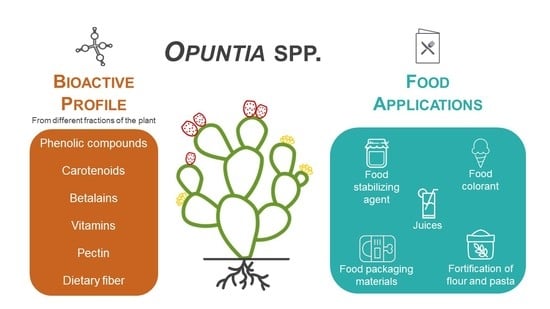
Opuntia spp.: An Overview of the Bioactive Profile and Food Applications of This Versatile Crop Adapted to Arid Lands
Abstract
:1. Introduction
2. Prickly Pear Plant as a Source of Bioactive Macromolecules
2.1. Cladodes
2.2. Fruits
2.3. Opuntia Flowers and Roots
3. Applications in Food Products
3.1. Food Applications of Cladodes
3.2. Food Applications of Prickly Pear Fruits
3.3. Food Applications of Opuntia spp. Flowers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, S.N.; Ahmad, M.; Zafar, M.; Rashid, S.; Sultana, S. Classification, Distribution and Morphological Characterization of Opuntia Species. In Opuntia spp.: Chemistry, Bioactivity and Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 109–119. [Google Scholar]
- Abouseadaa, H.H.; Atia, M.A.M.; Younis, I.Y.; Issa, M.Y.; Ashour, H.A.; Saleh, I.; Osman, G.H.; Arif, I.A.; Mohsen, E. Gene-targeted molecular phylogeny, phytochemical profiling, and antioxidant activity of nine species belonging to family Cactaceae. Saudi J. Biol. Sci. 2020, 27, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Agüero, J.I.; Galati, B.G.; Torretta, J.P. Structure and ultrastructure of floral nectaries of two Opuntia species (Cactaceae) in relation to their floral visitors. Plant Syst. Evol. 2018, 304, 1057–1067. [Google Scholar] [CrossRef]
- Edvan, R.L.; Mota, R.R.M.; Dias-Silva, T.P.; do Nascimento, R.R.; de Sousa, S.V.; da Silva, A.L.; de Araújo, M.J.; Araújo, J.S. Resilience of cactus pear genotypes in a tropical semi-arid region subject to climatic cultivation restriction. Sci. Rep. 2020, 10, 10040. [Google Scholar] [CrossRef] [PubMed]
- Khodaeiaminjan, M.; Nassrallah, A.A.; Kamal, K.Y. Potential Attribute of Crassulacean Acid Metabolism of Opuntia spp. Production in Water-Limited Conditions. In Opuntia spp.: Chemistry, Bioactivity and Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 201–218. [Google Scholar]
- Arba, M.; Falisse, A.; Choukr-Allah, R.; Sindic, M. Biology, flowering and fruiting of the cactus Opuntia spp.: A review and some observations on three varieties in Morocco. Braz. Arch. Biol. Technol. 2017, 60, e17160568. [Google Scholar] [CrossRef] [Green Version]
- Anderson, E.F. The Cactus Family. Timber Press: Portland, OR, USA, 2001; ISBN 0881924989. [Google Scholar]
- Khatabi, O.; Hanine, H.; Elothmani, D.; Hasib, A. Extraction and determination of polyphenols and betalain pigments in the Moroccan Prickly pear fruits (Opuntia ficus-indica). Arab. J. Chem. 2016, 9, S278–S281. [Google Scholar] [CrossRef] [Green Version]
- Dubeux, J.C.B.; dos Santos, M.V.F.; da Cunha, M.V.; dos Santos, D.C.; de Alemeida Souza, R.T.; de Mello, A.C.L.; de Souza, T.C. Cactus (Opuntia and Nopalea) nutritive value: A review. Anim. Feed Sci. Technol. 2021, 275, 114890. [Google Scholar] [CrossRef]
- Sá Souza, M.; Júnior, G.N.A.; Souza, L.S.B.; Ferraz Jardim, A.M.R.; da Silva, G.I.N.; Araújo, G.G.L.; Campos, F.S.; Leite, M.L.M.V.; Tabosa, J.N.; Silva, T.G.F. Forage yield, competition and economic benefit of intercropping cactus and millet with mulch in a semi-arid environment. Afr. J. Range Forage Sci. 2022, 1–12. [Google Scholar] [CrossRef]
- Stavi, I. Ecosystem services related with Opuntia ficus-indica (prickly pear cactus): A review of challenges and opportunities. Agroecol. Sustain. Food Syst. 2022, 46, 815–841. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Hamid, A.; Imtiaz, H.; Rizwan, M.; Imran, M.; Sheikh, U.A.A.; Saira, I. Cactus pear: A weed of dry-lands for supplementing food security under changing climate. Planta Daninha 2020, 38, e020191761. [Google Scholar] [CrossRef]
- Rocha Filho, R.R.; Santos, D.C.; Véras, A.S.C.; Siqueira, M.C.B.; Novaes, L.P.; Mora-Luna, R.; Monteiro, C.C.F.; Ferreira, M.A. Can spineless forage cactus be the queen of forage crops in dryland areas? J. Arid Environ. 2021, 186, 104426. [Google Scholar] [CrossRef]
- Lahbouki, S.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Ait-Rahou, Y.; Ait-El-Mokhtar, M.; El Gabardi, S.; Douira, A.; Wahbi, S.; Outzourhit, A. Arbuscular mycorrhizal fungi and/or organic amendment enhance the tolerance of prickly pear (Opuntia ficus-indica) under drought stress. J. Arid Environ. 2022, 199, 104703. [Google Scholar] [CrossRef]
- Scalisi, A.; Morandi, B.; Inglese, P.; Lo Bianco, R. Cladode growth dynamics in Opuntia ficus-indica under drought. Environ. Exp. Bot. 2016, 122, 158–167. [Google Scholar] [CrossRef]
