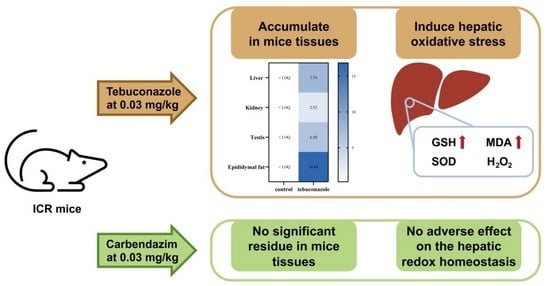
Low Dose of Carbendazim and Tebuconazole: Accumulation in Tissues and Effects on Hepatic Oxidative Stress in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Experimental Design
2.3. Biochemical Analysis
2.4. Pesticide Residue Analysis in the Tissues of Mice
2.5. LC–MS/MS Analysis
2.6. Analytical Method Validation
2.7. Statistical Analysis
3. Results
3.1. Method Validation
3.2. Accumulation and Distribution of Carbendazim and Tebuconazole in Mice
3.3. Effects of Carbendazim and Tebuconazole on Hepatic Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selmanoglu, G.; Barlas, N.; Songur, S.; Kockaya, E.A. Carbendazim-induced haematological, biochemical and histopathological changes to the liver and kidney of male rats. Hum. Exp. Toxicol. 2001, 20, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Tortella, G.R.; Mella-Herrera, R.A.; Sousa, D.Z.; Rubilar, O.; Briceno, G.; Parra, L.; Diez, M.C. Carbendazim dissipation in the biomixture of on-farm biopurification systems and its effect on microbial communities. Chemosphere 2013, 93, 1084–1093. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Wu, S.; Wang, Y.; An, X.; Cai, L.; Zhao, X.; Wu, C. Carbendazim has the potential to induce oxidative stress, apoptosis, immunotoxicity and endocrine disruption during zebrafish larvae development. Toxicol Vitr. 2015, 29, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Ezeoyili, I.C.; Mgbenka, B.O.; Atama, C.I.; Ngwu, G.I.; Madu, J.C.; Nwani, C.D. Changes in Brain Acetylcholinesterase and Oxidative Stress Biomarkers in African Catfish Exposed to Carbendazim. J. Aquat. Anim. Health 2019, 31, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Ebedy, Y.A.; Hassanen, E.I.; Hussien, A.M.; Ibrahim, M.A.; Elshazly, M.O. Neurobehavioral Toxicity Induced by Carbendazim in Rats and the Role of iNOS, Cox-2, and NF-kappaB Signalling Pathway. Neurochem. Res. 2022, 47, 1956–1971. [Google Scholar] [CrossRef]
- Ebedy, Y.A.; Elshazly, M.O.; Hassan, N.H.; Ibrahim, M.A.; Hassanen, E.I. Novel insights into the potential mechanisms underlying carbendazim-induced hepatorenal toxicity in rats. J. Biochem. Mol. Toxicol. 2022, 36, e23079. [Google Scholar] [CrossRef]
- Jin, C.; Zeng, Z.; Wang, C.; Luo, T.; Wang, S.; Zhou, J.; Ni, Y.; Fu, Z.; Jin, Y. Insights into a Possible Mechanism Underlying the Connection of Carbendazim-Induced Lipid Metabolism Disorder and Gut Microbiota Dysbiosis in Mice. Toxicol. Sci. 2018, 166, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Rama, E.M.; Bortolan, S.; Vieira, M.L.; Gerardin, D.C.; Moreira, E.G. Reproductive and possible hormonal effects of carbendazim. Regul. Toxicol. Pharmacol. 2014, 69, 476–486. [Google Scholar] [CrossRef]
- Markelewicz, R.J., Jr.; Hall, S.J.; Boekelheide, K. 2,5-hexanedione and carbendazim coexposure synergistically disrupts rat spermatogenesis despite opposing molecular effects on microtubules. Toxicol. Sci. 2004, 80, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Correa, L.M.; Nakai, M.; Strandgaard, C.S.; Hess, R.A.; Miller, M.G. Microtubules of the mouse testis exhibit differential sensitivity to the microtubule disruptors Carbendazim and colchicine. Toxicol. Sci. 2002, 69, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Rajeswary, S.; Kumaran, B.; Ilangovan, R.; Yuvaraj, S.; Sridhar, M.; Venkataraman, P.; Srinivasan, N.; Aruldhas, M.M. Modulation of antioxidant defense system by the environmental fungicide carbendazim in Leydig cells of rats. Reprod. Toxicol. 