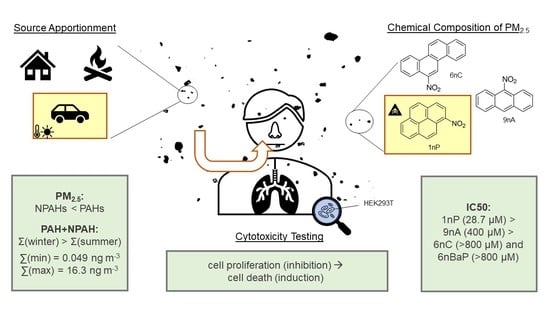
Seasonality of Polyaromatic Hydrocarbons (PAHs) and Their Derivatives in PM2.5 from Ljubljana, Combustion Aerosol Source Apportionment, and Cytotoxicity of Selected Nitrated Polyaromatic Hydrocarbons (NPAHs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Stock Solutions
2.2. Sample Collection and Sampling Site
2.3. TD/GC-MS Analysis
2.4. Cell Culture, Treatments, and Cell Counting
2.5. Statistical Analysis
3. Results and Discussion
3.1. Seasonal Variability of PAHs and NPAHs
3.2. Pollution Sources of Measured PAHs and NPAHs
3.3. Cytotoxic Activity on Human Cell Lines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Degrendele, C.; Kanduč, T.; Kocman, D.; Lammel, G.; Cambelová, A.; Dos Santos, S.G.; Horvat, M.; Kukučka, P.; Holubová Šmejkalová, A.; Mikeš, O.; et al. NPAHs and OPAHs in the Atmosphere of Two Central European Cities: Seasonality, Urban-to-Background Gradients, Cancer Risks and Gas-to-Particle Partitioning. Sci. Total Environ. 2021, 793, 148528. [Google Scholar] [CrossRef] [PubMed]
- Lara, S.; Villanueva, F.; Martín, P.; Salgado, S.; Moreno, A.; Sánchez-Verdú, P. Investigation of PAHs, Nitrated PAHs and Oxygenated PAHs in PM10 Urban Aerosols. A Comprehensive Data Analysis. Chemosphere 2022, 294, 133745. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jariyasopit, N.; Schrlau, J.; Jia, Y.; Tao, S.; Yu, T.-W.; Dashwood, R.H.; Zhang, W.; Wang, X.; Simonich, S.L.M. Concentration and Photochemistry of PAHs, NPAHs, and OPAHs and Toxicity of PM2.5 during the Beijing Olympic Games. Environ. Sci. Technol. 2011, 45, 6887–6895. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yang, L.; Bi, J.; Liu, Y.; Toriba, A.; Hayakawa, K.; Nagao, S.; Tang, N. Characteristics and Unique Sources of Polycyclic Aromatic Hydrocarbons and Nitro-Polycyclic Aromatic Hydrocarbons in PM2.5 at a Highland Background Site in Northwestern China☆. Environ. Pollut. 2021, 274, 116527. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczak, A.; Czech, B. Occurrence and Toxicity of Polycyclic Aromatic Hydrocarbons Derivatives in Environmental Matrices. Sci. Total Environ. 2021, 788, 147738. [Google Scholar] [CrossRef]
- Hayakawa, K. Recent Research Progress on Nitropolycyclic Aromatic Hydrocarbons in Outdoor and Indoor Environments. Appl. Sci. 2022, 12, 11259. [Google Scholar] [CrossRef]
- Fadel, M.; Ledoux, F.; Seigneur, M.; Oikonomou, K.; Sciare, J.; Courcot, D.; Afif, C. Chemical Profiles of PM2.5 Emitted from Various Anthropogenic Sources of the Eastern Mediterranean: Cooking, Wood Burning, and Diesel Generators. Environ. Res. 2022, 211, 113032. [Google Scholar] [CrossRef]
- Fadel, M.; Ledoux, F.; Farhat, M.; Kfoury, A.; Courcot, D.; Afif, C. PM2.5 Characterization of Primary and Secondary Organic Aerosols in Two Urban-Industrial Areas in the East Mediterranean. J. Environ. Sci. 2021, 101, 98–116. [Google Scholar] [CrossRef]
- Galarneau, E. Source Specificity and Atmospheric Processing of Airborne PAHs: Implications for Source Apportionment. Atmos. Environ. 2008, 42, 8139–8149. [Google Scholar] [CrossRef]
- Larsen, R.K.; Baker, J.E. Source Apportionment of Polycyclic Aromatic Hydrocarbons in the Urban Atmosphere: A Comparison of Three Methods. Environ. Sci. Technol. 2003, 37, 1873–1881. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namieśnik, J. PAH Diagnostic Ratios for the Identification of Pollution Emission Sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 Selection Guide of Classical- and Less Classical-Solvents. Green Chem. 2015, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- 12884:2000 ISO; Analyses, Ambient Air—Determination of Total (Gas and Particle-Phase) Polycyclic Aromatic Hydrocarbons—Collection on Sorbent-Backed Filters with Gas Chromatographic/Mass Spectrometric. International Standard ISO: Geneva, Switzerland, 2000; p. 27.
