex100MA20 | Dextran 100 kDa with a theoretical methacrylation degree of 20% * |
| Dex500MA20 | Dextran 500 kDa with a theoretical methacrylation degree of 20% * |
| PIS | Photoinitiator solution |
| IPN | Interpenetrating polymer network |
| BCP | Biphasic calcium phosphate |
| Theoretical Values | Experimental Data | |
|---|---|---|
| Polymer | % MA | % MA |
| Dex40MA20 | 20 | 18 |
| Dex100MA20 | 20 | 17 |
| Dex500MA20 | 20 | 19 |
| Photoinitiator Concentration (µL/mL) | |||||||
|---|---|---|---|---|---|---|---|
| 1.5 | 5 | ||||||
| Polymer * | Conc. % (w/v) | Gel Point (s) | Gel Time (s) | Shape | Gel Point (s) | Gel Time (s) | Shape |
| Dex100MA20 | 5 | / | / | / | / | / | / |
| 15 | / | / | / | / | / | / | |
| 30 | 150 | 210 | + | 90 | 150 | - | |
| Dex500MA20 | 5 | 210 | 240 | -- | 30 | 150 | -- |
| 15 | 90 | 150 | -- | 30 | 120 | + | |
| 30 | 60 | 60 | ++ | 30 | 90 | ++ | |
| Dextran | DexMA | Filtered DexMA | |
|---|---|---|---|
| Mw (kDa) | 500 | 702.8 | 803.4 |
| Mn (kDa) | 52.9 | 144.3 | 171.5 |
| Ɖ | 9.45 | 4.87 | 4.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chichiricco, P.M.; Matricardi, P.; Colaço, B.; Gomes, P.; Jérôme, C.; Lesoeur, J.; Veziers, J.; Réthoré, G.; Weiss, P.; Struillou, X.; et al. Injectable Hydrogel Membrane for Guided Bone Regeneration. Bioengineering 2023, 10, 94. https://doi.org/10.3390/bioengineering10010094
Chichiricco PM, Matricardi P, Colaço B, Gomes P, Jérôme C, Lesoeur J, Veziers J, Réthoré G, Weiss P, Struillou X, et al. Injectable Hydrogel Membrane for Guided Bone Regeneration. Bioengineering. 2023; 10(1):94. https://doi.org/10.3390/bioengineering10010094
Chicago/Turabian StyleChichiricco, Pauline Marie, Pietro Matricardi, Bruno Colaço, Pedro Gomes, Christine Jérôme, Julie Lesoeur, Joëlle Veziers, Gildas Réthoré, Pierre Weiss, Xavier Struillou, and et al. 2023. "Injectable Hydrogel Membrane for Guided Bone Regeneration" Bioengineering 10, no. 1: 94. https://doi.org/10.3390/bioengineering10010094