Emulsion-Based Delivery Systems to Enhance the Functionality of Bioactive Compounds: Towards the Use of Ingredients from Natural, Sustainable Sources
Abstract
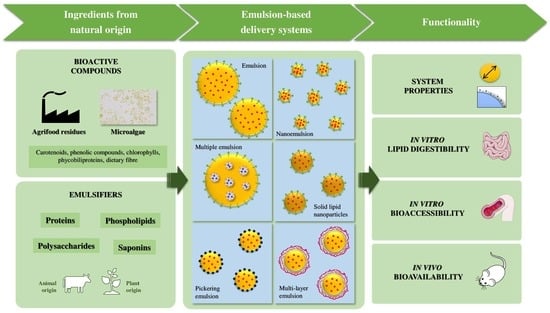
:1. Introduction
2. Bioactive Compounds
2.1. Novel Alternative Sources of Bioactive Compounds
2.1.1. Microalgae
- Carotenoids: It has been found that microalgae can produce and accumulate carotenoids, with Chlorophyceae being the dominant carotenoid-producing group of xanthophylls and carotenes [23]. Specifically, Dunaliella salina and Haematococcus pluvialis are commonly used for high-value carotenoid production due to their high content of carotenoids such as β-carotene and astaxanthin, which can represent up to 14% of the microalgae dry biomass [24]. Other compounds such as lutein have been identified in Muriellopsis sp., although the concentrations were lower (0.4% to 0.6% per dry biomass) [25].
- Polyunsaturated fatty acids: In microalgae, fatty acids cover the largest percentage of total lipids, with polyunsaturated fatty acids (PUFAs) representing 20–60% of the total lipids [26]. Spirulina and Chlorella are valuable sources of PUFAs such as docosahexaenoic acid (DHA), arachidonic acid (ARA), alpha-lipoic acid (ALA), and eicosapentaenoic acid (EPA) [27]. Microalgae can represent an interesting vegan source of fatty acids that, up to date, have been obtained mostly from animal sources such as fish oil.
- Phenolic compounds: Although high concentrations have been observed in macroalgae, microalgae such as Chlorella or Arthrospira have been found to contain appreciable levels of phenolic compounds. However, according to the literature, the concentration in microalgae present significant variations due to species type, cultivation conditions, and techniques used for extraction, identification, and quantification [22].
- Chlorophylls: These natural green pigments are crucial in photosynthetic organisms for harvesting energy from sunlight and can be classified as a, b, or c [22]. However, chlorophyll c is present only in brown algae and not in green algae. Among microalgae species, Chlorella is the main producer of chlorophyll, with other species such as Spirulina and Arthrospira producing limited concentrations [28].
- Peptides: Microalgal proteins have been demonstrated to be a source of bioactive peptides after enzymatic hydrolysis. Due to their differentiated sequential, structural, and compositional properties, microalgae peptides exert a list of positive health effects such as antioxidant, antihypertensive, antitumor, and immunomodulatory effects [27,29].
2.1.2. Co-Products from the Agrifood Industry
- Carotenoids: Various agrifood residues such as tomato peel [35], guarana peel [36] or peel, and the pulp of citrus fruits [37] have been found to contain carotenoids such as lycopene, β-carotene, or lutein. According to these authors, these agrifood residues can contain variable concentrations that can be up to 60% carotenoids per unit of dry weight.
- Chlorophylls: These green pigment compounds, which can be classified as chlorophyll a, b, or c, have been found in residues from different vegetables, especially in the leaves. As an example, chlorophyll a and b, in concentrations ranging from 1132.33 to 1795.93 ppm, were detected as co-products from olive leaves [42,43]. However, higher concentrations have been detected in the leaf residues from broccoli [44] (up to 4477.9 µg/g dry weight) or asparagus [45] (up to 5096 µg/g dry weight).
- Dietary fibre: Both soluble and insoluble fibre have been found in residues from vegetables such as artichoke, carrot, or pepper [46], as well as fruits such as guava or passion fruit [47]. However, most of these residues contained higher amounts of the insoluble fraction rather than the soluble one. In addition, diverse cereal residues have been found to contain soluble (beta-glucans) and insoluble dietary fibres (such as cellulose or lignin) in variable concentrations [48].
2.2. Extraction Methods of Bioactive Compounds
3. Emulsion-Based Delivery Systems to Carry Bioactive Compounds
| System Type | Preparation Techniques | Particle Size | Advantages | Limitations |
|---|---|---|---|---|
| Conventional emulsions |
| 500 nm–100 µm |
|
|
| Nanoemulsions |
| <500 nm |
|
|
| Multiple emulsions |
| Micrometric |
|
|
| Multilayer emulsions |
| Nanometric to micrometric |
|
|
| Pickering emulsions |
| Nanometric to micrometric |
|
|
| Solid-lipid nanoparticles (SLN) |
| 50 nm–1 µm |
|
|
| Nanostructured lipid carriers (NLC) |
| 10 nm–0.5 µm |
|
|
4. Natural-Based Stabilisers for Emulsion-Based Delivery Systems
4.1. Proteins
4.2. Phospholipids
4.3. Polysaccharides
4.4. Saponins
5. Functionality of Emulsion-Based Delivery Systems Containing Natural Emulsifiers
5.1. In Vitro Lipid Digestibility and Bioactive Compound Bioaccessibility
| System Type | Emulsifiers Used | Encapsulated Compound | Main Findings | Reference |
|---|---|---|---|---|
| O/W emulsion | Soybean lecithin (SBL), hydrolysed rice glutelin (HRG) | None | SBL-emulsion was more stable against flocculation under gastric conditions and presented higher digestibility than HRG-emulsion. | [131] |
| O/W emulsion | Lysolecithin (LL), Arabic gum (AG), caseinate (SC), quillaja saponin (QS), Tween 20 (T20) | β-carotene | Digestibility was lower for the emulsions stabilised by LL or SC, than those stabilised by AG, QS, or T20. β-carotene bioaccessibility increased in the following order: LL < AG < SC < QS < T20. | [127] |
| O/W emulsion | Quillaja saponin (QS), Arabic gum (AG), whey protein isolate (WPI) | Vitamin E | Lipid digestion was slower in QS-emulsions, presumably because the high surface activity of saponins inhibited their removal by bile acids and lipase. Vitamin E bioaccessibility was higher in WPI- than in QS- or AG-emulsions. | [129] |
| O/W emulsion | Cetyltrimethylammonium bromide (CTAB), Citrem, sodium caseinate (SC), fish gelatin (FG), Arabic gum (AG), or modified starch (MS) | Linseed oil (rich in omega-3 PUFA) | Emulsions prepared with CTAB and GA were the most stable under gastric conditions, while those stabilised by proteins (SC or FG) and MS showed aggregation with partial coalescence in the gastric phase. AG-emulsion showed the highest FFA extent, followed by CTAB- and SC- emulsions. | [125] |