- Elbana, M.; El-Gamal, E.; Mohamed, A.; Fernando, A.L.; Pari, L.; Outzourhit, A.; Elwakeel, M.; El-Sheikh, W.; Rashad, M. Effect of irrigation scheduling on canopy cover development and crop-water management related parameters of O. ficus-indica under prolonged drought conditions. Sci. J. Agric. Sci. 2020, 2, 113–122. [Google Scholar] [CrossRef]
- Yahia, E.M.; Mondragon-Jacobo, C. Nutritional components and anti-oxidant capacity of ten cultivars and lines of cactus pear fruit (Opuntia spp.). Food Res. Int. 2011, 44, 2311–2318. [Google Scholar] [CrossRef]
- Piga, A. Cactus Pear: A Fruit of Nutraceutical and Functional Importance. J. Prof. Assoc. Cactus Dev. 2004, 6, 9–22. [Google Scholar]
- Barba, F.J.; Garcia, C.; Fessard, A.; Munekata, P.E.S.; Lorenzo, J.M.; Aboudia, A.; Ouadia, A.; Remize, F. Opuntia ficus-indica Edible Parts: A Food and Nutritional Security Perspective. Food Rev. Int. 2020, 38, 930–952. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.; Kudanga, T. Phenolic compound profile and biological activities of Southern African Opuntia ficus-indica fruit pulp and peels. LWT 2019, 111, 337–344. [Google Scholar] [CrossRef]
- Bakar, B.; Çakmak, M.; Ibrahim, M.S.; Özer, D.; Saydam, S.; Karatas, F. Investigation of amounts of vitamins, lycopene, and elements in the fruits of Opuntia ficus-indica subjected to different pretreatments. Biol. Trace Elem. Res. 2020, 198, 315–323. [Google Scholar] [CrossRef]
- Gouws, C.A.; Georgousopoulou, E.N.; Mellor, D.D.; McKune, A.; Naumovski, N. Effects of the consumption of prickly pear cacti (Opuntia spp.) and its products on blood glucose levels and insulin: A systematic review. Medicina B. Aires 2019, 55, 138. [Google Scholar] [CrossRef] [Green Version]
- Gouws, C.A.; McKune, A.; Tee, N.; Somerset, S.; Mortazavi, R. Prickly pear juice consumption after fat intake affects postprandial heart rate variability but not traditional risk factors of cardiovascular disease in healthy men. Nutrition 2022, 96, 111555. [Google Scholar] [CrossRef]
- Lamia, I.; Zouhir, C.; Youcef, A. Characterization and transformation of the Opuntia ficus-indica fruits. J. Food Meas. Charact. 2018, 12, 2349–2357. [Google Scholar] [CrossRef]
- Hernández-Becerra, E.; de los Angeles Aguilera-Barreiro, M.; Contreras-Padilla, M.; Pérez-Torrero, E.; Rodriguez-Garcia, M.E. Nopal cladodes (Opuntia ficus-indica): Nutritional properties and functional potential. J. Funct. Foods 2022, 95, 105183. [Google Scholar] [CrossRef]
- Lahbouki, S.; Anli, M.; El Gabardi, S.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Boutasknit, A.; Ait-Rahou, Y.; Outzourhit, A.; Wahbi, S.; Douira, A. Evaluation of arbuscular mycorrhizal fungi and vermicompost supplementation on growth, phenolic content and antioxidant activity of prickly pear cactus (Opuntia ficus-indica). Plant Biosyst. Int. J. Deal. all Asp. Plant Biol. 2021, 156, 882–892. [Google Scholar] [CrossRef]
- Astello-García, M.G.; Cervantes, I.; Nair, V.; Santos-Díaz, M.d.S.; Reyes-Agüero, A.; Guéraud, F.; Negre-Salvayre, A.; Rossignol, M.; Cisneros-Zevallos, L.; Barba de la Rosa, A.P. Chemical composition and phenolic compounds profile of cladodes from Opuntia spp. cultivars with different domestication gradient. J. Food Compos. Anal. 2015, 43, 119–130. [Google Scholar] [CrossRef]
- Pandit, V.; Kashive, D.; Sharma, T.K. Formulation and Evaluation of Novel Formulation for Diabetes Induced Hypertension using Modified Innate Superdisintegrant. J. Drug Deliv. Ther. 2020, 10, 240–250. [Google Scholar] [CrossRef]
- Gouws, C.; Mortazavi, R.; Mellor, D.; McKune, A.; Naumovski, N. The effects of Prickly Pear fruit and cladode (Opuntia spp.) consumption on blood lipids: A systematic review. Complement. Ther. Med. 2020, 50, 102384. [Google Scholar] [CrossRef]
- Ennouri, M.; Fetoui, H.; Bourret, E.; Zeghal, N.; Attia, H. Evaluation of some biological parameters of Opuntia ficus-indica. 1. Influence of a seed oil supplemented diet on rats. Bioresour. Technol. 2006, 97, 1382–1386. [Google Scholar] [CrossRef]
- Sirotkin, A.V. Can nopal cactus (Opuntia ficus-indica L. Miller) treat obesity? Obes. Med. 2022, 30, 100390. [Google Scholar] [CrossRef]
- Contreras-Padilla, M.; Rodríguez-García, M.E.; Gutiérrez-Cortez, E.; Valderrama-Bravo, M.d.C.; Rojas-Molina, J.I.; Rivera-Muñoz, E.M. Physicochemical and rheological characterization of Opuntia ficus-indica mucilage at three different maturity stages of cladode. Eur. Polym. J. 2016, 78, 226–234. [Google Scholar] [CrossRef]