2007, 24, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, P.; Zhao, Y.; Zhang, H. Low dose carbendazim disrupts mouse spermatogenesis might Be through estrogen receptor related histone and DNA methylation. Ecotoxicol. Environ. Saf. 2019, 176, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, F.; Liu, X.; Xu, J.; Li, J.; Kong, Z.; Chen, X.; Liang, X.; Zheng, Y. Simultaneous enantioselective determination of triazole fungicides in soil and water by chiral liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2012, 1224, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Xu, H.; Yao, S.; He, Y.; Zhang, H.; Yu, Y. Chiral triazole fungicide tebuconazole: Enantioselective bioaccumulation, bioactivity, acute toxicity, and dissipation in soils. Environ. Sci. Pollut. Res. Int. 2018, 25, 25468–25475. [Google Scholar] [CrossRef]
- Kahle, M.; Buerge, I.J.; Hauser, A.; Muller, M.D.; Poiger, T. Azole fungicides: Occurrence and fate in wastewater and surface waters. Environ. Sci. Technol. 2008, 42, 7193–7200. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.; Zhou, M.; Hou, Y.; Xie, Y.; Li, G.; Sang, N. Tebuconazole induces liver injury coupled with ROS-mediated hepatic metabolism disorder. Ecotoxicol. Environ. Saf. 2021, 220, 112309. [Google Scholar] [CrossRef]
- Taxvig, C.; Hass, U.; Axelstad, M.; Dalgaard, M.; Boberg, J.; Andeasen, H.R.; Vinggaard, A.M. Endocrine-disrupting activities in vivo of the fungicides tebuconazole and epoxiconazole. Toxicol. Sci. 2007, 100, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Altenhofen, S.; Nabinger, D.D.; Wiprich, M.T.; Pereira, T.C.B.; Bogo, M.R.; Bonan, C.D. Tebuconazole alters morphological, behavioral and neurochemical parameters in larvae and adult zebrafish (Danio rerio). Chemosphere 2017, 180, 483–490. [Google Scholar] [CrossRef]
- Li, G.; Li, D.; Rao, H.; Liu, X. Potential neurotoxicity, immunotoxicity, and carcinogenicity induced by metribuzin and tebuconazole exposure in earthworms (Eisenia fetida) revealed by transcriptome analysis. Sci. Total Environ. 2022, 807, 150760. [Google Scholar] [CrossRef]
- Ben Othmene, Y.; Monceaux, K.; Belhadef, A.; Karoui, A.; Ben Salem, I.; Boussabbeh, M.; Abid-Essefi, S.; Lemaire, C. Triazole fungicide tebuconazole induces apoptosis through ROS-mediated endoplasmic reticulum stress pathway. Environ. Toxicol. Pharmacol. 2022, 94, 103919. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Li, F.; Liu, J. Triazole fungicide tebuconazole disrupts human placental trophoblast cell functions. J. Hazard. Mater. 2016, 308, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.M.P.; Linhares, B.S.; Oliveira, J.M.; Leite, J.P.V.; da Matta, S.L.P.; Goncalves, R.V.; Freitas, M.B. Tebuconazole-induced toxicity and the protective effect of Ficus carica extract in Neotropical fruit-eating bats. Chemosphere 2021, 275, 129985. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Y.; Sun, Q.; Coffin, S.; Chen, L.; Qiao, K.; Gui, W.; Zhu, G. Tebuconazole induced oxidative stress related hepatotoxicity in adult and larval zebrafish (Danio rerio). Chemosphere 2020, 241, 125129. [Google Scholar] [CrossRef] [PubMed]
- Othmene, Y.B.; Hamdi, H.; Salem, I.B.; Annabi, E.; Amara, I.; Neffati, F.; Najjar, M.F.; Abid-Essefi, S. Oxidative stress, DNA damage and apoptosis induced by tebuconazole in the kidney of male Wistar rat. Chem. Biol. Interact. 2020, 330, 109114. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Sun, W.; Liu, W.; Wang, Y.; Jia, M.; Tian, S.; Chen, X.; Zhu, W.; Zhou, Z. A common fungicide tebuconazole promotes colitis in mice via regulating gut microbiota. Environ. Pollut. 2022, 292, 118477. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Joint FAO/WHO Meeting on Pesticide Residues on Carbendazim; WHO: Geneva, Switzerland, 1995; pp. 50–53.
- FAO/WHO. Joint FAO/WHO Meeting on Pesticide Residues on Tebuconazole; WHO: Geneva, Switzerland, 2016; pp. 501–558.