| O/W emulsion | Arabic gum (AG), ghatti gum (GG), or sugar beet pectin (SBP) | None | The digestion rate decreased in the following order: AG > SBP > GG. Differences were attributed to the stability of the emulsified lipid droplets in the stimulated intestinal juice and the resistance of interfacial layer against displacement by bile salts. | [132] |
| O/W emulsion | Ulva fasciata polysaccharide (UFP), Arabic gum (AG), or beet pectin (BP) | β-carotene | UFP-stabilised emulsion showed higher release extent of free fatty acids and bioaccessibility of carotenoids compared to BP and AG-stabilised emulsions. | [115] |
| O/W emulsion | Tween 80 (TW), phosphatidylcholine (PC), or citrus pectin (CP) | β-carotene | T80-emulsion presented a higher β-carotene bioaccessibility than those with PC or CP, and it was associated with the higher concentration of incorporated MAG and FFA into the micellar fraction by using T80-emulsion. | [133] |
| W/O/W double emulsion | Lecithin (L), pectin (P), black bean protein (BBP), or Tween 80 (T80) | Insulin and quercetin | The BBP-stabilised double emulsion presented the lowest particle size during the GIT digestion. Moreover, it yielded a 2.60- and 4.56-fold increase in the bioaccessibility of insulin and quercetin, respectively, by increasing their chemical stability and solubility under simulated gastrointestinal conditions. | [66] |
| W/O/W double emulsion | gelatin-epigallocatechin gallate (EGCG)-high methoxyl pectin ternary complex | Vitamin C | Gelatin-EGCG-high methoxyl pectin ternary complex had a better protective effect on vitamin C in the internal aqueous phase during in vitro simulated digestion. Compared with the W1/O primary emulsion, the double emulsion effectively improved the bioavailability of vitamin C. | [67] |
| Multilayer emulsion | Quillaja saponin (QS), chitosan (CS), pectin (P) | Astaxanthin | Coating layers of CS and P improved the lipid stability during gastrointestinal digestion and reduced the release of free fatty acids (by nearly 20%). Meanwhile, the release of Astaxanthin was prolonged in the small intestine, and its final bioaccessibility was improved by the coating layers. | [134] |
| Multilayer emulsion | Sodium caseinate (SC), sulphated fucan (SF), ι-carrageenan (ICA), κ-carrageenan (KCA), or alginate (ALG) | None | All studied multilayer emulsions presented an increased digestibility compared to the primary emulsion. Moreover, the digestion rate and degree of multilayer emulsions decreased in the order of KCA > ALG ≈ ICA > SF. | [135] |
| Pickering emulsion | Nanochitin (NCh) | Vitamin D3 | NCh–Pickering emulsions presented lower digestibility and vitamin bioaccessibility than T80-emulsions as a consequence of flocculation, hindered access for lipase to reach lipid, and precipitation of mixed micelles. | [136] |
| Pickering emulsion | Chitosan (CS) | Roasted coffee oil | CS nanoparticles were shown to be able to adsorb onto oil droplet surfaces, providing efficiency in encapsulating and protecting bioactive compounds during lipid digestion and increasing the bioaccessibility of phenolic compounds. | [137] |
| Pickering emulsion | Nanofibrillated cellulose (NFC) or whey protein isolate (WPI) | Astaxanthin | Pickering emulsions containing 0.7% NFC presented higher compound stability during digestion than emulsions with 0.7% WPI. However, they presented the same astaxanthin bioaccessibility due to the reduced digestibility of NFC emulsions. | [138] |
5.2. In Vivo Bioavailability
| Bioactive Compound | Dose | System Type | Animal Model | Ingredients | Outcomes | Reference |
|---|---|---|---|---|---|---|
| β-carotene | 1 mg/kg BW | O/W nanoemulsion | mice | 10% corn oil; 2% whey protein isolate | Nanoemulsions increased transportation and absorption of β-carotene in the digestive tract compared to macroemulsions. | [142] |
| 60 mg/kg BW | O/W nanoemulsion | rat | 30% corn oil; 12% whey protein isolate; or soybean lecithin | Nanoemulsions containing protein-based emulsifiers better increased the bioavailability of β-carotene than those containing soybean lecithin. | [59] | |
| Carotenoids (from fresh spinach puree) | 1.0, 0.6, 0.2, and 0 g/kg BW | O/W nanoemulsion (excipient) | rat | 10% oil (medium-chain triglyceride and long-chain triglyceride 1:1); 1% sodium caseinate | Carotenoid bioavailability was enhanced by increasing the lipid content due to the higher transfer efficiency of the carotenoids from spinach to fat droplets and mixed micelles. | [11] |
| Cholecalciferol (VD3) | 4000 IU kg−1 supplementation | O/W emulsion or nanoemulsion | mice | 10% corn oil; 2% quillaja saponin | Nano-based delivery system improved the bioavailability and homogeneity of VD absorption. | [61] |
| Coenzyme Q10 | 30 mg/kg BW | O/W nanoemulsion | rat | 10% soybean oil; 1–10% lecithin | Incorporation of Coenzyme Q10 to nanoemulsions increased the bioavailability of the bioactive compound by 1.8-fold. | [146] |
| Tangeretin | 100 mg/kg BW | O/W emulsion | rat | 20% medium-chain triglyceride oil, whey protein concentrate, and gum Arabic, or cinnamaldehyde, or hydroxypropyl methylcellulose | Tangeretin bioavailability increased from 4- to 20-fold after encapsulation, especially in the presence of hydroxypropyl methylcellulose. | [147] |
| α-tocopherol | 100 mg/kg BW | O/W emulsion, submicron emulsion and nanoemulsion | rat | 10% sunflower oil; 0.1% saponins | By reducing the particle size of emulsions, the bioavailability of α-tocopherol was enhanced, which was 3 times higher when the nanoemulsion was used than when the emulsion was used. | [145] |
| EPA | 60 mg/kg BW | Pickering emulsion | mice | 60% oil; 4% pea protein–chitosan nanoparticles | EPA-loaded Pickering emulsions containing pea protein —chitosan nanoparticles were shown to be more effective in increasing EPA bioavailability than an emulsion containing Tween 80. | [144] |