- Nharingo, T.; Moyo, M. Application of Opuntia ficus-indica in bioremediation of wastewaters. A critical review. J. Environ. Manag. 2016, 166, 55–72. [Google Scholar] [CrossRef]
- del Socorro Santos-Díaz, M.; Barba de la Rosa, A.P.; Héliès-Toussaint, C.; Guéraud, F.; Nègre-Salvayre, A. Opuntia spp.: Characterization and Benefits in Chronic Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginestra, G.; Parker, M.L.; Bennett, R.N.; Robertson, J.; Mandalari, G.; Narbad, A.; Lo Curto, R.B.; Bisignano, G.; Faulds, C.B.; Waldron, K.W. Anatomical, chemical, and biochemical characterization of cladodes from prickly pear [Opuntia ficus-indica (L.) Mill.]. J. Agric. Food Chem. 2009, 57, 10323–10330. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Cactus stems (Opuntia spp.): A review on their chemistry, technology, and uses. Mol. Nutr. Food Res. 2005, 49, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Albuquerque, T.G.; Pereira, P.; Ramalho, R.; Vicente, F.; Oliveira, M.B.P.P.; Costa, H.S. Opuntia ficus-indica (L.) Mill.: A Multi-Benefit Potential to Be Exploited. Molecules 2021, 26, 951. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Urbiola, M.I.; Pérez-Torrero, E.; Rodríguez-García, M.E. Chemical Analysis of Nutritional Content of Prickly Pads (Opuntia ficus-indica) at Varied Ages in an Organic Harvest. Int. J. Environ. Res. Public Health 2011, 8, 1287–1295. [Google Scholar] [CrossRef] [Green Version]
- Aragona, M.; Lauriano, E.R.; Pergolizzi, S.; Faggio, C. Opuntia ficus-indica (L.) Miller as a source of bioactivity compounds for health and nutrition. Nat. Prod. Res. 2018, 32, 2037–2049. [Google Scholar] [CrossRef]
- Uebelhack, R.; Busch, R.; Alt, F.; Beah, Z.M.; Chong, P.W. Effects of Cactus Fiber on the Excretion of Dietary Fat in Healthy Subjects: A Double Blind, Randomized, Placebo-Controlled, Crossover Clinical Investigation. Curr. Ther. Res. Clin. Exp. 2014, 76, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Méndez, L.P.; Flores, F.T.; Martín, J.D.; Rodríguez Rodríguez, E.M.; Díaz Romero, C. Physicochemical characterization of cactus pads from Opuntia dillenii and Opuntia ficus-indica. Food Chem. 2015, 188, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Feugang, J.M.; Konarski, P.; Zou, D.; Stintzing, F.C.; Zou, C. Nutritional and medicinal use of Cactus pear (Opuntia spp.) cladodes and fruits. Front. Biosci. 2006, 11, 2574–2589. [Google Scholar] [CrossRef]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; Saïd, M.H.; Kebbaj, E.; Latruffe, N.; Lizard, G.; Nasser, B.; et al. Nopal Cactus (Opuntia ficus-indica) as a Source of Bioactive Compounds for Nutrition, Health and Disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [Green Version]
- Ciriminna, R.; Chavarría-Hernández, N.; Rodríguez-Hernández, A.I.; Pagliaro, M. Toward unfolding the bioeconomy of nopal (Opuntia spp.). Biofuels Bioprod. Biorefining 2019, 13, 1417–1427. [Google Scholar] [CrossRef] [Green Version]
- Msaddak, L.; Abdelhedi, O.; Kridene, A.; Rateb, M.; Belbahri, L.; Ammar, E.; Nasri, M.; Zouari, N. Opuntia ficus-indica cladodes as a functional ingredient: Bioactive compounds profile and their effect on antioxidant quality of bread. Lipids Health Dis. 2017, 16, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Lataief, S.; Zourgui, M.N.; Rahmani, R.; Najjaa, H.; Gharsallah, N.; Zourgui, L. Chemical composition, antioxidant, antimicrobial and cytotoxic activities of bioactive compounds extracted from Opuntia dillenii cladodes. J. Food Meas. Charact. 2020, 15, 782–794. [Google Scholar] [CrossRef]
- Missaoui, M.; D′Antuono, I.; D′Imperio, M.; Linsalata, V.; Boukhchina, S.; Logrieco, A.F.; Cardinali, A. Characterization of micronutrients, bioaccessibility and antioxidant activity of prickly pear cladodes as functional ingredient. Molecules 2020, 25, 2176. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Pellizzoni, M.; Montesano, D.; Lucini, L. Italian opuntia ficus-indica cladodes as rich source of bioactive compounds with health-promoting properties. Foods 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antunes-Ricardo, M.; Mendiola, J.A.; García-Cayuela, T.; Ibañez, E.; Gutiérrez-Uribe, J.A.; Pilar Cano, M.; Guajardo-Flores, D. Enzyme-assisted supercritical fluid extraction of antioxidant isorhamnetin conjugates from Opuntia ficus-indica (L.) Mill. J. Supercrit. Fluids 2020, 158, 104713. [Google Scholar] [CrossRef]