- Ibrahim, I.; Togola, A.; Gonzalez, C. In-situ calibration of POCIS for the sampling of polar pesticides and metabolites in surface water. Talanta 2013, 116, 495–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakanov, N.; Wieczorek, M.V.; Schulz, R. The role of organic matrices in the fate of hydrophobic pesticides: An outdoor stream mesocosm study. Chemosphere 2020, 259, 127459. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, Y.; Yu, Y.; Li, Z.; Lin, L.; Chen, Q.; Xu, Q.; Pan, P.; Wang, Y.; Ge, R.S. Gestational exposure to tebuconazole affects the development of rat fetal Leydig cells. Chemosphere 2021, 262, 127792. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Q.; Li, X.; Huang, T.; Wang, S.; Wang, Y.; Chen, X.; Lin, Z.; Ge, R.S. Pubertal exposure to tebuconazole increases testosterone production via inhibiting testicular aromatase activity in rats. Chemosphere 2019, 230, 519–526. [Google Scholar] [CrossRef]
- Abolaji, A.O.; Awogbindin, I.O.; Adedara, I.A.; Farombi, E.O. Insecticide chlorpyrifos and fungicide carbendazim, common food contaminants mixture, induce hepatic, renal, and splenic oxidative damage in female rats. Hum. Exp. Toxicol. 2017, 36, 483–493. [Google Scholar] [CrossRef]
- Mirzaei, A.; Sepehri, S.; Sadeghi, H.; Alamdari, A. Protecting impact of Jaft against carbendazim induced biochemical changes in male Wistar rats. J. Med. Life 2015, 8, 96–100. [Google Scholar] [PubMed]
- Yang, J.D.; Liu, S.H.; Liao, M.H.; Chen, R.M.; Liu, P.Y.; Ueng, T.H. Effects of tebuconazole on cytochrome P450 enzymes, oxidative stress, and endocrine disruption in male rats. Environ. Toxicol. 2018, 33, 899–907. [Google Scholar] [CrossRef]
- Othmene, Y.B.; Hamdi, H.; Amara, I.; Abid-Essefi, S. Tebuconazole induced oxidative stress and histopathological alterations in adult rat heart. Pestic. Biochem. Physiol. 2020, 170, 104671. [Google Scholar] [CrossRef] [PubMed]
- Moser, V.C.; Barone, S., Jr.; Smialowicz, R.J.; Harris, M.W.; Davis, B.J.; Overstreet, D.; Mauney, M.; Chapin, R.E. The effects of perinatal tebuconazole exposure on adult neurological, immunological, and reproductive function in rats. Toxicol. Sci. 2001, 62, 339–352. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ji, B.; Hao, X.; Li, X.; Eisele, F.; Nystrom, T.; Petranovic, D. FMN reduces Amyloid-beta toxicity in yeast by regulating redox status and cellular metabolism. Nat. Commun. 2020, 11, 867. [Google Scholar] [CrossRef] [Green Version]
- Appenzeller-Herzog, C. Glutathione- and non-glutathione-based oxidant control in the endoplasmic reticulum. J. Cell Sci. 2011, 124, 847–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, R.S.F.; Venancio, C.A.S.; Felix, L.M. Behavioural impairment and oxidative stress by acute exposure of zebrafish to a commercial formulation of tebuconazole. Environ. Toxicol. Pharmacol. 2022, 91, 103823. [Google Scholar] [CrossRef]
- Burckhardt, I.C.; Gozal, D.; Dayyat, E.; Cheng, Y.; Li, R.C.; Goldbart, A.D.; Row, B.W. Green tea catechin polyphenols attenuate behavioral and oxidative responses to intermittent hypoxia. Am. J. Respir. Crit. Care Med. 2008, 177, 1135–1141. [Google Scholar] [CrossRef] [Green Version]
- Chaabane, M.; Koubaa, M.; Soudani, N.; Elwej, A.; Grati, M.; Jamoussi, K.; Boudawara, T.; Ellouze Chaabouni, S.; Zeghal, N. Nitraria retusa fruit prevents penconazole-induced kidney injury in adult rats through modulation of oxidative stress and histopathological changes. Pharm. Biol. 2017, 55, 1061–1073. [Google Scholar] [CrossRef] [Green Version]