| Curcumin | 12 mg/kg BW | Multilayer emulsion | mice | 10% medium chain oil and 90% WPI aqueous solution (1%), 0.2% chitosan (CS), and 0.1 carboxymethyl konjac glucomannan (CKG) | Emulsions coater with CKG or CS + CKG conferred a higher Cmax value and improved the bioavailability of curcumin by up to 5-fold compared with free curcumin. | [143] |
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potter, J.D. Vegetables, Fruit, and Cancer. Lancet 2005, 366, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, L.A.; Serdula, M.K.; Liu, S. Dietary Intake of Fruits and Vegetables and Risk of Cardiovascular Disease. Curr. Atheroscler. Rep. 2003, 5, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Dietary Bioactive Compounds and Their Health Implications. J. Food Sci. 2013, 78, A18–A25. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in Micro and Nano-Encapsulation of Bioactive Compounds Using Biopolymer and Lipid-Based Transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Hu, D.; Xu, Y.; Xie, J.; Sun, C.; Zheng, X.; Chen, W. Systematic Evaluation of Phenolic Compounds and Protective Capacity of a New Mulberry Cultivar J33 against Palmitic Acid-Induced Lipotoxicity Using a Simulated Digestion Method. Food Chem. 2018, 258, 43–50. [Google Scholar] [CrossRef]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef]
- Baysal, G.; Olcay, H.S.; Keresteci, B.; Özpinar, H. The Antioxidant and Antibacterial Properties of Chitosan Encapsulated with the Bee Pollen and the Apple Cider Vinegar. J. Biomater. Sci. Polym. Ed. 2022, 33, 995–1011. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, J.; Zhang, Y.; Wen, Z.; Zhao, L. Physicochemical Stability and in Vitro Bioaccessibility of β-Carotene Nanoemulsions Stabilized with Whey Protein-Dextran Conjugates. Food Hydrocoll. 2017, 63, 256–264. [Google Scholar] [CrossRef]
- Zheng, B.; Zhang, X.; Peng, S.; McClements, D.J. Impact of Curcumin Delivery System Format on Bioaccessibility: Nanocrystals, Nanoemulsion Droplets, and Natural Oil Bodies. Food Funct. 2019, 10, 4339–4349. [Google Scholar] [CrossRef]
- Yao, K.; McClements, D.J.; Yan, C.; Xiao, J.; Liu, H.; Chen, Z.; Hou, X.; Cao, Y.; Xiao, H.; Liu, X. In Vitro and in Vivo Study of the Enhancement of Carotenoid Bioavailability in Vegetables Using Excipient Nanoemulsions: Impact of Lipid Content. Food Res. Int. 2021, 141, 110162. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Montoliu-Boneu, J.; Salvia-Trujillo, L.; Martín-Belloso, O. Factors Affecting the Formation of Highly Concentrated Emulsions and Nanoemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123577. [Google Scholar] [CrossRef]
- Giroux, H.J.; Robitaille, G.; Britten, M. Controlled Release of Casein-Derived Peptides in the Gastrointestinal Environment by Encapsulation in Water-in-Oil-in-Water Double Emulsions. LWT—Food Sci. Technol. 2016, 69, 225–232. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Molet-Rodríguez, A.; Salvia-Trujillo, L.; Martín-Belloso, O. Formation of Double (W 1/O/W 2) Emulsions as Carriers of Hydrophilic and Lipophilic Active Compounds. Food Bioprocess Technol. 2019, 12, 422–435. [Google Scholar] [CrossRef] [Green Version]
- Aditya, N.P.; Aditya, S.; Yang, H.; Kim, H.W.; Park, S.O.; Ko, S. Co-Delivery of Hydrophobic Curcumin and Hydrophilic Catechin by a Water-in-Oil-in-Water Double Emulsion. Food Chem. 2015, 173, 7–13. [Google Scholar] [CrossRef]
- Dammak, I.; Sobral, P.J.d.A.; Aquino, A.; das Neves, M.A.; Conte-Junior, C.A. Nanoemulsions: Using Emulsifiers from Natural Sources Replacing Synthetic Ones—A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2721–2746. [Google Scholar] [CrossRef]
- Gao, H.T.; Xu, R.; Cao, W.X.; Zhou, X.; Yan, Y.H.M.; Lu, L.; Xu, Q.; Shen, Y. Food Emulsifier Glycerin Monostearate Increases Internal Exposure Levels of Six Priority Controlled Phthalate Esters and Exacerbates Their Male Reproductive Toxicities in Rats. PLoS ONE 2016, 11, e0161253. [Google Scholar] [CrossRef] [Green Version]
- Garcia, M.T.; Kaczerewska, O.; Ribosa, I.; Brycki, B.; Materna, P.; Drgas, M. Biodegradability and Aquatic Toxicity of Quaternary Ammonium-Based Gemini Surfactants: Effect of the Spacer on Their Ecological Properties. Chemosphere 2016, 154, 155–160. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J.; Bai, L.; Chung, C. Recent Advances in the Utilization of Natural Emulsifiers to Form and Stabilize Emulsions. Annu. Rev. Food Sci. Technol. 2017, 8, 205–236. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X.; Chen, J.; Wang, T.; Huang, X.; Chen, G. The Extraction of β-Carotene from Microalgae for Testing Their Health Benefits. Foods 2022, 11, 502. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.d.l.L.; Villegas-Aguilar, M.d.C.; Leyva-Jiménez, F.J.; Pimentel-Moral, S.; Fernández-Ochoa, Á.; Alañón, M.E.; Segura-Carretero, A. Revalorization of Bioactive Compounds from Tropical Fruit By-Products and Industrial Applications by Means of Sustainable Approaches. Food Res. Int. 2020, 138, 109786. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, J.; Abbey, L. Microalgae: Bioactive Composition, Health Benefits, Safety and Prospects as Potential High-Value Ingredients for the Functional Food Industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Mazzucchi, L.; Acharya, P.; Xu, Y.; Morgan, G.; Harvey, P.J. A Comparison of β-Carotene, Phytoene and Amino Acids Production in Dunaliella Salina DF 15 (CCAP 19/41) and Dunaliella Salina CCAP 19/30 Using Different Light Wavelengths. Foods 2021, 10, 2824. [Google Scholar] [CrossRef]
- Tamaki, S.; Mochida, K.; Suzuki, K. Diverse Biosynthetic Pathways and Protective Functions against Environmental Stress of Antioxidants in Microalgae. Plants 2021, 10, 1250. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, E.B.; Antoniosi Filho, N.R. Concepts and Studies on Lipid and Pigments of Microalgae: A Review. Renew. Sustain. Energy Rev. 2016, 58, 832–841. [Google Scholar] [CrossRef]
- Liang, M.H.; Wang, L.; Wang, Q.; Zhu, J.; Jiang, J.G. High-Value Bioproducts from Microalgae: Strategies and Progress. Crit. Rev. Food Sci. Nutr. 2019, 59, 2423–2441. [Google Scholar] [CrossRef]