- De Santiago, E.; Juániz, I.; Cid, C.; De Peña, M.P. Extraction of (Poly)phenolic Compounds of Cactus (Opuntia ficus-indica (L.) Mill.) Cladodes. Food Anal. Methods 2021, 14, 1167–1175. [Google Scholar] [CrossRef]
- Ammar, I.; Ennouri, M.; Attia, H. Phenolic content and antioxidant activity of cactus (Opuntia ficus-indica L.) flowers are modified according to the extraction method. Ind. Crops Prod. 2015, 64, 97–104. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; García-Cayuela, T.; Mendiola, J.A.; Ibañez, E.; Gutiérrez-Uribe, J.A.; Cano, M.P.; Guajardo-Flores, D. Supercritical CO2 enzyme hydrolysis as a pretreatment for the release of isorhamnetin conjugates from Opuntia ficus-indica (L.) Mill. J. Supercrit. Fluids 2018, 141, 21–28. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Opuntia (Cactaceae) plant compounds, biological activities and prospects—A comprehensive review. Food Res. Int. 2018, 112, 328–344. [Google Scholar] [CrossRef]
- Dick, M.; Dal Magro, L.; Rodrigues, R.C.; Rios, A.d.O.; Flôres, S.H. Valorization of Opuntia monacantha (Willd.) Haw. cladodes to obtain a mucilage with hydrocolloid features: Physicochemical and functional performance. Int. J. Biol. Macromol. 2019, 123, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, C.; Sepúlveda, E.; Matsuhiro, B. Opuntia spp. mucilage’s: A functional component with industrial perspectives. J. Arid Environ. 2004, 57, 275–290. [Google Scholar] [CrossRef]
- Rodríguez-González, S.; Martínez-Flores, H.E.; Chávez-Moreno, C.K.; Macías-Rodríguez, L.I.; Zavala-Mendoza, E.; Garnica-Romo, M.G.; Chacõn-García, L. Extraction and characterization of mucilage from wild species of Opuntia. J. Food Process Eng. 2014, 37, 285–292. [Google Scholar] [CrossRef]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction and characterization of three polysaccharides extracted from Opuntia ficus-indica cladodes. Int. J. Biol. Macromol. 2016, 92, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, E.; Sáenz, C.; Aliaga, E.; Aceituno, C. Extraction and characterization of mucilage in Opuntia spp. J. Arid Environ. 2007, 68, 534–545. [Google Scholar] [CrossRef]
- Rodrigues, C.; Souza, V.G.L.; Rashad, M.; Pari, L.; Outzourhit, A.; Fernando, A.L. Mucilage extraction from Opuntia spp. for production of biofilms. In Proceedings of the 27th European Biomass Conference and Exhibition, Lisbon, Portugal, 27–30 May 2019; pp. 1456–1459. [Google Scholar]
- Lefsih, K.; Delattre, C.; Pierre, G.; Michaud, P.; Aminabhavi, T.M.; Dahmoune, F.; Madani, K. Extraction, characterization and gelling behavior enhancement of pectins from the cladodes of Opuntia ficus-indica. Int. J. Biol. Macromol. 2016, 82, 645–652. [Google Scholar] [CrossRef]
- Gheribi, R.; Puchot, L.; Verge, P.; Jaoued-Grayaa, N.; Mezni, M.; Habibi, Y.; Khwaldia, K. Development of plasticized edible films from Opuntia ficus-indica mucilage: A comparative study of various polyol plasticizers. Carbohydr. Polym. 2018, 190, 204–211. [Google Scholar] [CrossRef]
- Bayar, N.; Bouallegue, T.; Achour, M.; Kriaa, M.; Bougatef, A.; Kammoun, R. Ultrasonic extraction of pectin from Opuntia ficus-indica cladodes after mucilage removal: Optimization of experimental conditions and evaluation of chemical and functional properties. Food Chem. 2017, 235, 275–282. [Google Scholar] [CrossRef]
- Felkai-Haddache, L.; Remini, H.; Dulong, V.; Mamou-Belhabib, K.; Picton, L.; Madani, K.; Rihouey, C. Conventional and Microwave-Assisted Extraction of Mucilage from Opuntia ficus-indica Cladodes: Physico-Chemical and Rheological Properties. Food Bioprocess Technol. 2016, 9, 481–492. [Google Scholar] [CrossRef]
- Kalegowda, P.; Chauhan, A.S.; Nanjaraj Urs, S.M. Opuntia dillenii (Ker-Gawl) Haw cladode mucilage: Physico-chemical, rheological and functional behavior. Carbohydr. Polym. 2017, 157, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Bayar, N.; Friji, M.; Kammoun, R. Optimization of enzymatic extraction of pectin from Opuntia ficus-indica cladodes after mucilage removal. Food Chem. 2018, 241, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Chaouch, M.A.; Hafsa, J.; Rihouey, C.; Le Cerf, D.; Majdoub, H. Effect of pH during Extraction on the Antioxidant and Antiglycated Activities of Polysaccharides from Opuntia ficus-indica. J. Food Biochem. 2016, 40, 316–325. [Google Scholar] [CrossRef]
