A New Method for Ecological Risk Assessment of Combined Contaminated Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soils
2.1.1. Soil pH
2.1.2. Organic Matter Content
2.1.3. Clay Content
2.1.4. Cation Exchange Capacity
2.1.5. Mn, Fe, and Al Content
2.1.6. Hg, As and Sb Content
2.1.7. Cr, Pb, Cu, Zn and Cd Content
2.2. Test Organisms
2.3. Toxicity Tests
2.3.1. E. fetida Toxicity Tests
2.3.2. F. candida Toxicity Tests
2.3.3. C. elegans Toxicity Tests
2.4. Ecological Risk Assessment Based on RSVs
2.5. Ecological Risk Assessment Based on Toxicity Tests
2.5.1. Toxicity Effect Index
2.5.2. Cumulative Probability Distribution Curve of EI and Risk Probability
2.6. Data Processing and Statistical Analysis
3. Results and Discussion
3.1. Ecological Risk Assessment Based on RSVs
3.2. Ecological Risk Assessment Based on Toxicity Tests
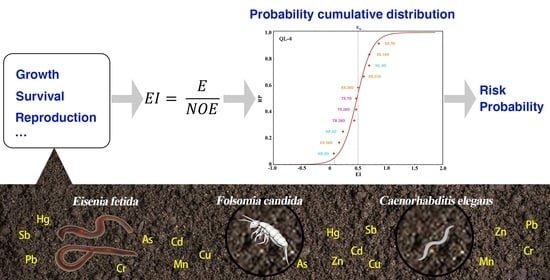
- What is the overall risk probability of the soil to the subject organism? For example, RP = 0.877 for BY−2 in Figure 3;
- For which toxicity endpoints was the risk of the soil acceptable or unacceptable? For example, the risk of soil QL−6 for ES.7D, NL.4D and TS.7D was acceptable (EI > 0.5), while the risk for other toxicity endpoints was unacceptable (EI < 0.5);
- Relative sensitivity distribution among toxicity endpoints in the risk assessment of soil. For different soil, the sensitivity order of the toxicity endpoints varies among species (Figure 3). For instance, the sensitivity order of the toxicity endpoints in soil BY−2 was NR.4D > ES.28D > TR.28D. While in soil ZS−3, the order was TR.28D > ES.28D > NR.4D. This indicates that if only one toxicity endpoint was used for soil ecological risk assessment, the choice of toxicity endpoint would bring about a great influence on the assessment results.
3.3. Comparative Analysis between NRI and RP
3.4. Prospects for Risk Assessment Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, X.; Duan, Z.; Wang, J. Status Assessment, Spatial Distribution and HealthRisk of Heavy Metals in Agricultural Soils AroundMining-Impacted Communities in China. Pol. J. Environ. Stud. 2021, 30, 993–1002. [Google Scholar] [CrossRef]
- Zhao, S.W.; Qin, L.Y.; Wang, L.F.; Sun, X.Y.; Yu, L.; Wang, M.; Chen, S.B. Ecological risk thresholds for Zn in Chinese soils. Sci. Total Environ. 2022, 833, 9. [Google Scholar] [CrossRef] [PubMed]
- Song, W.E.; Chen, S.B.; Liu, J.F.; Chen, L.; Song, N.N.; Li, N.; Liu, B. Variation of Cd concentration in various rice cultivars and derivation of cadmium toxicity thresholds for paddy soil by species-sensitivity distribution. J. Integr. Agric. 2015, 14, 1845–1854. [Google Scholar] [CrossRef]
- Qin, L.Y.; Wang, L.F.; Sun, X.Y.; Yu, L.; Wang, M.; Chen, S.B. Ecological toxicity (ECx) of Pb and its prediction models in Chinese soils withdifferent physiochemical properties. Sci. Total Environ. 2022, 853, 10. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.Y.; Sun, X.Y.; Yu, L.; Wang, J.; Modabberi, S.; Wang, M.; Chen, S.B. Ecological risk threshold for Pb in Chinese soils. J. Hazard. Mater. 2023, 444, 10. [Google Scholar] [CrossRef]
- Jiang, B.; Ma, Y.B.; Zhu, G.Y.; Li, J. Prediction of soil copper phytotoxicity to barley root elongation by an EDTA extraction method. J. Hazard. Mater. 2020, 389, 8. [Google Scholar] [CrossRef]
- Gao, J.T.; Ye, X.X.; Wang, X.Y.; Jiang, Y.J.; Li, D.C.; Ma, Y.B.; Sun, B. Derivation and validation of thresholds of cadmium, chromium, lead, mercury and arsenic for safe rice production in paddy soil. Ecotoxicol. Environ. Saf. 2021, 220, 10. [Google Scholar] [CrossRef]
- Holtra, A.; Zamorska-Wojdyla, D. Application of individual and integrated pollution indices of trace elements to evaluate the noise barrier impact on the soil environment in Wroclaw (Poland). Environ. Sci. Pollut. Res. 2023, 30, 26858–26873. [Google Scholar] [CrossRef]