- Zhou, L.; Li, K.; Duan, X.; Hill, D.; Barrow, C.; Dunshea, F.; Martin, G.; Suleria, H. Bioactive Compounds in Microalgae and Their Potential Health Benefits. Food Biosci. 2022, 49, 101932. [Google Scholar] [CrossRef]
- Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.N.; Gayen, K.; Bhowmick, T.K. Downstream Processing of Microalgae for Pigments, Protein and Carbohydrate in Industrial Application: A Review. Food Bioprod. Process. 2018, 110, 60–84. [Google Scholar] [CrossRef]
- Hamidi, M.; Safarzadeh Kozani, P.; Safarzadeh Kozani, P.; Pierre, G.; Michaud, P.; Delattre, C. Marine Bacteria versus Microalgae: Who Is the Best for Biotechnological Production of Bioactive Compounds with Antioxidant Properties and Other Biological Applications? Mar. Drugs 2019, 18, 28. [Google Scholar] [CrossRef] [Green Version]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Larkum, A.W.D. Limitations and Prospects of Natural Photosynthesis for Bioenergy Production. Curr. Opin. Biotechnol. 2010, 21, 271–276. [Google Scholar] [CrossRef]
- Lemes, A.C.; Egea, M.B.; Oliveira Filho, J.G.d.; Gautério, G.V.; Ribeiro, B.D.; Coelho, M.A.Z. Biological Approaches for Extraction of Bioactive Compounds From Agro-Industrial By-Products: A Review. Front. Bioeng. Biotechnol. 2022, 9, 1413. [Google Scholar] [CrossRef]
- Chauhan, C.; Dhir, A.; Akram, M.U.; Salo, J. Food Loss and Waste in Food Supply Chains. A Systematic Literature Review and Framework Development Approach. J. Clean. Prod. 2021, 295, 126438. [Google Scholar] [CrossRef]
- Chaouch, M.A.; Benvenuti, S. The Role of Fruit By-Products as Bioactive Compounds for Intestinal Health. Foods 2020, 9, 1716. [Google Scholar] [CrossRef]
- Silva, Y.P.A.; Borba, B.C.; Pereira, V.A.; Reis, M.G.; Caliari, M.; Brooks, M.S.L.; Ferreira, T.A.P.C. Characterization of Tomato Processing By-Product for Use as a Potential Functional Food Ingredient: Nutritional Composition, Antioxidant Activity and Bioactive Compounds. Int. J. Food Sci. Nutr. 2019, 70, 150–160. [Google Scholar] [CrossRef]
- Pinho, L.S.; da Silva, M.P.; Thomazini, M.; Cooperstone, J.L.; Campanella, O.H.; da Costa Rodrigues, C.E.; Favaro-Trindade, C.S. Guaraná (Paullinia Cupana) by-Product as a Source of Bioactive Compounds and as a Natural Antioxidant for Food Applications. J. Food Process. Preserv. 2021, 45, e15854. [Google Scholar] [CrossRef]
- Agócs, A.; Nagy, V.; Szabó, Z.; Márk, L.; Ohmacht, R.; Deli, J. Comparative Study on the Carotenoid Composition of the Peel and the Pulp of Different Citrus Species. Innov. Food Sci. Emerg. Technol. 2007, 8, 390–394. [Google Scholar] [CrossRef]
- Castellanos-Gallo, L.; Ballinas-Casarrubias, L.; Espinoza-Hicks, J.C.; Hernández-Ochoa, L.R.; Muñoz-Castellanos, L.N.; Zermeño-Ortega, M.R.; Borrego-Loya, A.; Salas, E. Grape Pomace Valorization by Extraction of Phenolic Polymeric Pigments: A Review. Processes 2022, 10, 469. [Google Scholar] [CrossRef]
- Baccouri, B.; Mechi, D.; Rajhi, I.; Vertedor, D.M. Tunisian Wild Olive Leaves: Phenolic Compounds and Antioxidant Activity as an Important Step Toward Their Valorization. Food Anal. Methods 2023, 16, 436–444. [Google Scholar] [CrossRef]
- Da Costa, R.S.; Santos, O.V.D.; da Silva Lannes, S.C.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Ribeiro-Costa, R.M.; Converti, A.; Silva Júnior, J.O.C. Bioactive Compounds and Value-Added Applications of Cupuassu (Theobroma grandiflorum Schum.) Agroindustrial by-Product. Food Sci. Technol. 2020, 40, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Nazeam, J.A.; AL-Shareef, W.A.; Helmy, M.W.; El-Haddad, A.E. Bioassay-Guided Isolation of Potential Bioactive Constituents from Pomegranate Agrifood by-Product. Food Chem. 2020, 326, 126993. [Google Scholar] [CrossRef] [PubMed]
- Bahloul, N.; Kechaou, N.; Mihoubi, N.B. Comparative Investigation of Minerals, Chlorophylls Contents, Fatty Acid Composition and Thermal Profiles of Olive Leaves (Olea europeae L.) as by-Product. Grasas y Aceites 2014, 65, e035. [Google Scholar] [CrossRef] [Green Version]
- Flamminii, F.; Di Mattia, C.D.; Difonzo, G.; Neri, L.; Faieta, M.; Caponio, F.; Pittia, P. From By-Product to Food Ingredient: Evaluation of Compositional and Technological Properties of Olive-Leaf Phenolic Extracts. J. Sci. Food Agric. 2019, 99, 6620–6627. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, L.; Ser, S.L.; Cumming, J.R.; Ku, K.M. Comparative Phytonutrient Analysis of Broccoli By-Products: The Potentials for Broccoli by-Product Utilization. Molecules 2018, 23, 900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitrakar, B.; Zhang, M.; Zhang, X.; Devahastin, S. Bioactive Dietary Fiber Powder from Asparagus Leaf By-Product: Effect of Low-Temperature Ball Milling on Physico-Chemical, Functional and Microstructural Characteristics. Powder Technol. 2020, 366, 275–282. [Google Scholar] [CrossRef]
- Vaz, A.A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Physicochemical Properties and Bioaccessibility of Phenolic Compounds of Dietary Fibre Concentrates from Vegetable By-Products. Foods 2022, 11, 2578. [Google Scholar] [CrossRef]
- Casarotti, S.N.; Borgonovi, T.F.; Batista, C.L.F.M.; Penna, A.L.B. Guava, Orange and Passion Fruit by-Products: Characterization and Its Impacts on Kinetics of Acidification and Properties of Probiotic Fermented Products. LWT 2018, 98, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Fărcaș, A.C.; Socaci, S.A.; Nemeș, S.A.; Pop, O.L.; Coldea, T.E.; Fogarasi, M.; Biriș-Dorhoi, E.S. An Update Regarding the Bioactive Compound of Cereal By-Products: Health Benefits and Potential Applications. Nutrients 2022, 14, 3470. [Google Scholar] [CrossRef]
- Banožić, M.; Babić, J.; Jokić, S. Recent Advances in Extraction of Bioactive Compounds from Tobacco Industrial Waste-a Review. Ind. Crops Prod. 2020, 144, 112009. [Google Scholar] [CrossRef]