- Ben Othmene, Y.; Monceaux, K.; Karoui, A.; Ben Salem, I.; Belhadef, A.; Abid-Essefi, S.; Lemaire, C. Tebuconazole induces ROS-dependent cardiac cell toxicity by activating DNA damage and mitochondrial apoptotic pathway. Ecotoxicol. Environ. Saf. 2020, 204, 111040. [Google Scholar] [CrossRef]

| Compounds | Parent Mass (m/z) | Product Mass (m/z) | Collision Energy (eV) |
|---|---|---|---|
| Carbendazim | 192.10 | 160.10 * | −18.0 |
| 132.10 | −30.0 | ||
| 105.15 | −39.0 | ||
| Tebuconazole | 308.10 | 70.05 * | −24.0 |
| 125.05 | −37.0 | ||
| 151.05 | −26.0 |
| Sample | Concentration (μg/kg) | Carbendazim | Tebuconazole |
|---|---|---|---|
| Recovery ± RSD * (%) | Recovery ± RSD (%) | ||
| Liver | 100 | 86.8 ± 2.4 | 93.2 ± 2.7 |
| 10 | 92.2 ± 3.5 | 91.7 ± 2.6 | |
| 1 | 89.5 ± 5.5 | 88.9 ± 6.2 | |
| Kidney | 50 | 93.8 ± 1.6 | 92.2 ± 3.6 |
| 5 | 87.2 ± 6.3 | 90.7 ± 4.7 | |
| 0.5 | 86.5 ± 5.7 | 87.6 ± 6.4 | |
| Testis | 50 | 91.3 ± 2.6 | 90.0 ± 3.5 |
| 5 | 87.9 ± 5.8 | 84.6 ± 4.9 | |
| 0.5 | 84.3 ± 6.7 | 79.7 ± 5.5 | |
| Epididymal fat | 50 | 90.0 ± 2.1 | 93.5 ± 2.8 |
| 5 | 86.4 ± 2.2 | 89.2 ± 3.1 | |
| 0.5 | 76.4 ± 7.6 | 73.9 ± 8.9 |
| Sample | Carbendazim | Tebuconazole | |
|---|---|---|---|
| Liver | Linear calibration curve | y = 5861.5x + 10661 | y = 7526.2x + 7837.6 |
| Linearity range (μg/kg) | 1–100 | 1–100 | |
| R2 * | 0.9997 | 0.9999 | |
| LOQ (μg/kg) | 1 | 1 | |
| Kidney | Linear calibration curve | y = 6785.2x + 8507.2 | y = 8335.2x + 11684 |
| Linearity range (μg/kg) | 0.5–50 | 0.5–50 | |
| R2 | 0.9997 | 0.9994 | |
| LOQ (μg/kg) | 0.5 | 0.5 | |
| Testis | Linear calibration curve | y = 7972.5x + 11211 | y = 7906.2x + 8596.5 |
| Linearity range (μg/kg) | 0.5–50 | 0.5–50 | |
| R2 | 0.9996 | 0.9978 | |
| LOQ (μg/kg) | 0.5 | 0.5 | |
| Epididymal fat | Linear calibration curve | y = 11256x − 10722 | y = 8573.1x − 6992.8 |
| Linearity range (μg/kg) | 0.5–50 | 0.5–50 | |
| R2 | 0.9937 | 0.9955 | |
| LOQ (μg/kg) | 0.5 | 0.5 |
| Sample | Carbendazim Residual Range (μg/kg) | Tebuconazole Residual Range (μg/kg) |
|---|---|---|
| Liver | ND * | ND-24.33 |
| Kidney | ND | ND |
| Testis | ND | ND-15.61 |
| Epididymal fat | ND | 12.71–21.98 |
| Sample | Carbendazim Residual Range (μg/kg) | Tebuconazole Residual Range (μg/kg) |
|---|---|---|
| Liver | ND * | ND-20.30 |
| Kidney | ND | ND |
| Testis | ND | ND-53.97 |
| Epididymal fat | ND | 14.93–55.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Chen, X.; Hou, H.; Liu, D.; Liu, X.; Wang, P.; Zhou, Z. Low Dose of Carbendazim and Tebuconazole: Accumulation in Tissues and Effects on Hepatic Oxidative Stress in Mice. Toxics 2023, 11, 326. https://doi.org/10.3390/toxics11040326
Ma X, Chen X, Hou H, Liu D, Liu X, Wang P, Zhou Z. Low Dose of Carbendazim and Tebuconazole: Accumulation in Tissues and Effects on Hepatic Oxidative Stress in Mice. Toxics. 2023; 11(4):326. https://doi.org/10.3390/toxics11040326
Chicago/Turabian StyleMa, Xiaoran, Xin Chen, Haonan Hou, Donghui Liu, Xueke Liu, Peng Wang, and Zhiqiang Zhou. 2023. "Low Dose of Carbendazim and Tebuconazole: Accumulation in Tissues and Effects on Hepatic Oxidative Stress in Mice" Toxics 11, no. 4: 326. https://doi.org/10.3390/toxics11040326