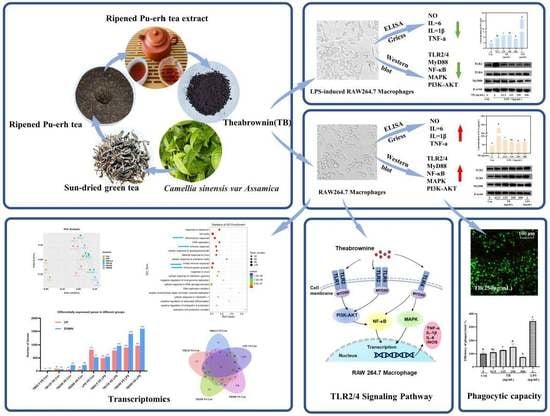
Theabrownin Isolated from Pu-Erh Tea Enhances the Innate Immune and Anti-Inflammatory Effects of RAW264.7 Macrophages via the TLR2/4-Mediated Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction of Theabrownin
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Morphologic Observation
2.5.1. Immune Enhancement Groups
2.5.2. Anti-Inflammatory Groups
2.6. Measurement of Pinocytic and Phagocytic Capacity
2.6.1. Pinocytic Capacity
2.6.2. Phagocytic Capacity
2.7. Determination of NO and Cytokines
2.8. Reverse Transcription and Real-Time Quantitative PCR
2.9. Investigation of Membrane Receptors (TLR2/4)
2.10. Western Blot Analysis
2.11. mRNA-seq and Transcriptome Analysis
2.12. Statistical Analysis and Graphics Processing
3. Results
3.1. Effects of TB on RAW264.7 Cell Viability
3.2. Effect of TB on RAW264.7 Morphology
3.3. TB Enhances the Phagocytic Capacity of Macrophages
3.4. Immune-Enhancing Effects of TB on NO and Cytokine Production
3.5. Anti-Inflammatory Effects of TB on NO and Cytokine Production
3.6. TLRs of TB on RAW264.7 Macrophages
3.7. TB Enhances the Immune Function of RAW264.7 Cells via the TLR2/4-Mediated MAPK/NF-κB/PI3K–AKT Signaling Pathway
3.8. Anti-Inflammatory Effect of TB on the TLR2/4-Mediated MAPK/NF-κB/PI3K–AKT Signaling Pathway of RAW264.7 Cells
3.9. Effect of TB on Transcriptomics of RAW264.7 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Janeway, C.A., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989, 54, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Xu, D.; Liu, H.; Komai-Koma, M. Direct and indirect role of Toll-like receptors in T cell mediated immunity. Cell. Mol. Immunol. 2004, 1, 239–246. [Google Scholar] [PubMed]
- Kim, J.H.; Kim, D.H.; Jo, S.; Cho, M.J.; Cho, Y.R.; Lee, Y.J.; Byun, S. Immunomodulatory functional foods and their molecular mechanisms. Exp. Mol. Med. 2022, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, Y.; Liu, R.; Xu, H.; Mei, X.; Shang, Q.; Liu, S.; Yu, D.; Li, W. Lactobacillus rhamnosus GG promotes M1 polarization in murine bone marrow-derived macrophages by activating TLR2/MyD88/MAPK signaling pathway. Anim. Sci. J. 2020, 91, e13439. [Google Scholar] [CrossRef]
- Yang, F.; Li, X.; Yang, Y.; Ayivi-Tosuh, S.M.; Wang, F.; Li, H.; Wang, G. A polysaccharide isolated from the fruits of Physalis alkekengi L. induces RAW264.7 macrophages activation via TLR2 and TLR4-mediated MAPK and NF-κB signaling pathways. Int. J. Biol. Macromol. 2019, 140, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Um, Y.; Eo, H.J.; Kim, H.J.; Kim, K.; Jeon, K.S.; Jeong, J.B. Wild simulated ginseng activates mouse macrophage, RAW264.7 cells through TRL2/4-dependent activation of MAPK, NF-κB and PI3K/AKT pathways. J. Ethnopharmacol. 2020, 263, 113218. [Google Scholar] [CrossRef]
- Ren, D.Y.; Lin, D.H.; Alim, A.; Zheng, Q.; Yang, X.B. Chemical characterization of a novel polysaccharide ASKP-1 from Artemisia sphaerocephala Krasch seed and its macrophage activation via MAPK, PI3K/Akt and NF-κB signaling pathways in RAW264.7 cells. Food Funct. 2017, 8, 1299–1312. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, Z.; Chen, J.; Song, D.; Liu, B.; Li, J.; Liu, R.; Niu, J.; Wang, D.; Ling, N.; et al. Immune-Enhancing Effects of a Novel Glucan from Purple Sweet Potato Ipomoea batatas (L.) Lam on RAW264.7 Macrophage Cells via TLR2- and TLR4-Mediated Pathways. J. Agric. Food Chem. 2021, 69, 9313–9325. [Google Scholar] [CrossRef]