- Nikolova, R.; Boteva, S.; Kenarova, A.; Dinev, N.; Radeva, G. Enzyme activities in soils under heavy metal pollution: A case study from the surroundings of a non−ferrous metal plant in Bulgaria. Biotechnol. Biotechnol. Equip. 2023, 37, 49–57. [Google Scholar] [CrossRef]
- Bai, Z.; Wu, F.; He, Y.; Han, Z. Pollution and risk assessment of heavy metals in Zuoxiguo antimony mining area, southwest China. Environ. Pollut. Bioavailab. 2023, 35, 1–11. [Google Scholar] [CrossRef]
- Shen, G.; Ru, X.; Gu, Y.; Liu, W.; Wang, K.; Li, B.; Guo, Y.; Han, J. Pollution Characteristics, Spatial Distribution, and Evaluation of Heavy Metal(loid)s in Farmland Soils in a Typical Mountainous Hilly Area in China. Foods 2023, 12, 681. [Google Scholar] [CrossRef] [PubMed]
- Senoro, D.B.; Monjardin, C.E.F.; Fetalvero, E.G.; Benjamin, Z.E.C.; Gorospe, A.F.B.; de Jesus, K.L.M.; Ical, M.L.G.; Wong, J.P. Quantitative Assessment and Spatial Analysis of Metals and Metalloids in Soil Using the Geo-Accumulation Index in the Capital Town of Romblon Province, Philippines. Toxics 2022, 10, 633. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Huang, M.; You, W.; Cai, L.; Hong, Y.; Xiao, Q.; Zheng, X.; Lin, R. Spatial analysis and risk assessment of heavy metal pollution in rice in Fujian Province, China. Front. Environ. Sci. 2022, 10, 2422. [Google Scholar] [CrossRef]
- Shifaw, E. Review of Heavy Metals Pollution in China in Agricultural and Urban Soils. J. Health Pollut. 2020, 8, 180607. [Google Scholar] [CrossRef]
- Adnan, M.; Xiao, B.; Xiao, P.; Zhao, P.; Li, R.; Bibi, S. Research Progress on Heavy Metals Pollution in the Soil of Smelting Sites in China. Toxics 2022, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lin, X.; Zhao, L.; Zhang, J.; Fan, W.; Hou, H. Toxicity threshold and prediction model for zinc in soil-dwelling springtails in Chinese soils. J. Agro−Environ. Sci. 2021, 40, 766–773. [Google Scholar]
- Zhang, J.; Liu, Z.; Tian, B.; Li, J.; Luo, J.; Wang, X.; Ai, S.; Wang, X. Assessment of soil heavy metal pollution in provinces of China based on different soil types: From normalization to soil quality criteria and ecological risk assessment. J. Hazard. Mater. 2023, 441, 129891. [Google Scholar] [CrossRef]
- Han, D.; Zhao, L.; Zhang, N.; Hou, H.; Sun, Z. Classification of Cd Contaminated Paddy Soils in Carbonate Parent Material Area of Southwest China by Species Sensitivity Distribution Method (SSD). Res. Environ. Sci. 2021, 34, 409–418. [Google Scholar]
- Sun, X.Y.; Qin, L.Y.; Wang, L.F.; Zhao, S.W.; Yu, L.; Wang, M.; Chen, S.B. Aging factor and its prediction models of chromium ecotoxicity in soils with various properties. Sci. Total Environ. 2022, 847, 8. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.N.; Jiang, B.; Wei, D.P.; Ma, Y.B. Ecological criteria for zinc in Chinese soil as affected by soil properties. Ecotoxicol. Environ. Saf. 2020, 194, 7. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, T.; Faust, M. Predictive environmental risk assessment of chemical mixtures: A conceptual framework. Environ. Sci. Technol. 2012, 46, 2564–2573. [Google Scholar] [CrossRef]