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A Review of Airborne Polycyclic Aromatic Hydrocarbons (PAHs) and Their Human Health Effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- Lagerqvist, A.; Håkansson, D.; Lundin, C.; Prochazka, G.; Dreij, K.; Segerbäck, D.; Jernström, B.; Törnqvist, M.; Frank, H.; Seidel, A.; et al. DNA Repair and Replication Influence the Number of Mutations per Adduct of Polycyclic Aromatic Hydrocarbons in Mammalian Cells. DNA Repair (Amst) 2011, 10, 877–886. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Zhang, X.; Bai, P.; Zhang, L.; Huang, S.J.; Pointing, S.B.; Nagao, S.; Chen, B.; Toriba, A.; et al. Abundance, Source Apportionment and Health Risk Assessment of Polycyclic Aromatic Hydrocarbons and Nitro-Polycyclic Aromatic Hydrocarbons in PM2.5 in the Urban Atmosphere of Singapore. Atmosphere 2022, 13, 1420. [Google Scholar] [CrossRef]
- Andersson, H.; Piras, E.; Demma, J.; Hellman, B.; Brittebo, E. Low Levels of the Air Pollutant 1-Nitropyrene Induce DNA Damage, Increased Levels of Reactive Oxygen Species and Endoplasmic Reticulum Stress in Human Endothelial Cells. Toxicology 2009, 262, 57–64. [Google Scholar] [CrossRef]
- Nepali, K.; Lee, H.Y.; Liou, J.P. Nitro-Group-Containing Drugs. J. Med. Chem. 2019, 62, 2851–2893. [Google Scholar] [CrossRef]
- Bandowe, B.A.M.; Meusel, H. Nitrated Polycyclic Aromatic Hydrocarbons (Nitro-PAHs) in the Environment—A Review. Sci. Total Environ. 2017, 581–582, 237–257. [Google Scholar] [CrossRef]
- Umbuzeiro, G.A.; Franco, A.; Martins, M.H.; Kummrow, F.; Carvalho, L.; Schmeiser, H.H.; Leykauf, J.; Stiborova, M.; Claxton, L.D. Mutagenicity and DNA Adduct Formation of PAH, Nitro-PAH, and Oxy-PAH Fractions of Atmospheric Particulate Matter from São Paulo, Brazil. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2008, 652, 72–80. [Google Scholar] [CrossRef]
- Gooch, A.; Sizochenko, N.; Rasulev, B.; Gorb, L.; Leszczynski, J. In Vivo Toxicity of Nitroaromatics: A Comprehensive Quantitative Structure–Activity Relationship Study. Environ. Toxicol. Chem. 2017, 36, 2227–2233. [Google Scholar] [CrossRef]
- Liu, D.; Lin, T.; Syed, J.H.; Cheng, Z.; Xu, Y.; Li, K.; Zhang, G.; Li, J. Concentration, Source Identification, and Exposure Risk Assessment of PM2.5-Bound Parent PAHs and Nitro-PAHs in Atmosphere from Typical Chinese Cities. Sci. Rep. 2017, 7, 10398. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, N.V.; Vieira, C.L.Z.; Saldiva, P.H.N.; De André, C.D.S.; Mazzilli, B.P.; de Fátima Andrade, M.; Saueia, C.H.; Saiki, M.; Veras, M.M.; Koutrakis, P. Accumulation of Trace Element Content in the Lungs of Sao Paulo City Residents and Its Correlation to Lifetime Exposure to Air Pollution. Sci. Rep. 2022, 12, 11083. [Google Scholar] [CrossRef] [PubMed]
- Moller, W.; Felten, K.; Sommerer, K.; Scheuch, G.; Meyer, G.; Meyer, P.; Häussinger, K.; Kreyling, W.G. Deposition, Retention, and Translocation of Ultrafine Particles from the Central Airways and Lung Periphery. Am. J. Respir. Crit. Care Med. Crit. Care Med. 2006, 177, 7. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.K.; Zhang, Q.; Qiu, Z.; Chung, K.F. Mechanistic Impact of Outdoor Air Pollution on Asthma and Allergic Diseases. J. Thorac. Dis. 2015, 7, 23–33. [Google Scholar] [CrossRef]