- Quinzio, C.; Ayunta, C.; Alancay, M.; de Mishima, B.L.; Iturriaga, L. Physicochemical and rheological properties of mucilage extracted from Opuntia ficus-indica (L. Miller). Comparative study with guar gum and xanthan gum. J. Food Meas. Charact. 2017, 12, 459–470. [Google Scholar] [CrossRef]
- Madera-Santana, T.J.; Vargas-Rodríguez, L.; Núñez-Colín, C.A.; González-García, G.; Peña-Caballero, V.; Núñez-Gastélum, J.A.; Gallegos-Vázquez, C.; Rodríguez-Núñez, J.R. Mucilage from cladodes of Opuntia spinulifera Salm-Dyck: Chemical, morphological, structural and thermal characterization. CyTA-J. Food 2018, 16, 650–657. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Martínez, J.D.; Sánchez-Becerril, M.; Ornelas-Paz, J.J.; González-Chávez, M.M.; Ibarra-Junquera, V.; Escalante-Minakata, P. The Effect of Extraction Conditions on the Chemical Characteristics of Pectin from Opuntia ficus-indica Cladode Flour. J. Polym. Environ. 2013, 21, 1040–1051. [Google Scholar] [CrossRef]
- Inglese, P.; Saenz, C.; Mondragon, C.; Nefzaoui, A.; Louhaichi, M. Crop Ecology, Cultivation and Uses of Cactus Pear; FAO: Rome, Italy, 2017; ISBN 978-92-5-109860-8. [Google Scholar]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V.; Maran, J.P. Microwave-assisted extraction of polysaccharides from mulberry leaves. Int. J. Biol. Macromol. 2015, 72, 1–5. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef] [Green Version]
- Monrroy, M.; García, E.; Ríos, K.; García, J.R. Extraction and Physicochemical Characterization of Mucilage from Opuntia cochenillifera (L.) Miller. J. Chem. 2017, 2017, 4301901. [Google Scholar] [CrossRef] [Green Version]
- Gheribi, R.; Khwaldia, K. Cactus Mucilage for Food Packaging Applications. Coatings 2019, 9, 655. [Google Scholar] [CrossRef] [Green Version]
- Tegegne, F. Nutritional value of Opuntia ficus-indica as a ruminant feed in Ethiopia. In Cactus (Opuntia spp.) as Forage; Mondragon, C., Gonzalez, S.P., Eds.; Food and Agriculture Organization: Rome, Italy, 2001; pp. 91–100. [Google Scholar]
- Melgar, B.; Inês, M.; Ciric, A.; Sokovic, M.; Garcia-castello, E.M.; Rodriguez-lopez, A.D.; Barros, L.; Ferreira, I. By-product recovery of Opuntia spp. peels: Betalainic and phenolic profiles and bioactive properties. Ind. Crops Prod. 2017, 107, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Kuti, J.O. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem. 2004, 85, 527–533. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: A comparative study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Chougui, N.; Tamendjari, A.; Hamidj, W.; Hallal, S.; Barras, A.; Richard, T.; Larbat, R. Oil composition and characterisation of phenolic compounds of Opuntia ficus-indica seeds. Food Chem. 2013, 139, 796–803. [Google Scholar] [CrossRef]
- Chiteva, R.; Wairagu, N. Chemical and nutritional content of Opuntia ficus-indica (L.). Afr. J. Biotechnol. 2013, 12, 3309–3312. [Google Scholar]
- Barba, F.J.; Putnik, P.; Bursać Kovačević, D.; Poojary, M.M.; Roohinejad, S.; Lorenzo, J.M.; Koubaa, M. Impact of conventional and non-conventional processing on prickly pear (Opuntia spp.) and their derived products: From preservation of beverages to valorization of by-products. Trends Food Sci. Technol. 2017, 67, 260–270. [Google Scholar] [CrossRef]
- Severo, C.; Anjos, I.; Souza, V.G.L.; Canejo, J.P.; Bronze, M.R.; Fernando, A.L.; Coelhoso, I.; Bettencourt, A.F.; Ribeiro, I.A.C. Development of cranberry extract films for the enhancement of food packaging antimicrobial properties. Food Packag. Shelf Life 2021, 28, 100646. [Google Scholar] [CrossRef]
- Andrade, M.A.; Barbosa, C.H.; Souza, V.G.L.; Coelhoso, I.M.; Reboleira, J.; Bernardino, S.; Ganhão, R.; Mendes, S.; Fernando, A.L.; Vilarinho, F.; et al. Novel Active Food Packaging Films Based on Whey Protein Incorporated with Seaweed Extract: Development, Characterization, and Application in Fresh Poultry Meat. Coatings 2021, 11, 229. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, C.; Ferreira, L.; Pires, J.R.A.; Duarte, M.P.; Coelhoso, I.; Fernando, A.L. In vitro bioactivity of novel chitosan bionanocomposites incorporated with different essential oils. Ind. Crops Prod. Prod. 2019, 140, 111563. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, P.F.; Duarte, M.P.; Fernando, A.L. Antioxidant Migration Studies in Chitosan Films Incorporated with Plant Extracts. J. Renew. Mater. 2018, 6, 548–558. [Google Scholar] [CrossRef]