- Jiang, R.; Wang, M.; Chen, W.; Li, X. Ecological risk evaluation of combined pollution of herbicide siduron and heavy metals in soils. Sci. Total Environ. 2018, 626, 1047–1056. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Qian, Y.; Zhao, X.; Wang, Q. The synergistic toxicity of the multiple chemical mixtures: Implications for risk assessment in the terrestrial environment. Environ. Int. 2015, 77, 95–105. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, A.; Etxebarria, J.; Boatti, L.; Marigomez, I. Scenario-targeted toxicity assessment through multiple endpoint bioassays in a soil posing unacceptable environmental risk according to regulatory screening values. Environ. Sci. Pollut. Res. Int. 2015, 22, 13344–13361. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Resende, A.P.; Santos, V.S.V.; Campos, C.F.; Morais, C.R.d.; de Campos Júnior, E.O.; Oliveira, A.M.M.d.; Pereira, B.B. Ecotoxicological risk assessment of contaminated soil from a complex of ceramic industries using earthworm Eisenia fetida. J. Toxicol. Environ. Health Part A 2018, 81, 1058–1065. [Google Scholar] [CrossRef]
- Sujetoviene, G.; Cesynaite, J. Assessment of Toxicity to Earthworm Eisenia fetida of Lead Contaminated Shooting Range Soils with Different Properties. Bull. Environ. Contam. Toxicol. 2019, 103, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Marchand, C.; Jani, Y.; Kaczala, F.; Hijri, M.; Hogland, W. Physicochemical and Ecotoxicological Characterization of Petroleum Hydrocarbons and Trace Elements Contaminated Soil. Polycycl. Aromat. Compd. 2018, 40, 967–978. [Google Scholar] [CrossRef]
- Gruss, I.; Stefanovska, T.; Twardowski, J.; Pidlisnyuk, V.; Shapoval, P. The ecological risk assessment of soil contamination with Ti and Fe at military sites in Ukraine: Avoidance and reproduction tests with Folsomia candida. Rev. Environ. Health 2019, 34, 303–307. [Google Scholar] [CrossRef]
- HJ 1068−2019; Soil—Determination of Particle Size Distribution—Pipette Method and Hydrometer Method. Ministry of Ecology and Environment: Beijing, China, 2019.
- OECD. Test No. 207: Earthworm, Acute Toxicity Tests; OECD: Paris, France, 1984. [Google Scholar]
- OECD. Test No. 222: Earthworm Reproduction Test (Eisenia fetida/Eisenia andrei); OECD: Paris, France, 2016. [Google Scholar]
- EPA. Ecological Soil Screening Levels for Zinc (Interim Final): OSWER Directive 9285.7−73; EPA: Washington, DC, USA, 2007.
- EPA. Ecological Soil Screening Levels for Copper (Interim Final): OSWER Directive 9285.7−68; EPA: Washington, DC, USA, 2007.
- EPA. Ecological Soil Screening Levels for Lead (Interim Final): OSWER Directive 9285.7−70; EPA: Washington, DC, USA, 2005.
- EPA. Ecological Soil Screening Levels for Cadmium (Interim Final): OSWER Directive 9285.7−65; EPA: Washington, DC, USA, 2005.
- EPA. Ecological Soil Screening Levels for Antimony (Interim Final): OSWER Directive 9285.7−61; EPA: Washington, DC, USA, 2005.
- EPA. Ecological Soil Screening Levels for Manganese (Interim Final): OSWER Directive 9285.7−71; EPA: Washington, DC, USA, 2007.
- GB 15618–2018; Soil Environmental Quality: Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment: Beijing, China, 2018.
- Song, Z.; Dang, X.; Zhao, L.; Hou, H.; Wang, X.; Lu, H. Toxic effects of antimony on Caenorhabditis elegans in soils. J. Agro-Environ. Sci. 2022, 41, 1917–1925. [Google Scholar]
- Lin, X.; Sun, Z.; Zhao, L.; Ma, J.; Li, X.; He, F.; Hou, H. The toxicity of exogenous arsenic to soil−dwelling springtail Folsomia candida in relation to soil properties and aging time. Ecotoxicol. Environ. Saf. 2019, 171, 530–538. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, Z.; Liu, L.; Ma, Q.; Xu, X. Toxicological and biochemical responses of the earthworm Eisenia fetida exposed to contaminated soil: Effects of arsenic species. Chemosphere 2016, 154, 161–170. [Google Scholar] [CrossRef]
- Delistraty, D.; Yokel, J. Ecotoxicological study of arsenic and lead contaminated soils in former orchards at the Hanford Site, USA. Environ. Toxicol. 2014, 29, 10–20. [Google Scholar] [CrossRef]
- Porfido, C.; Allegretta, I.; Panzarino, O.; Laforce, B.; Vekemans, B.; Vincze, L.; de Lillo, E.; Terzano, R.; Spagnuolo, M. Correlations between As in Earthworms’ Coelomic Fluid and As Bioavailability in Highly Polluted Soils as Revealed by Combined Laboratory X−ray Techniques. Environ. Sci. Technol. 2019, 53, 10961–10968. [Google Scholar] [CrossRef]