- More, P.R.; Jambrak, A.R.; Arya, S.S. Green, Environment-Friendly and Sustainable Techniques for Extraction of Food Bioactive Compounds and Waste Valorization. Trends Food Sci. Technol. 2022, 128, 296–315. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A Comparative Study on Different Extraction Techniques to Recover Red Grape Pomace Polyphenols from Vinification Byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Fan, C.; Liu, Y.; Shan, Y.; Cao, X. A Priori Design of New Natural Deep Eutectic Solvent for Lutein Recovery from Microalgae. Food Chem. 2022, 376, 131930. [Google Scholar] [CrossRef]
- Low, K.L.; Idris, A.; Mohd Yusof, N. Novel Protocol Optimized for Microalgae Lutein Used as Food Additives. Food Chem. 2020, 307, 125631. [Google Scholar] [CrossRef]
- Kubiak, T. Polymeric Capsules and Micelles as Promising Carriers of Anticancer Drugs. Polim. Med. 2022, 52, 35–48. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, J.; Jiang, Y.; Meng, Y.; Zhang, K.; Ban, Q.; Wang, X. Emulsions Stabilized by Casein and Hyaluronic Acid: Effects of High Intensity Ultrasound on the Stability and Vitamin E Digestive Characteristics. Ultrason. Sonochem. 2023, 94, 106314. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Feng, Z.; An, T.; Liu, F. A Stable Peony Seed Oil Emulsion That Enhances the Stability, Antioxidant Activity, and Bioaccessibility of Curcumin. LWT 2023, 173, 114408. [Google Scholar] [CrossRef]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, Structure, and Physical Properties. J. Phys. Condens. Matter 2006, 18, R635. [Google Scholar] [CrossRef] [Green Version]
- Salvia-Trujillo, L.; Qian, C.; Martín-Belloso, O.; McClements, D.J. Influence of Particle Size on Lipid Digestion and β-Carotene Bioaccessibility in Emulsions and Nanoemulsions. Food Chem. 2013, 141, 1472–1480. [Google Scholar] [CrossRef]
- Teixé-Roig, J.; Oms-Oliu, G.; Odriozola-Serrano, I.; Martín-Belloso, O. Enhancing in Vivo Retinol Bioavailability by Incorporating β-Carotene from Alga Dunaliella Salina into Nanoemulsions Containing Natural-Based Emulsifiers. Food Res. Int. 2023, 164, 112359. [Google Scholar] [CrossRef]
- Luo, H.; Li, Z.; Yao, M.; McClements, D.J.; Xiao, H. Impact of Excipient Emulsions Made from Different Types of Oils on the Bioavailability and Metabolism of Curcumin in Gastrointestinal Tract. Food Chem. 2022, 370, 130980. [Google Scholar] [CrossRef]
- Kadappan, A.S.; Guo, C.; Gumus, C.E.; Bessey, A.; Wood, R.J.; McClements, D.J.; Liu, Z. The Efficacy of Nanoemulsion-Based Delivery to Improve Vitamin D Absorption: Comparison of In Vitro and In Vivo Studies. Mol. Nutr. Food Res. 2018, 62, 1700836. [Google Scholar] [CrossRef] [PubMed]
- Harwansh, R.K.; Deshmukh, R.; Rahman, M.A. Nanoemulsion: Promising Nanocarrier System for Delivery of Herbal Bioactives. J. Drug Deliv. Sci. Technol. 2019, 51, 224–233. [Google Scholar] [CrossRef]
- Teixé-Roig, J.; Oms-Oliu, G.; Ballesté-Muñoz, S.; Odriozola-Serrano, I.; Martín-Belloso, O. Improving the in Vitro Bioaccessibility of β-Carotene Using Pectin Added Nanoemulsions. Foods 2020, 9, 447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozogul, Y.; Karsli, G.T.; Durmuş, M.; Yazgan, H.; Oztop, H.M.; McClements, D.J.; Ozogul, F. Recent Developments in Industrial Applications of Nanoemulsions. Adv. Colloid Interface Sci. 2022, 304, 102685. [Google Scholar] [CrossRef] [PubMed]
- Muschiolik, G.; Dickinson, E. Double Emulsions Relevant to Food Systems: Preparation, Stability, and Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 532–555. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Lu, K.; Zhou, S.; Qi, B.; Li, Y. Co-Delivery of Insulin and Quercetin in W/O/W Double Emulsions Stabilized by Different Hydrophilic Emulsifiers. Food Chem. 2022, 369, 130918. [Google Scholar] [CrossRef]
- Huang, X.; Tu, R.; Song, H.; Dong, K.; Geng, F.; Chen, L.; Huang, Q.; Wu, Y. Gelatin-EGCG-High Methoxyl Pectin Ternary Complex Stabilized W1/O/W2 Double Emulsions Loaded with Vitamin C: Formation, Structure, Stability, in Vitro Gastrointestinal Digestion. Int. J. Biol. Macromol. 2022, 216, 891–905. [Google Scholar] [CrossRef]
- Heidari, F.; Jafari, S.M.; Ziaiifar, A.M.; Malekjani, N. Stability and Release Mechanisms of Double Emulsions Loaded with Bioactive Compounds; a Critical Review. Adv. Colloid Interface Sci. 2022, 299, 102567. [Google Scholar] [CrossRef]
- Eisinaite, V.; Duque Estrada, P.; Schroën, K.; Berton-Carabin, C.; Leskauskaite, D. Tayloring W/O/W Emulsion Composition for Effective Encapsulation: The Role of PGPR in Water Transfer-Induced Swelling. Food Res. Int. 2018, 106, 722–728. [Google Scholar] [CrossRef]
- Kartal, C.; Unal, M.K.; Otles, S. Production and Stabilization of a Flaxseed Oil Multi-Layer Emulsion Containing Sodium Caseinate and Pectin. Int. J. Food Prop. 2017, 20, 833–844. [Google Scholar] [CrossRef] [Green Version]
- Guzey, D.; McClements, D.J. Formation, Stability and Properties of Multilayer Emulsions for Application in the Food Industry. Adv. Colloid Interface Sci. 2006, 128–130, 227–248. [Google Scholar] [CrossRef]
- Gasa-Falcon, A.; Acevedo-Fani, A.; Oms-Oliu, G.; Odriozola-Serrano, I.; Martín-Belloso, O. Development, Physical Stability and Bioaccessibility of β-Carotene-Enriched Tertiary Emulsions. J. Funct. Foods 2020, 64, 103615. [Google Scholar] [CrossRef]
- Gasa-Falcon, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Nanostructured Lipid-Based Delivery Systems as a Strategy to Increase Functionality of Bioactive Compounds. Foods 2020, 9, 325. [Google Scholar] [CrossRef] [Green Version]
- Berton-Carabin, C.C.; Schroën, K. Pickering Emulsions for Food Applications: Background, Trends, and Challenges. Annu. Rev. Food Sci. Technol. 2015, 6, 263–297. [Google Scholar] [CrossRef]
- Kubiak, T.; Zubko, M.; Józefczak, A. Ultrasound-Triggered Directional Release from Turmeric Capsules. Particuology 2021, 57, 19–27. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Wang, C.; Mu, C.; Ngai, T.; Lin, W. Advances in Pickering Emulsions Stabilized by Protein Particles: Toward Particle Fabrication, Interaction and Arrangement. Food Res. Int. 2022, 157, 111380. [Google Scholar] [CrossRef]