- De Santiago, E.; Domínguez-Fernández, M.; Cid, C.; De Peña, M.P. Impact of cooking process on nutritional composition and antioxidants of cactus cladodes (Opuntia ficus-indica). Food Chem. 2018, 240, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Souza, V.G.L.; Coelhoso, I.; Fernando, A.L. Bio-Based Sensors for Smart Food Packaging—Current Applications and Future Trends. Sensors 2021, 21, 2148. [Google Scholar] [CrossRef] [PubMed]
- Kanatt, S.R. Development of active/intelligent food packaging film containing Amaranthus leaf extract for shelf life extension of chicken/fish during chilled storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Sáenz, C.; Berger, H.; Rodríguez-Félix, A.; Galletti, L.; García, J.C.; Sepúlveda, E.; Teresa, M.; Víctor, V.; De Cortázar, G.; Cuevas García, R.; et al. Agro-Industrial Utilization of Cactus Pear; Food and Agriculture Organization: Rome, Italy, 2013; ISBN 978-92-5-107987-4. [Google Scholar]
- Ammar, I.; Ennouri, M.; Bali, O.; Attia, H. Characterization of two prickly pear species flowers growing in Tunisia at four flowering stages. LWT-Food Sci. Technol. 2014, 59, 448–454. [Google Scholar] [CrossRef]
- Ammar, I.; Ennouri, M.; Khemakhem, B.; Yangui, T.; Attia, H. Variation in chemical composition and biological activities of two species of Opuntia flowers at four stages of flowering. Ind. Crops Prod. 2012, 37, 34–40. [Google Scholar] [CrossRef]
- Ammar, I.; Bardaa, S.; Mzid, M.; Sahnoun, Z.; Rebaii, T.; Attia, H.; Ennouri, M. Antioxidant, antibacterial and in vivo dermal wound healing effects of Opuntia flower extracts. Int. J. Biol. Macromol. 2015, 81, 483–490. [Google Scholar] [CrossRef]
- De Leo, M.; De Abreu, M.B.; Pawlowska, A.M.; Cioni, P.L.; Braca, A. Profiling the chemical content of Opuntia ficus-indica flowers by HPLC–PDA-ESI-MS and GC/EIMS analyses. Phytochem. Lett. 2010, 3, 48–52. [Google Scholar] [CrossRef]
- Alimi, H.; Hfaiedh, N.; Bouoni, Z.; Hfaiedh, M.; Sakly, M.; Zourgui, L.; Rhouma, K. Ben Antioxidant and antiulcerogenic activities of Opuntia ficus-indica f. inermis root extract in rats. Phytomedicine 2010, 17, 1120–1126. [Google Scholar] [CrossRef]
- Ventura-Aguilar, R.I.; Bosquez-Molina, E.; Bautista-Baños, S.; Rivera-Cabrera, F. Cactus stem (Opuntia ficus-indica Mill): Anatomy, physiology and chemical composition with emphasis on its biofunctional properties. J. Sci. Food Agric. 2017, 97, 5065–5073. [Google Scholar] [CrossRef]
- Sciacca, F.; Palumbo, M.; Pagliaro, A.; Di Stefano, V.; Scandurra, S.; Virzì, N.; Melilli, M.G. Opuntia cladodes as functional ingredient in durum wheat bread: Rheological, sensory, and chemical characterization. CYTA-J. Food 2021, 19, 96–104. [Google Scholar] [CrossRef]
- Attanzio, A.; Diana, P.; Barraja, P.; Carbone, A.; Spanò, V.; Parrino, B.; Cascioferro, S.M.; Allegra, M.; Cirrincione, G.; Tesoriere, L.; et al. Quality, functional and sensory evaluation of pasta fortified with extracts from Opuntia ficus-indica cladodes. J. Sci. Food Agric. 2019, 99, 4242–4247. [Google Scholar] [CrossRef] [PubMed]
- Msaddak, L.; Siala, R.; Fakhfakh, N.; Ayadi, M.A.; Nasri, M.; Zouari, N. Cladodes from prickly pear as a functional ingredient: Effect on fat retention, oxidative stability, nutritional and sensory properties of cookies. Int. J. Food Sci. Nutr. 2015, 66, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Moreno, E.; Cordoba-Díaz, M.; de Cortes Sánchez-Mata, M.; Marqués, C.D.; Goñi, I. The addition of cladodes (Opuntia ficus-indica L. Miller) to instant maize flour improves physicochemical and nutritional properties of maize tortillas. LWT 2015, 62, 675–681. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Pitacas, F.I.; Reis, C.M.G.; Blasco, M. Nutritional value of Opuntia ficus-indica cladodes from Portuguese ecotypes. Bulg. J. Agric. Sci. 2016, 22, 40–45. [Google Scholar]
- Rezende, F.M.; Véras, A.S.C.; Siqueira, M.C.B.; Conceição, M.G.; Lima, C.L.; Almeida, M.P.; Mora-Luna, R.E.; Neves, M.L.M.W.; Monteiro, C.C.F.; Ferreira, M.A. Nutritional effects of using cactus cladodes (Opuntia stricta Haw Haw) to replace sorghum silage in sheep diet. Trop. Anim. Health Prod. 2020, 52, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Mokoboki, K.; Sebola, N. Chemical composition and feed intake of Opuntia cladodes varieties offered to goats. J. Anim. Plant Sci. 2017, 32, 5096–5103. [Google Scholar]