- Santorufo, L.; Van Gestel, C.A.M.; Maisto, G. Ecotoxicological assessment of metal−polluted urban soils using bioassays with three soil invertebrates. Chemosphere 2012, 88, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, M.; Zhou, J.; Zhou, D.; Wang, Y. Effects of soil properties and aging process on the acute toxicity of cadmium to earthworm Eisenia fetida. Environ. Sci. Pollut. Res. Int. 2018, 25, 3708–3717. [Google Scholar] [CrossRef] [PubMed]
- Nahmani, J.; Hodson, M.E.; Black, S. Effects of metals on life cycle parameters of the earthworm Eisenia fetida exposed to field-contaminated, metal−polluted soils. Environ. Pollut. 2007, 149, 44–58. [Google Scholar] [CrossRef]
- Römbke, J.; Jänsch, S.; Junker, T.; Pohl, B.; Scheffczyk, A.; Schallnaß, H. Improvement of the applicability of ecotoxicological tests with earthworms, springtails, and plants for the assessment of metals in natural soils. Environ. Toxicol. Chem. 2006, 25, 776–787. [Google Scholar] [CrossRef]
- Bonnard, M.; Eom, I.-C.; Morel, J.-L.; Vasseur, P. Genotoxic and reproductive effects of an industrially contaminated soil on the earthwormEisenia Fetida. Environ. Mol. Mutagen. 2009, 50, 60–67. [Google Scholar] [CrossRef]
- Crouau, Y.; Pinelli, E. Comparative ecotoxicity of three polluted industrial soils for the Collembola Folsomia candida. Ecotoxicol. Environ. Saf. 2008, 71, 643–649. [Google Scholar] [CrossRef]
- Yu, F.; Wei, C.; Deng, P.; Peng, T.; Hu, X. Deep exploration of random forest model boosts the interpretability of machine learning studies of complicated immune responses and lung burden of nanoparticles. Sci. Adv. 2021, 7, eabf4130. [Google Scholar] [CrossRef]
- Hao, Y.; Yu, F.; Hu, X. Multiple factors drive imbalance in the global microbial assemblage in soil. Sci. Total Environ. 2022, 831, 154920. [Google Scholar] [CrossRef]










| Zn | Cu | Cr | Pb | Cd | As | Sb | Hg | Mn |
|---|---|---|---|---|---|---|---|---|
| 120 1 | 80 1 | 150 2 | 1700 1 | 140 1 | 25 2 | 78 1 | 1.3 2 | 450 1 |
| Sample Point | RMSE | p (K−S) | RP (EI = 0.5) | Sample Point | RMSE | p (K−S) | RP (EI = 0.5) |
|---|---|---|---|---|---|---|---|
| BY−1 | 0.098 | >0.05 | 1.000 | QL−1 | 0.061 | >0.05 | 0.759 |
| BY−2 | 0.060 | >0.05 | 0.877 | QL−2 | 0.154 | >0.05 | 1.000 |
| BY−3 | 0.075 | >0.05 | 0.697 | QL−3 | 0.230 | <0.05 | 1.000 |
| BY−4 | 0.079 | >0.05 | 0.755 | QL−4 | 0.061 | >0.05 | 0.550 |
| BY−5 | 0.051 | >0.05 | 0.971 | QL−5 | 0.053 | >0.05 | 0.777 |
| BY−6 | 0.057 | >0.05 | 0.711 | QL−6 | 0.058 | >0.05 | 0.759 |
| DQ−1 | 0.054 | >0.05 | 0.668 | ZS−1 | 0.090 | >0.05 | 0.601 |
| DQ−2 | 0.068 | >0.05 | 0.696 | ZS−2 | 0.048 | >0.05 | 0.737 |
| DQ−3 | 0.097 | >0.05 | 0.675 | ZS−3 | 0.086 | >0.05 | 0.469 |
| DQ−4 | 0.078 | >0.05 | 0.735 | ZS−4 | 0.080 | >0.05 | 0.803 |
| DQ−5 | 0.068 | >0.05 | 0.940 | ZS−5 | 0.068 | >0.05 | 0.931 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Wang, J.; Cheng, J.; Zhu, Y.; Geng, J.; Wang, X.; Feng, X.; Hou, H. A New Method for Ecological Risk Assessment of Combined Contaminated Soil. Toxics 2023, 11, 411. https://doi.org/10.3390/toxics11050411
Wang Q, Wang J, Cheng J, Zhu Y, Geng J, Wang X, Feng X, Hou H. A New Method for Ecological Risk Assessment of Combined Contaminated Soil. Toxics. 2023; 11(5):411. https://doi.org/10.3390/toxics11050411
Chicago/Turabian StyleWang, Qiaoping, Junhuan Wang, Jiaqi Cheng, Yingying Zhu, Jian Geng, Xin Wang, Xianjie Feng, and Hong Hou. 2023. "A New Method for Ecological Risk Assessment of Combined Contaminated Soil" Toxics 11, no. 5: 411. https://doi.org/10.3390/toxics11050411