- Kim, D.; Chen, Z.; Zhou, L.-F.; Huang, S.-X. Air Pollutants and Early Origins of Respiratory Diseases. Chronic Dis. Transl. Med. 2018, 4, 75–94. [Google Scholar] [CrossRef]
- Hwang, S.-L.; Guo, S.-E.; Chi, M.-C.; Chou, C.-T.; Lin, Y.-C.; Lin, C.-M.; Chou, Y.-L. Association between Atmospheric Fine Particulate Matter and Hospital Admissions for Chronic Obstructive Pulmonary Disease in Southwestern Taiwan: A Population-Based Study. Int. J. Environ. Res. Public Health 2016, 13, 366. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Zhou, R.; Zhang, J. Function of PM2.5 in the Pathogenesis of Lung Cancer and Chronic Airway Inflammatory Diseases (Review). Oncol. Lett. 2018, 15, 7506–7514. [Google Scholar] [CrossRef] [Green Version]
- Vinikoor-Imler, L.C.; Davis, J.A.; Luben, T.J. An Ecologic Analysis of County-Level PM2.5 Concentrations and Lung Cancer Incidence and Mortality. Int. J. Environ. Res. Public Health 2011, 8, 1865–1871. [Google Scholar] [CrossRef] [Green Version]
- Vineis, P.; Forastiere, F.; Hoek, G.; Lipsett, M. Outdoor Air Pollution and Lung Cancer: Recent Epidemiologic Evidence. Int. J. Cancer 2004, 111, 647–652. [Google Scholar] [CrossRef]
- Liu, C.; Guo, H.; Cheng, X.; Shao, M.; Wu, C.; Wang, S.; Li, H.; Wei, L.; Gao, Y.; Tan, W.; et al. Exposure to Airborne PM2.5 Suppresses MicroRNA Expression and Deregulates Target Oncogenes That Cause Neoplastic Transformation in NIH3T3 Cells. Oncotarget 2015, 6, 29428–29439. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yang, L.; Zhang, H.; Xing, W.; Wang, Y.; Bai, P.; Zhang, L.; Hayakawa, K.; Toriba, A.; Wei, Y.; et al. Assessing Approaches of Human Inhalation Exposure to Polycyclic Aromatic Hydrocarbons: A Review. Int. J. Environ. Res. Public Health 2021, 18, 3124. [Google Scholar] [CrossRef]
- Mashroofeh, A.; Bakhtiari, A.R.; Pourkazemi, M. Distribution and Composition Pattern of Polycyclic Aromatic Hydrocarbons in Different Tissues of Sturgeons Collected from Iranian Coastline of the Caspian Sea. Chemosphere 2015, 120, 575–583. [Google Scholar] [CrossRef]
- Moulton, P.V.; Yang, W. Air Pollution, Oxidative Stress, and Alzheimer’s Disease. J. Environ. Public Health 2012, 2012, 472751. [Google Scholar] [CrossRef] [Green Version]
- Beamish, L.A.; Osornio-Vargas, A.R.; Wine, E. Air Pollution: An Environmental Factor Contributing to Intestinal Disease. J. Crohn’s Colitis 2011, 5, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Hamanaka, R.B.; Mutlu, G.M. Particulate Matter Air Pollution: Effects on the Cardiovascular System. Front. Endocrinol. 2018, 9, 680. [Google Scholar] [CrossRef] [Green Version]
- Marris, C.R.; Kompella, S.N.; Miller, M.R.; Incardona, J.P.; Brette, F.; Hancox, J.C.; Sørhus, E.; Shiels, H.A. Polyaromatic Hydrocarbons in Pollution: A Heart-Breaking Matter. J. Physiol. 2020, 598, 227–247. [Google Scholar] [CrossRef]