- Souza, V.G.L.; Mello, I.P.; Khalid, O.; Pires, J.R.A.; Rodrigues, C.; Alves, M.M.; Santos, C.; Fernando, A.L.; Coelhoso, I. Strategies to Improve the Barrier and Mechanical Properties of Pectin Films for Food Packaging: Comparing Nanocomposites with Bilayers. Coatings 2022, 12, 108. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Del-Valle, V.; Hernández-Muñoz, P.; Guarda, A.; Galotto, M.J. Development of a cactus-mucilage edible coating (Opuntia ficus-indica) and its application to extend strawberry (Fragaria ananassa) shelf-life. Food Chem. 2005, 91, 751–756. [Google Scholar] [CrossRef]
- Allegra, A.; Inglese, P.; Sortino, G.; Settanni, L.; Todaro, A.; Liguori, G. The influence of Opuntia ficus-indica mucilage edible coating on the quality of “Hayward” kiwifruit slices. Postharvest Biol. Technol. 2016, 120, 45–51. [Google Scholar] [CrossRef]
- Allegra, A.; Sortino, G.; Inglese, P.; Settanni, L.; Todaro, A.; Gallotta, A. The effectiveness of Opuntia ficus-indica mucilage edible coating on post-harvest maintenance of ‘Dottato’ fig (Ficus carica L.) fruit. Food Packag. Shelf Life 2017, 12, 135–141. [Google Scholar] [CrossRef]
- Espino-Díaz, M.; De Jesús Ornelas-Paz, J.; Martínez-Téllez, M.A.; Santillán, C.; Barbosa-Cánovas, G.V.; Zamudio-Flores, P.B.; Olivas, G.I. Development and characterization of edible films based on mucilage of Opuntia ficus-indica (L.). J. Food Sci. 2010, 75, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.R.A.; de Souza, V.G.L.; Fernando, A.L. Production of Nanocellulose from Lignocellulosic Biomass Wastes: Prospects and Limitations; Springer: Cham, Switzerland, 2019; pp. 719–725. [Google Scholar]
- Pires, J.; Paula, C.D.; Souza, V.G.L.; Fernando, A.L.; Coelhoso, I. Understanding the barrier and mechanical behavior of different nanofillers in chitosan films for food packaging. Polymers 2021, 13, 721. [Google Scholar] [CrossRef] [PubMed]
- De Farias, P.M.; de Vasconcelos, L.B.; Ferreira, M.E.S.; Pascall, M.; Tapia-Blácido, D.R. Nopal cladode (Opuntia ficus-indica) flour: Production, characterization, and evaluation for producing bioactive film. Food Packag. Shelf Life 2021, 29, 100703. [Google Scholar] [CrossRef]
- Lira-Vargas, A.A.; Corrales-Garcia, J.J.E.; Valle-Guadarrama, S.; Peña-Valdivia, C.B.; Trejo-Marquez, M.A. Biopolymeric films based on cactus (Opuntia ficus-indica) mucilage incorporated with gelatin and beeswax. J. Prof. Assoc. Cactus Dev. 2014, 16, 51–70. [Google Scholar]
- Souza, V.G.L.; Ferreira, L.S.; Rodrigues, C.P.; Pires, J.R.; Duarte, M.P.; Coelhoso, I.M.; Fernando, A.L. Antimicrobial and antioxidant activity of novel biocomposites incorporated with essential oils. In Proceedings of the 27th European Biomass Conference and Exhibition Proceedings, Lisbon, Portugal, 27–30 May 2019; pp. 1083–1086. [Google Scholar]
- García-García, R.; Escobedo-Avellaneda, Z.; Tejada-Ortigoza, V.; Martín-Belloso, O.; Valdez-Fragoso, A.; Welti-Chanes, J. Hurdle technology applied to prickly pear beverages for inhibiting Saccharomyces cerevisiae and Escherichia coli. Lett. Appl. Microbiol. 2015, 60, 558–564. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Wójtowicz, A.; Oniszczuk, T.; Matwijczuk, A.; Dib, A.; Markut-Miotła, E. Opuntia fruits as food enriching ingredient, the first step towards new functional food products. Molecules 2020, 25, 916. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, N.; Stefanoni, W.; Latterini, F.; Pari, L. An Italian explorative study of willingness to pay for a new functional pasta featuring Opuntia ficus-indica. Agriculture 2021, 11, 701. [Google Scholar] [CrossRef]
- Palmieri, N.; Suardi, A.; Stefanoni, W.; Pari, L. Opuntia ficus-indica as an ingredient in new functional pasta: Consumer preferences in Italy. Foods 2021, 10, 803. [Google Scholar] [CrossRef]
- Elhassaneen, Y. Improvement of Bioactive Compounds Content and Antioxidant Properties in Crackers with the Incorporation of Prickly Pear and Potato Peels Powder. Int. J. Nutr. Food Sci. 2016, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Manzur-Valdespino, S.; Ramírez-Moreno, E.; Arias-Rico, J.; Jaramillo-Morales, O.A.; Calderón-Ramos, Z.G.; Delgado-Olivares, L.; Córdoba-Díaz, M.; Córdoba-Díaz, D.; Cruz-Cansino, N.d.S. Opuntia ficus-indica L. Mill residues-Properties and application possibilities in food supplements. Appl. Sci. 2020, 10, 3260. [Google Scholar] [CrossRef]
- Chougui, N.; Djerroud, N.; Naraoui, F.; Hadjal, S.; Aliane, K.; Zeroual, B.; Larbat, R. Physicochemical properties and storage stability of margarine containing Opuntia ficus-indica peel extract as antioxidant. Food Chem. 2015, 173, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Parafati, L.; Restuccia, C.; Palmeri, R.; Fallico, B.; Arena, E. Impact of prickly pear extract on the quality parameters of beef burger patties after cooking. Food Biosci. 2021, 42, 101146. [Google Scholar] [CrossRef]