- Khobaib, K.; Mikkelsen, A.; Vincent-Dospital, T.; Rozynek, Z. Electric-Field-Induced Deformation, Yielding, and Crumpling of Jammed Particle Shells Formed on Non-Spherical Pickering Droplets. Soft Matter 2021, 17, 5006–5017. [Google Scholar] [CrossRef]
- Mikkelsen, A.; Rozynek, Z. Mechanical Properties of Particle Films on Curved Interfaces Probed through Electric Field-Induced Wrinkling of Particle Shells. ACS Appl. Mater. Interfaces 2019, 11, 29396–29407. [Google Scholar] [CrossRef]
- Wu, J.; Ma, G.H. Recent Studies of Pickering Emulsions: Particles Make the Difference. Small 2016, 12, 4633–4648. [Google Scholar] [CrossRef]
- Kubiak, T.; Banaszak, J.; Józefczak, A.; Rozynek, Z. Direction-Specific Release from Capsules with Homogeneous or Janus Shells Using an Ultrasound Approach. ACS Appl. Mater. Interfaces 2020, 12, 15810–15822. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid Lipid Nanoparticles: Production, Characterization and Applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Harde, H.; Das, M.; Jain, S. Solid Lipid Nanoparticles: An Oral Bioavailability Enhancer Vehicle. Expert Opin. Drug Deliv. 2011, 8, 1407–1424. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.S.; Cho, C.W. Surface Modification of Solid Lipid Nanoparticles for Oral Delivery of Curcumin: Improvement of Bioavailability through Enhanced Cellular Uptake, and Lymphatic Uptake. Eur. J. Pharm. Biopharm. 2017, 117, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ng, W.K.; Tan, R.B.H. Are Nanostructured Lipid Carriers (NLCs) Better than Solid Lipid Nanoparticles (SLNs): Development, Characterizations and Comparative Evaluations of Clotrimazole-Loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef]
- Shu, X.; Zhang, L.; Liao, W.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Nanostructured Lipid Carriers (NLCs) Stabilized by Natural or Synthetic Emulsifiers for Lutein Delivery: Improved Physicochemical Stability, Antioxidant Activity, and Bioaccessibility. Food Chem. 2022, 403, 134465. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-Based Delivery Systems for Lipophilic Bioactive Components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering Emulsions: Preparation Processes, Key Parameters Governing Their Properties and Potential for Pharmaceutical Applications. J. Control. Release 2019, 309, 302–332. [Google Scholar] [CrossRef]
- McClements, D.J.; Gumus, C.E. Natural Emulsifiers—Biosurfactants, Phospholipids, Biopolymers, and Colloidal Particles: Molecular and Physicochemical Basis of Functional Performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J. Food Emulsions: Principles, Practice, and Techniques; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780849320231. [Google Scholar]
- Yuan, Y.; Gao, Y.; Zhao, J.; Mao, L. Characterization and Stability Evaluation of β-Carotene Nanoemulsions Prepared by High Pressure Homogenization under Various Emulsifying Conditions. Food Res. Int. 2008, 41, 61–68. [Google Scholar] [CrossRef]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Faisal, M.; Shafiq, S. Stability Evaluation of Celecoxib Nanoemultion Containing Tween 80. Thai J. Pharm. Sci. 2008, 32, 4–9. [Google Scholar]
- Salvia-Trujillo, L.; Qian, C.; Martín-Belloso, O.; McClements, D.J. Modulating β-Carotene Bioaccessibility by Controlling Oil Composition and Concentration in Edible Nanoemulsions. Food Chem. 2013, 139, 878–884. [Google Scholar] [CrossRef]
- Grumezescu, A.M. Nano- and Microscale Drug Delivery Systems: Design and Fabrication, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780323527279. [Google Scholar]
- Lam, R.S.H.; Nickerson, M.T. Food Proteins: A Review on Their Emulsifying Properties Using a Structure-Function Approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef]
- Delahaije, R.J.B.M.; Wierenga, P.A.; Van Nieuwenhuijzen, N.H.; Giuseppin, M.L.F.; Gruppen, H. Protein Concentration and Protein-Exposed Hydrophobicity as Dominant Parameters Determining the Flocculation of Protein-Stabilized Oil-in-Water Emulsions. Langmuir 2013, 29, 11567–11574. [Google Scholar] [CrossRef]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D.J. Formation and Stabilization of Nanoemulsion-Based Vitamin e Delivery Systems Using Natural Surfactants: Quillaja Saponin and Lecithin. J. Food Eng. 2014, 142, 57–63. [Google Scholar] [CrossRef]
- Flores-Andrade, E.; Allende-Baltazar, Z.; Sandoval-González, P.E.; Jiménez-Fernández, M.; Beristain, C.I.; Pascual-Pineda, L.A. Carotenoid Nanoemulsions Stabilized by Natural Emulsifiers: Whey Protein, Gum Arabic, and Soy Lecithin. J. Food Eng. 2021, 290, 110208. [Google Scholar] [CrossRef]
- Qi, H.; Chen, S.; Zhang, J.; Liang, H. Robust Stability and Antimicrobial Activity of D-Limonene Nanoemulsion by Sodium Caseinate and High Pressure Homogenization. J. Food Eng. 2022, 334, 111159. [Google Scholar] [CrossRef]
- Yerramilli, M.; Ghosh, S. Long-Term Stability of Sodium Caseinate-Stabilized Nanoemulsions. J. Food Sci. Technol. 2017, 54, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; McClements, D.J. Plant-Based Colloidal Delivery Systems for Bioactives. Molecules 2021, 26, 6895. [Google Scholar] [CrossRef]
- Jiang, S.; Yildiz, G.; Ding, J.; Andrade, J.; Rababahb, T.M.; Almajwalc, A.; Abulmeatyc, M.M.; Feng, H. Pea Protein Nanoemulsion and Nanocomplex as Carriers for Protection of Cholecalciferol (Vitamin D3). Food Bioprocess Technol. 2019, 12, 1031–1040. [Google Scholar] [CrossRef]
- Chen, W.; Ju, X.; Aluko, R.E.; Zou, Y.; Wang, Z.; Liu, M.; He, R. Rice Bran Protein-Based Nanoemulsion Carrier for Improving Stability and Bioavailability of Quercetin. Food Hydrocoll. 2020, 108, 106042. [Google Scholar] [CrossRef]
- Teuling, E.; Schrama, J.W.; Gruppen, H.; Wierenga, P.A. Characterizing Emulsion Properties of Microalgal and Cyanobacterial Protein Isolates. Algal Res. 2019, 39, 101471. [Google Scholar] [CrossRef]