- Drventić, I.; Šala, M.; Vidović, K.; Kroflič, A. Direct Quantification of PAHs and Nitro-PAHs in Atmospheric PM by Thermal Desorption Gas Chromatography with Electron Ionization Mass Spectroscopic Detection. Talanta 2022, 251, 123761. [Google Scholar] [CrossRef]
- Ogrizek, M.; Gregorič, A.; Ivančič, M.; Contini, D.; Skube, U.; Vidović, K.; Bele, M.; Šala, M.; Gunde, M.K.; Rigler, M.; et al. Characterization of Fresh PM Deposits on Calcareous Stone Surfaces: Seasonality, Source Apportionment and Soiling Potential. Sci. Total Environ. 2023, 856, 159012. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, C.; Tang, M.; Yang, Z.; Jia, W.; Ma, Y.; Jia, P.; Pei, D.; Wang, H. Nanotoxicity of Silver Nanoparticles on HEK293T Cells: A Combined Study Using Biomechanical and Biological Techniques. ACS Omega 2018, 3, 6770–6778. [Google Scholar] [CrossRef]
- Mayati, A.; Levoin, N.; Paris, H.; N’Diaye, M.; Courtois, A.; Uriac, P.; Lagadic-Gossmann, D.; Fardel, O.; Le Ferrec, E. Induction of Intracellular Calcium Concentration by Environmental Benzo(a)Pyrene Involves a Β2-Adrenergic Receptor/Adenylyl Cyclase/Epac-1/Inositol 1,4,5-Trisphosphate Pathway in Endothelial Cells. J. Biol. Chem. 2012, 287, 4041–4052. [Google Scholar] [CrossRef] [Green Version]
- Manoli, E.; Kouras, A.; Karagkiozidou, O.; Argyropoulos, G.; Voutsa, D.; Samara, C. Polycyclic Aromatic Hydrocarbons (PAHs) at Traffic and Urban Background Sites of Northern Greece: Source Apportionment of Ambient PAH Levels and PAH-Induced Lung Cancer Risk. Environ. Sci. Pollut. Res. 2016, 23, 3556–3568. [Google Scholar] [CrossRef] [PubMed]
- Lammel, G.; Kitanovski, Z.; Kukučka, P.; Novák, J.; Arangio, A.M.; Codling, G.P.; Filippi, A.; Hovorka, J.; Kuta, J.; Leoni, C.; et al. Oxygenated and Nitrated Polycyclic Aromatic Hydrocarbons in Ambient Air—Levels, Phase Partitioning, Mass Size Distributions, and Inhalation Bioaccessibility. Environ. Sci. Technol. 2020, 54, 2615–2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, P.K.; Sah, D.; Kumari, K.M.; Lakhani, A. Atmospheric Concentrations and Gas-Particle Partitioning of Polycyclic Aromatic Hydrocarbons (PAHs) and Nitro-PAHs at Indo-Gangetic Sites. Environ. Sci. Process. Impacts 2017, 19, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Pratt, G.C.; Herbrandson, C.; Krause, M.J.; Schmitt, C.; Lippert, C.J.; McMahon, C.R.; Ellickson, K.M. Measurements of gas and particle polycyclic aromatic hydrocarbons (PAHs) in air at urban, rural and near-roadway sites. Atmos. Environ. 2018, 179, 268–278. [Google Scholar] [CrossRef]
- Siudek, P. Compositional and seasonal differences of gas and particle phase polycyclic aromatic hydrocarbons (PAHs) over the southern Baltic Sea coast. Sci. Rep. 2022, 12, 21005. [Google Scholar] [CrossRef]
- Alves, C.A.; Vicente, A.M.; Custódio, D.; Cerqueira, M.; Nunes, T.; Pio, C.; Lucarelli, F.; Calzolai, G.; Nava, S.; Diapouli, E.; et al. Polycyclic Aromatic Hydrocarbons and Their Derivatives (Nitro-PAHs, Oxygenated PAHs, and Azaarenes) in PM2.5 from Southern European Cities. Sci. Total Environ. 2017, 595, 494–504. [Google Scholar] [CrossRef]