- Chowdhury, P.; Barooah, A.K. Tea Bioactive Modulate Innate Immunity: In Perception to COVID-19 Pandemic. Front. Immunol. 2020, 11, 590716. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Ma, Y.; Liu, Y.; Lin, C.C.; Li, S.; Zhan, J.; Ho, C.T. Immunomodulatory Effects of Green Tea Polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef]
- Muta, T.; Takeshige, K. Essential roles of CD14 and lipopolysaccharide-binding protein for activation of toll-like receptor (TLR)2 as well as TLR4 Reconstitution of TLR2- and TLR4-activation by distinguishable ligands in LPS preparations. Eur. J. Biochem. 2001, 268, 4580–4589. [Google Scholar] [CrossRef] [PubMed]
- Sunan Wang, S.A.; Qiu, Y.; Gan, R.Y.; Zhu, F. Chemical constituents and biological properties of Pu-erh tea. Food Res. Int. 2022, 154, 110899. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.J.; Shan, B.; Peng, C.X.; Tan, C.; Wang, Q.P.; Gong, J.S. Theabrownin-targeted regulation of intestinal microorganisms to improve glucose and lipid metabolism in Goto-Kakizaki rats. Food Funct. 2022, 13, 1921–1940. [Google Scholar] [CrossRef]
- Wang, Q.P.; Belščak, C.A.; Durgo, K.; Chisti, Y.; Gong, J.S.; Sirisansaneeyakul, S. Physicochemical properties and biological activities of a high-theabrownins instant Pu-Erh tea produced using Aspergillus tubingensis. LWT-Food Sci. Technol. 2018, 90, 598–605. [Google Scholar] [CrossRef]
- Deng, X.; Hou, Y.; Zhou, H.; Li, Y.; Xue, Z.; Xue, X.; Huang, G.; Huang, K.; He, X.; Xu, W. Hypolipidemic, anti-inflammatory, and anti-atherosclerotic effects of tea before and after microbial fermentation. Food Sci. Nutr. 2021, 9, 1160–1170. [Google Scholar] [CrossRef]
- Gong, J.S.; Peng, C.X.; Chen, T.T.; Gao, B.; Zhou, H.J. Effects of theabrownin from Pu-erh Tea on the metabolism of serum lipids in Rats: Mechanism of action. J. Food Sci. 2010, 75, H182–H189. [Google Scholar] [CrossRef]
- Yue, S.J.; Zhao, D.; Peng, C.X.; Tan, C.; Wang, Q.P.; Gong, J.S. Effects of theabrownin on serum metabolite and gut microbiome in rats with high-sugar diet. Food Funct. 2019, 10, 7063–7080. [Google Scholar] [CrossRef]
- Fu, J.Y.; Jiang, C.X.; Wu, M.Y.; Mei, R.Y.; Yang, A.F.; Tao, H.P.; Chen, X.J.; Zhang, J.; Huang, L.; Zhao, X.F. Theabrownin Induces Cell Apoptosis and Cell Cycle Arrest of Oligodendroglioma and Astrocytoma in Different Pathways. Front. Pharmacol. 2021, 12, 664003. [Google Scholar] [CrossRef]
- Lv, H.P.; Zhang, Y.; Shi, J.; Lin, Z. Phytochemical profiles and antioxidant activities of Chinese dark teas obtained by different processing technologies. Food Res. Int. 2016, 100, 486–493. [Google Scholar] [CrossRef]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Wang, G.; Li, C.; Zhang, M.; Zhao, H.; Sheng, J.; Shi, W. Pu-erh tea reduces nitric oxide levels in rats by inhibiting inducible nitric oxide synthase expression through toll-like receptor 4. Int. J. Mol. Sci. 2012, 13, 7174–7185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, S.W.; Zhang, Z.F.; Xie, G.H.; Zhao, C.Y.; Miao, Y.; Chen, D.H.; Zhang, G.G.; Liu, H.; Peng, C.X.; Hou, Y.; et al. Theabrownin modulates the gut microbiome and serum metabolome in aging mice induced by D-galactose. J. Funct. Foods 2022, 89, 104941. [Google Scholar] [CrossRef]