- Böcker, L.; Bertsch, P.; Wenner, D.; Teixeira, S.; Bergfreund, J.; Eder, S.; Fischer, P.; Mathys, A. Effect of Arthrospira Platensis Microalgae Protein Purification on Emulsification Mechanism and Efficiency. J. Colloid Interface Sci. 2021, 584, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.C.; Almeida, T.; Colucci, G.; Santamaria-Echart, A.; Manrique, Y.A.; Dias, M.M.; Barros, L.; Fernandes, Â.; Colla, E.; Barreiro, M.F. Spirulina (Arthrospira Platensis) Protein-Rich Extract as a Natural Emulsifier for Oil-in-Water Emulsions: Optimization through a Sequential Experimental Design Strategy. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129264. [Google Scholar] [CrossRef]
- Gao, W.; Jiang, Z.; Du, X.; Zhang, F.; Liu, Y.; Bai, X.; Sun, G. Impact of Surfactants on Nanoemulsions Based on Fractionated Coconut Oil: Emulsification Stability and In Vitro Digestion. J. Oleo Sci. 2020, 69, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, R.A.; Cavallieri, Â.L.F.; Netto, F.M.; Cunha, R.L. Stability and in Vitro Digestibility of Emulsions Containing Lecithin and Whey Proteins. Food Funct. 2013, 4, 1322–1331. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Lanjari-Pérez, Y.; Martín-Belloso, O. Curcumin-Loaded Nanoemulsions Stability as Affected by the Nature and Concentration of Surfactant. Food Chem. 2018, 266, 466–474. [Google Scholar] [CrossRef]
- Teixé-Roig, J.; Oms-Oliu, G.; Ballesté-Muñoz, S.; Odriozola-Serrano, I.; Martín-Belloso, O. Encapsulation and Controlled Release of Phycocyanin during the in Vitro Digestion Using Polysaccharide-Added Double Emulsions (W1/O/W2). Food Struct. 2022, 31, 100249. [Google Scholar] [CrossRef]
- Shao, P.; Feng, J.; Sun, P.; Xiang, N.; Lu, B.; Qiu, D. Recent Advances in Improving Stability of Food Emulsion by Plant Polysaccharides. Food Res. Int. 2020, 137, 109376. [Google Scholar] [CrossRef]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D.J. Formation and Stabilization of Nanoemulsion-Based Vitamin E Delivery Systems Using Natural Biopolymers: Whey Protein Isolate and Gum Arabic. Food Chem. 2015, 188, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Castel, V.; Rubiolo, A.C.; Carrara, C.R. Droplet Size Distribution, Rheological Behavior and Stability of Corn Oil Emulsions Stabilized by a Novel Hydrocolloid (Brea Gum) Compared with Gum Arabic. Food Hydrocoll. 2017, 63, 170–177. [Google Scholar] [CrossRef]
- Verkempinck, S.H.E.; Kyomugasho, C.; Salvia-Trujillo, L.; Denis, S.; Bourgeois, M.; Van Loey, A.M.; Hendrickx, M.E.; Grauwet, T. Emulsion Stabilizing Properties of Citrus Pectin and Its Interactions with Conventional Emulsifiers in Oil-in-Water Emulsions. Food Hydrocoll. 2018, 85, 144–157. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Salvia-Trujillo, L.; González-Aguilar, G.A.; Martín-Belloso, O. Interfacial Activity of Phenolic-Rich Extracts from Avocado Fruit Waste: Influence on the Colloidal and Oxidative Stability of Emulsions and Nanoemulsions. Innov. Food Sci. Emerg. Technol. 2021, 69, 102665. [Google Scholar] [CrossRef]
- Shao, P.; Qiu, Q.; Xiao, J.; Zhu, Y.; Sun, P. Chemical Stability and in Vitro Release Properties of β-Carotene in Emulsions Stabilized by Ulva Fasciata Polysaccharide. Int. J. Biol. Macromol. 2017, 102, 225–231. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Hamzeh, A.; Tabarsa, M.; Cravotto, G. Cellular Antioxidant and Emulsifying Activities of Fucoidan Extracted from Nizamuddinia Zanardinii Using Different Green Extraction Methods. J. Food Process. Preserv. 2022, 46, e17238. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.J.; Park, Y.B.; Woo, H.C.; Chun, B.S. Structural, Antioxidant, and Emulsifying Activities of Fucoidan from Saccharina Japonica Using Pressurized Liquid Extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef]
- Oliyaei, N.; Moosavi-Nasab, M.; Tanideh, N. Preparation of Fucoxanthin Nanoemulsion Stabilized by Natural Emulsifiers: Fucoidan, Sodium Caseinate, and Gum Arabic. Molecules 2022, 27, 6713. [Google Scholar] [CrossRef]
- Jamshidi, A.; Shabanpour, B.; Pourashouri, P.; Raeisi, M. Using WPC-Inulin-Fucoidan Complexes for Encapsulation of Fish Protein Hydrolysate and Fish Oil in W1/O/W2 Emulsion: Characterization and Nutritional Quality. Food Res. Int. 2018, 114, 240–250. [Google Scholar] [CrossRef]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular Activities, Biosynthesis and Evolution of Triterpenoid Saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Gu, J.; McClements, D.J. Fabrication of Oil-in-Water Nanoemulsions by Dual-Channel Microfluidization Using Natural Emulsifiers: Saponins, Phospholipids, Proteins, and Polysaccharides. Food Hydrocoll. 2016, 61, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Schreiner, T.B.; Colucci, G.; Santamaria-Echart, A.; Fernandes, I.P.; Dias, M.M.; Pinho, S.P.; Barreiro, M.F. Evaluation of Saponin-Rich Extracts as Natural Alternative Emulsifiers: A Comparative Study with Pure Quillaja Bark Saponin. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126748. [Google Scholar] [CrossRef]
- Zhu, Z.; Wen, Y.; Yi, J.; Cao, Y.; Liu, F.; McClements, D.J. Comparison of Natural and Synthetic Surfactants at Forming and Stabilizing Nanoemulsions: Tea Saponin, Quillaja Saponin, and Tween 80. J. Colloid Interface Sci. 2019, 536, 80–87. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Li, Y. Structured Emulsion-Based Delivery Systems: Controlling the Digestion and Release of Lipophilic Food Components. Adv. Colloid Interface Sci. 2010, 159, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Lamothe, S.; Jolibois, E.; Britten, M. Effect of Emulsifiers on Linseed Oil Emulsion Structure, Lipolysis and Oxidation during: In Vitro Digestion. Food Funct. 2020, 11, 10126–10136. [Google Scholar] [CrossRef] [PubMed]
- Gasa-Falcon, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Impact of Emulsifier Nature and Concentration on the Stability of β-Carotene Enriched Nanoemulsions during: In Vitro Digestion. Food Funct. 2019, 10, 713–722. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Zhang, Z.; Muriel Mundo, J.; McClements, D.J. Factors Impacting Lipid Digestion and Nutraceutical Bioaccessibility Assessed by Standardized Gastrointestinal Model (INFOGEST): Emulsifier Type. Food Res. Int. 2020, 137, 109739. [Google Scholar] [CrossRef]