- Lee, Y.; Hsieh, Y.; Huang, B.; Mutuku, J.K. An Overview: PAH and Nitro-PAH Emission from the Stationary Sources and Their Transformations in the Atmosphere. Aerosol Air Qual. Res. 2021, 22, 220164. [Google Scholar] [CrossRef]
- Dvorská, A.; Lammel, G.; Klánová, J. Use of Diagnostic Ratios for Studying Source Apportionment and Reactivity of Ambient Polycyclic Aromatic Hydrocarbons over Central Europe. Atmos. Environ. 2011, 45, 420–427. [Google Scholar] [CrossRef]
- Biache, C.; Mansuy-Huault, L.; Faure, P. Impact of oxidation and biodegradation on the most commonly used polycyclic aromatic hydrocarbon (PAH) diagnostic ratios: Implications for the source identifications. J. Hazard. Mater. 2014, 267, 31–39. [Google Scholar] [CrossRef]
- Cervena, T.; Rossnerova, A.; Sikorova, J.; Beranek, V.; Vojtisek-Lom, M.; Ciganek, M.; Topinka, J.; Rossner, P. DNA Damage Potential of Engine Emissions Measured In Vitro by Micronucleus Test in Human Bronchial Epithelial Cells. Basic Clin. Pharmacol. Toxicol. 2017, 121, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Calderón-segura, M.E.; Gómez-arroyo, S.; Villalobos-pietrini, R.; Butterworth, F.M.; Amador-muñoz, O. The Effects of Seasonal Weather on the Genotoxicity, Cytokinetic Properties, Cytotoxicity and Organochemical Content of Extracts of Airborne Particulates in Mexico City. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2004, 558, 7–17. [Google Scholar] [CrossRef]
- Li, R.; Zhao, L.; Zhang, L.; Chen, M.; Shi, J.; Dong, C.; Cai, Z. Effects of Ambient PM 2.5 and 9-Nitroanthracene on DNA Damage and Repair, Oxidative Stress and Metabolic Enzymes in the Lungs of Rats. Toxicol. Res. 2017, 6, 654–663. [Google Scholar] [CrossRef] [Green Version]
- Hustedt, N.; Durocher, D. The Control of DNA Repair by the Cell Cycle. Nat. Cell Biol. 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Roos, W.P.; Thomas, A.D.; Kaina, B. DNA Damage and the Balance between Survival and Death in Cancer Biology. Nat. Rev. Cancer 2016, 16, 20–33. [Google Scholar] [CrossRef]
- Torgovnick, A.; Schumacher, B. DNA Repair Mechanisms in Cancer Development and Therapy. Front. Genet. 2015, 6, 157. [Google Scholar] [CrossRef] [Green Version]
- Watt, D.L.; Utzat, C.D.; Hilario, P.; Basu, A.K. Mutagenicity of the 1-Nitropyrene-DNA Adduct N -(Deoxyguanosin-8-Yl)-1-Aminopyrene in Mammalian Cells. Chem. Res. Toxicol. 2007, 20, 1658–1664. [Google Scholar] [CrossRef] [Green Version]
- Dihl, R.R.; Bereta, M.S.; do Amaral, V.S.; Lehmann, M.; Reguly, M.L.; de Andrade, H.H.R. Nitropolycyclic Aromatic Hydrocarbons Are Inducers of Mitotic Homologous Recombination in the Wing-Spot Test of Drosophila Melanogaster. Food Chem. Toxicol. 2008, 46, 2344–2348. [Google Scholar] [CrossRef]





| Target Compound | Abr | Summer, ng m−3 | Autumn, ng m−3 | Winter, ng m−3 | Spring, ng m−3 | |
|---|---|---|---|---|---|---|
| PAH | Acenaphthylene | Ace | n.d. | 0.031 (0.004–0.109) | 0.038 (0.001–0.064) | n.d. |
| Fluorene | Flu | 0.003 (0.001–0.011) | n.d. | n.d. | 0.002 (0.001–0.010) | |