- Cheng, X.D.; Wu, Q.X.; Zhao, J.; Su, T.; Lu, Y.M.; Zhang, W.N.; Wang, Y.; Chen, Y. Immunomodulatory effect of a polysaccharide fraction on RAW 264.7 macrophages extracted from the wild Lactarius deliciosus. Int. J. Biol. Macromol. 2019, 128, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.K.; Zhao, Y.; Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 2019, 20, 527–533. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Lei, L.; Li, F.; Tang, Y.; Yuan, Y.; Zhang, Y.; Wu, S.; Yin, R.; Ming, J. Acetylation of polysaccharide from Morchella angusticeps peck enhances its immune activation and anti-inflammatory activities in macrophage RAW264.7 cells. Food Chem. Toxicol. 2019, 125, 38–45. [Google Scholar] [CrossRef]
- Byun, E.B.; Sung, N.Y.; Yang, M.S.; Lee, B.S.; Song, D.S.; Park, J.N.; Kim, J.H.; Jang, B.S.; Choi, D.S.; Park, S.H.; et al. Anti-inflammatory effect of gamma-irradiated genistein through inhibition of NF-κB and MAPK signaling pathway in lipopolysaccharide-induced macrophages. Food Chem. Toxicol. 2014, 74, 255–264. [Google Scholar] [CrossRef]
- Sun, H.; Ni, X.Q.; Zeng, D.; Zou, F.L.; Yang, M.Y.; Peng, Z.R.; Zhou, Y.; Zeng, Y.; Zhu, H.; Wang, H.S.; et al. Bidirectional immunomodulating activity of fermented polysaccharides from Yupingfeng. Res. Vet. Sci. 2016, 110, 22–28. [Google Scholar] [CrossRef]
- Gramlich, R.; Aliahmadi, E.; Peiser, M. In Vitro Induction of T Helper 17 Cells by Synergistic Activation of Human Monocyte-Derived Langerhans Cell-Like Cells with Bacterial Agonists. Int. J. Mol. Sci. 2019, 20, 1367. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Q.; Mao, J.B.; Zhou, M.Q.; Jin, Y.W.; Lou, C.H.; Dong, Y.; Shou, D.; Hu, Y.; Yang, B.; Jin, C.Y.; et al. Polysaccharide from Phellinus Igniarius activates TLR4-mediated signaling pathways in macrophages and shows immune adjuvant activity in mice. Int. J. Biol. Macromol. 2019, 123, 157–166. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, G.; Lai, F.; Wu, H. Structural Characterization and Immunomodulatory Activity of a Novel Polysaccharide from Lepidium meyenii. J. Agric. Food Chem. 2016, 64, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Chen, Y.; Luo, Z.H.; Shen, X.L.; Hu, Y.J. 7-methoxyflavanone alleviates neuroinflammation in lipopolysaccharide-stimulated microglial cells by inhibiting TLR4/MyD88/MAPK signalling and activating the Nrf2/NQO-1 pathway. J. Pharm. Pharmacol. 2020, 72, 385–395. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, Y.; Deng, B.; Lin, A.; Zhang, G.; Ma, M.; Wang, Y.; Yang, Y.; Kang, X. The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: Mechanisms and therapeutic opportunities. Cell Prolif. 2022, 55, e13275. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Hao, B.; Pang, D.; Li, Q.; Li, E.; Yang, Q.; Zou, Y.; Liao, S.; Liu, F. Diverse Galactooligosaccharides Differentially Reduce LPS-Induced Inflammation in Macrophages. Foods 2022, 11, 3973. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, R.; Peng, Z.; Hu, B.; Rao, X.; Li, J. HMGB1 participates in LPS-induced acute lung injury by activating the AIM2 inflammasome in macrophages and inducing polarization of M1 macrophages via TLR2, TLR4, and RAGE/NF-κB signaling pathways. Int. J. Mol. Med. 2020, 45, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Peng, C.X.; Gao, B.; Gong, J.S. Spectroscopic and structural characteristics of the main components of Theabrownin in Pu-erh tea. Spectrosc. Spectr. Anal. 2012, 32, 1051–1056. (In Chinese) [Google Scholar]