- Chen, Z.; Shu, G.; Taarji, N.; Barrow, C.J.; Nakajima, M.; Khalid, N.; Neves, M.A. Gypenosides as Natural Emulsifiers for Oil-in-Water Nanoemulsions Loaded with Astaxanthin: Insights of Formulation, Stability and Release Properties. Food Chem. 2018, 261, 322–328. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Tan, H.; Zhang, R.; McClements, D.J. Vitamin e Encapsulation within Oil-in-Water Emulsions: Impact of Emulsifier Type on Physicochemical Stability and Bioaccessibility. J. Agric. Food Chem. 2019, 67, 1521–1529. [Google Scholar] [CrossRef]
- Teixé-Roig, J.; Oms-Oliu, G.; Odriozola-Serrano, I.; Martín-Belloso, O. Enhancing the Gastrointestinal Stability of Curcumin by Using Sodium Alginate-Based Nanoemulsions Containing Natural Emulsifiers. Int. J. Mol. Sci. 2022, 24, 498. [Google Scholar] [CrossRef]
- Yan, J.; Yang, Z.; Qiao, X.; Kong, Z.; Dai, L.; Wu, J.; Xu, X.; McClements, D.J. Interfacial Characteristics and in Vitro Digestion of Emulsion Coated by Single or Mixed Natural Emulsifiers: Lecithin and/or Rice Glutelin Hydrolysates. J. Sci. Food Agric. 2022, 102, 2990–2999. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, W.; Nie, K.; Gao, Z.; Fang, Y.; Nishinari, K.; Phillips, G.O.; Jiang, F. Effect of Gum Arabic, Gum Ghatti and Sugar Beet Pectin as Interfacial Layer on Lipid Digestibility in Oil-in-Water Emulsions. Food Biophys. 2016, 11, 292–301. [Google Scholar] [CrossRef]
- Verkempinck, S.H.E.; Salvia-Trujillo, L.; Denis, S.; Van Loey, A.M.; Hendrickx, M.E.; Grauwet, T. Pectin Influences the Kinetics of in Vitro Lipid Digestion in Oil-in-Water Emulsions. Food Chem. 2018, 262, 150–161. [Google Scholar] [CrossRef]
- Sun, X.; Liu, C.; Wang, D.; Xu, Y.; Wang, C.Y. Effects of Coating Layers Chitosan/Pectin on Lipid Stability and in Vitro Digestion of Astaxanthin-Loaded Multilayer Emulsions. LWT 2023, 173, 114282. [Google Scholar] [CrossRef]
- Shi, F.; Chang, Y.; Shen, J.; Chen, G.; Xue, C. A Comparative Investigation of Anionic Polysaccharides (Sulfated Fucan, ι-Carrageenan, κ-Carrageenan, and Alginate) on the Fabrication, Stability, Rheology, and Digestion of Multilayer Emulsion. Food Hydrocoll. 2023, 134, 108081. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, Y.; Lv, S.; Liu, J.; Muriel Mundo, J.L.; Bai, L.; Rojas, O.J.; McClements, D.J. Nanochitin-Stabilized Pickering Emulsions: Influence of Nanochitin on Lipid Digestibility and Vitamin Bioaccessibility. Food Hydrocoll. 2020, 106, 105878. [Google Scholar] [CrossRef]
- Ribeiro, E.F.; Borreani, J.; Moraga, G.; Nicoletti, V.R.; Quiles, A.; Hernando, I. Digestibility and Bioaccessibility of Pickering Emulsions of Roasted Coffee Oil Stabilized by Chitosan and Chitosan-Sodium Tripolyphosphate Nanoparticles. Food Biophys. 2020, 15, 196–205. [Google Scholar] [CrossRef]
- Saechio, S.; Akanitkul, P.; Thiyajai, P.; Jain, S.; Tangsuphoom, N.; Suphantharika, M.; Winuprasith, T. Astaxanthin-Loaded Pickering Emulsions Stabilized by Nanofibrillated Cellulose: Impact on Emulsion Characteristics, Digestion Behavior, and Bioaccessibility. Polymers 2023, 15, 901. [Google Scholar] [CrossRef]
- Nazari-Vanani, R.; Moezi, L.; Heli, H. In Vivo Evaluation of a Self-Nanoemulsifying Drug Delivery System for Curcumin. Biomed. Pharmacother. 2017, 88, 715–720. [Google Scholar] [CrossRef]
- Meng, Q.; Long, P.; Zhou, J.; Ho, C.T.; Zou, X.; Chen, B.; Zhang, L. Improved Absorption of β-Carotene by Encapsulation in an Oil-in-Water Nanoemulsion Containing Tea Polyphenols in the Aqueous Phase. Food Res. Int. 2019, 116, 731–736. [Google Scholar] [CrossRef]
- Pandita, D.; Kumar, S.; Poonia, N.; Lather, V. Solid Lipid Nanoparticles Enhance Oral Bioavailability of Resveratrol, a Natural Polyphenol. Food Res. Int. 2014, 62, 1165–1174. [Google Scholar] [CrossRef]
- Chen, L.; Yokoyama, W.; Alves, P.; Tan, Y.; Pan, J.; Zhong, F. Effect of Encapsulation on β-Carotene Absorption and Metabolism in Mice. Food Hydrocoll. 2021, 121, 107009. [Google Scholar] [CrossRef]
- Wang, L.H.; Xiao, J.X.; Li, X.D.; Huang, G.Q. Carboxymethyl Konjac Glucomannan Coating on Multilayered Emulsions for Improved Bioavailability and Targeted Delivery of Curcumin. Food Funct. 2021, 12, 5429–5439. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Han, C.; Liu, E.; Li, X.; Meng, X.; Liu, B. Pickering Emulsions Stabilized by Pea Protein Isolate-Chitosan Nanoparticles: Fabrication, Characterization and Delivery EPA for Digestion in Vitro and in Vivo. Food Chem. 2022, 378, 132090. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, S.; Muthukumar, S.P.; Anandharamakrishnan, C. The Influence of Droplet Size on the Stability,: In Vivo Digestion, and Oral Bioavailability of Vitamin E Emulsions. Food Funct. 2016, 7, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Acevedo-Fani, A.; McDowell, A.; Barnett, A.; Loveday, S.M.; Singh, H. Nanoemulsion Structure and Food Matrix Determine the Gastrointestinal Fate and in Vivo Bioavailability of Coenzyme Q10. J. Control. Release 2020, 327, 444–455. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, F.; Pang, J.; McClements, D.J.; Zhou, Z.; Li, B.; Li, Y. Biopolymer Additives Enhance Tangeretin Bioavailability in Emulsion-Based Delivery Systems: An in Vitro and in Vivo Study. J. Agric. Food Chem. 2021, 69, 730–740. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixé-Roig, J.; Oms-Oliu, G.; Odriozola-Serrano, I.; Martín-Belloso, O. Emulsion-Based Delivery Systems to Enhance the Functionality of Bioactive Compounds: Towards the Use of Ingredients from Natural, Sustainable Sources. Foods 2023, 12, 1502. https://doi.org/10.3390/foods12071502
Teixé-Roig J, Oms-Oliu G, Odriozola-Serrano I, Martín-Belloso O. Emulsion-Based Delivery Systems to Enhance the Functionality of Bioactive Compounds: Towards the Use of Ingredients from Natural, Sustainable Sources. Foods. 2023; 12(7):1502. https://doi.org/10.3390/foods12071502
Chicago/Turabian StyleTeixé-Roig, Júlia, Gemma Oms-Oliu, Isabel Odriozola-Serrano, and Olga Martín-Belloso. 2023. "Emulsion-Based Delivery Systems to Enhance the Functionality of Bioactive Compounds: Towards the Use of Ingredients from Natural, Sustainable Sources" Foods 12, no. 7: 1502. https://doi.org/10.3390/foods12071502