| Phenanthrene | Phe | 0.008 (0.000–0.065) | 0.107 (0.006–0.262) | 0.135 (0.022–0.207) | 0.016 (0.010–0.081) | |
| Anthracene | Ant | 0.005 (0.003–0.019) | 0.008 (0.002–0.033) | 0.005 (0.002–0.032) | 0.004 (0.002–0.009) | |
| Pyrene | Pyr | 0.020 (0.010–0.105) | 0.519 (0.091–2.788) | 0.764 (0.190–1.917) | 0.038 (0.006–0.137) | |
| Benzo(a)anthracene | BaA | 0.030 (0.007–0.241) | 1.119 (0.117–2.979) | 0.868 (0.110–2.239) | 0.057 (0.008–0.242) | |
| Chrysene | Cry | 0.010 (0.001–0.138) | 1.518 (0.192–5.711) | 1.408 (0.278–2.776) | 0.020 (0.001–0.116) | |
| Benzo(b)fluoranthene | BbF | 0.044 (0.007–0.627) | 1.063 (0.062–2.490) | 1.476 (0.351–2.316) | 0.115 (0.022–0.488) | |
| Benzo(k)fluoranthene | BkF | 0.045 (0.002–0.691) | 1.372 (0.291–2.764) | 1.701 (0.201–2.361) | 0.122 (0.010–0.533) | |
| Benzo(a)pyrene | BaP | 0.026 (0.002–0.264) | 1.088 (0.178–2.538) | 0.987 (0.016–2.085) | 0.028 (0.004–0.219) | |
| Indeno(1,2,3-cd)pyrene | Ind | 0.035 (0.004–0.433) | 0.742 (0.143–1.779) | 0.646 (0.009–2.004) | 0.041 (0.013–0.329) | |
| Dibenz(a,h)anthracene | Dba | 0.016 (0.002–0.167) | 0.232 (0.056–0.819) | 0.264 (0.028–0.540) | 0.013 (0.003–0.076) | |
| Benzo(g,h,i)perylene | Bgp | 0.048 (0.005–0.377) | 0.576 (0.134–1.597) | 0.391 (0.011–1.375) | 0.067 (0.014–0.344) | |
| NPAH | 9-nitroanthracene | 9-nA | 0.004 (0.002–0.007) | 0.076 (0.028–0.204) | 0.138 (0.029–0.334) | n.d. |
| 1-nitropyrene | 1-nP | 0.009 (0.001–0.034) | 0.059 (0.025–0.184) | 0.071 (0.017–0.159) | n.d. | |
| 6-nitrochrysene | 6-nC | 0.007 (0.001–0.057) | 0.128 (0.051–0.588) | 0.096 (0.030–0.203) | 0.005 (0.001–0.053) | |
| ΣPAH | 0.208 | 8.807 | 9.234 | 0.516 | ||
| (0.043–3.122) | (1.516–15.285) | (1.518–15.831) | (0.050–2.584) | |||
| ΣNPAH | 0.008 | 0.284 | 0.347 | 0.005 | ||
| (0.001–0.063) | (0.153–0.706) | (0.079–0.545) | (0.001–0.053) | |||
| ΣNPAH/ΣPAH | 0.022 | 0.033 | 0.044 | 0.008 | ||
| ΣNPAH/ΣPAHparent | 0.148 | 0.159 | 0.178 | 0.071 | ||
| Σ(PAH + NPAH) | 0.211 | 9.121 | 9.741 | 0.519 | ||
| (0.049–3.139) | (1.875–15.728) | (1.597–16.300) | (0.055–2.637) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drventić, I.; Glumac, M.; Carev, I.; Kroflič, A. Seasonality of Polyaromatic Hydrocarbons (PAHs) and Their Derivatives in PM2.5 from Ljubljana, Combustion Aerosol Source Apportionment, and Cytotoxicity of Selected Nitrated Polyaromatic Hydrocarbons (NPAHs). Toxics 2023, 11, 518. https://doi.org/10.3390/toxics11060518
Drventić I, Glumac M, Carev I, Kroflič A. Seasonality of Polyaromatic Hydrocarbons (PAHs) and Their Derivatives in PM2.5 from Ljubljana, Combustion Aerosol Source Apportionment, and Cytotoxicity of Selected Nitrated Polyaromatic Hydrocarbons (NPAHs). Toxics. 2023; 11(6):518. https://doi.org/10.3390/toxics11060518
Chicago/Turabian StyleDrventić, Ivana, Mateo Glumac, Ivana Carev, and Ana Kroflič. 2023. "Seasonality of Polyaromatic Hydrocarbons (PAHs) and Their Derivatives in PM2.5 from Ljubljana, Combustion Aerosol Source Apportionment, and Cytotoxicity of Selected Nitrated Polyaromatic Hydrocarbons (NPAHs)" Toxics 11, no. 6: 518. https://doi.org/10.3390/toxics11060518