- Gong, J.S.; Zhang, Q.; Peng, C.X.; Fan, J.P.; Dong, W.M. Curie-point pyrolysis-gas chromatography-mass spectroscopic analysis of theabrownins from fermented Zijuan tea. J. Anal. Appl. Pyrolysis 2012, 97, 171–180. [Google Scholar] [CrossRef]
- Ying, M.; Yu, Q.; Zheng, B.; Wang, H.; Wang, J.; Chen, S.; Nie, S.; Xie, M. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr. Polym. 2020, 235, 115957. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Li, Q.; Wang, J.; Nie, S.; Xie, M. Influence of Natural Polysaccharides on Intestinal Microbiota in Inflammatory Bowel Diseases: An Overview. Foods 2022, 11, 1084. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.C.; Lin, R.Y.; Hou, X.N.; Wu, J.; Zhao, W.B.; Ma, H.H.; Fan, Z.Y.; Li, S.J.; Zhu, Y.; Zhang, D.Y. Immunomodulatory mechanism of a purified polysaccharide isolated from Isaria cicadae Miquel on RAW264.7 cells via activating TLR4-MAPK-NF-κB signaling pathway. Int. J. Biol. Macromol. 2020, 164, 4329–4338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, A.; Zhao, Y.; Feng, X.H.; Sheng, Y.Y.; Zhang, H.L. Expressions of TLR4, MyD88, IRAK4 and NF-κB in the oviduct of chinese brown frog (Rana dybowskii). Eur. J. Histochem. 2019, 63, 3050. [Google Scholar] [CrossRef]
- Zheng, K.; Lv, B.; Wu, L.; Wang, C.; Xu, H.; Li, X.; Wu, Z.; Zhao, Y.; Zheng, Z. Protecting effect of emodin in experimental autoimmune encephalomyelitis mice by inhibiting microglia activation and inflammation via Myd88/PI3K/Akt/NF-κB signalling pathway. Bioengineered 2022, 13, 9322–9344. [Google Scholar] [CrossRef] [PubMed]





| Gene | Primer Sequence | |
|---|---|---|
| GAPDH | Forward | 5′-GCAGTGGCAAAGTGGAGATT-3′ |
| Reverse | 5′-CGCTCCTGGAAGATGGTGAT-3′ | |
| Nitric oxide synthase (iNOs) | Forward | 5′-CTTGGAGCGAGTTGTGGATTGTC-3′ |
| Reverse | 5′-AATGTCCAGGAAGTAGGTGAGGGCT -3′ | |
| TNF-α | Forward | 5′-AAAAGCAAGCAGCCAACCAG-3′ |
| Reverse | 5′-GCCACAAGCAGGAATGAGAA-3′ | |
| IL-6 | Forward | 5′-CCATCTCTCCGTCTCTCACC-3′ |
| Reverse | 5′- AGACCGCTGCCTGTCTAAAA-3′ | |
| IL-1β | Forward | 5′-TGAAGGGCTGCTTCCAAACCTTTGACC-3′ |
| Reverse | 5′-TGTCCATTGAGGTGGAGAGCTTTCAGC-3′ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Miao, Y.; Shan, B.; Zhao, C.; Peng, C.; Gong, J. Theabrownin Isolated from Pu-Erh Tea Enhances the Innate Immune and Anti-Inflammatory Effects of RAW264.7 Macrophages via the TLR2/4-Mediated Signaling Pathway. Foods 2023, 12, 1468. https://doi.org/10.3390/foods12071468
Zhao L, Miao Y, Shan B, Zhao C, Peng C, Gong J. Theabrownin Isolated from Pu-Erh Tea Enhances the Innate Immune and Anti-Inflammatory Effects of RAW264.7 Macrophages via the TLR2/4-Mediated Signaling Pathway. Foods. 2023; 12(7):1468. https://doi.org/10.3390/foods12071468
Chicago/Turabian StyleZhao, Lei, Yue Miao, Bo Shan, Chunyan Zhao, Chunxiu Peng, and Jiashun Gong. 2023. "Theabrownin Isolated from Pu-Erh Tea Enhances the Innate Immune and Anti-Inflammatory Effects of RAW264.7 Macrophages via the TLR2/4-Mediated Signaling Pathway" Foods 12, no. 7: 1468. https://doi.org/10.3390/foods12071468