- Ciriminna, R.; Delisi, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Opuntia ficus-indica seed oil: Biorefinery and bioeconomy aspects. Eur. J. Lipid Sci. Technol. 2017, 119, 1700013. [Google Scholar] [CrossRef]
- Berrabah, H.; Taïbi, K.; Ait Abderrahim, L.; Boussaid, M. Phytochemical composition and antioxidant properties of prickly pear (Opuntia ficus-indica L.) flowers from the Algerian germplasm. J. Food Meas. Charact. 2019, 13, 1166–1174. [Google Scholar] [CrossRef]
- Ammar, I.; Ennouri, M.; Bouaziz, M.; Amira, A. Ben Phenolic Profiles, Phytchemicals and Mineral Content of Decoction and Infusion of Opuntia ficus-indica Flowers. Plant Foods Hum. Nutr. 2015, 70, 388–394. [Google Scholar] [CrossRef]
- Ammar, I.; BenAmira, A.; Khemakem, I.; Attia, H.; Ennouri, M. Effect of Opuntia ficus-indica flowers maceration on quality and on heat stability of olive oil. J. Food Sci. Technol. 2017, 54, 1502–1510. [Google Scholar] [CrossRef] [Green Version]

| Species/Variety | Extraction Procedure | Main Compounds Found | Conclusions | Ref. |
|---|---|---|---|---|
| Opuntia ficus-indica f. inermis | Cladodes were washed, cut, dried, ground, and sieved. Maceration was performed with ethanol. The solvent was evaporated under vacuum. Theextract was dissolved in methanol and properly kept in Liquid Cromatography-mass spectrometry (LC-MS) vials until further analysis. | Quercetin Quercetin 3-O-glucoside Kaempferol Kaempferol 3-O-glucoside Kaempferol 3-O-rutinoside Isorhamnetin Isorhamnetin 3-O-glucoside Isorhamnetin 3-O-glucoside Isorhamnetin 3-O-neohesperidoside 3,3′,4′,5,7-Pentahydroxy-flavanone p-Coumaric acid Zataroside-A Indicaxanthin β-Sitosterol | The analysis of cladode extracts allowed the identification of 13 bioactive compounds, several of them with antioxidant potential. | [45] |
| Opuntia dillenii | Aqueous extract: Extraction with water (24 h, room temperature). Macerates were filtered, lyophilized, and kept at −20 °C. | Quinic acid Protocatechuic acid Caffeic acid Syringic acid p-coumaric acid Naringin Trans ferulic acid Cinnamic acid | Both aqueous and ethanolic cladode extracts presented a good polyphenol profile, with the ethanolic cladode extract presenting highest variability of these bioactive compounds. The aqueous cladode extract was poor in flavonoid compounds. | [46] |
| Ethanolic extract: Extraction with ethanol (24 h, room temperature). The macerates were filtered and evaporated in a vacuum rotary evaporator and kept below −20 °C. | Quinic acid Gallic acid Protocatechuic acid Rutin Hyperoside p-coumaric acid Naringin Quercetrin 1,3-di-O-caffeoylquinic acid Apegenin-7-O-glucoside Trans ferulic acid Salviolonic acid Quercetin Kampherol Naringenin Apeginin Luteolin Cirsiliol Cirsilineol Acacetin | |||
| Opuntia ficus-indica (L.) Mill | Cladode powder was extracted in an ultrasonic bath with a mixture of methanol/water 80:20 v/v. Then it was centrifuged, diluted, and filtered. | Piscidic acid I Piscidic acid II 2 Ferulic acid derivative I Eucomic acid Ferulic acid derivative II Kaempferol derivative I Isorhamnetin derivative I Isorhamnetin derivative II Kaempferol derivative II Isorhamnetin derivative III Isorhamnetin 3-O rutinoside (a narcissin) | The analysis of cladode extracts through HPLC-DAD allowed the identification of 11 classes of bioactive compounds. The authors highlighted the presence of piscidic acid (a polyphenol) and isorhamnetin (a flavonoid). | [47] |
| Opuntia ficus-indica (L.) Mill | Extraction was performed in an Ultra-turrax with a mixture of methanol/water 80:20 v/v and 0.1% formic acid. The resulting extracts were centrifuged, filtered, and finally collected until analysis. | Cyanidin-Glu Pelargonidin-Glu Petunidin-Glu Delphinidin-Glu Malvidin-Glu Luteolin-Glu Apigenin-Glu Isoflavonoids Myricetin-Glu Quercetin-Glu Kaempferol-Glu Isorhamnetin-Glu Furofurans Dibenzylbutyrolactone Alkylphenols Hydroxybenzaldehydes Hydroxycoumarins Tyrosols Hydroxybenzoics Hydroxyphenylpropanoics Hydroxycinnamics | The analysis of cladode extracts through UHPLC-ESI-QTOF-MS allowed the identification of the principal phenolic classes and subclasses. The authors highlighted that cladodes are a good source of anthocyanins and phenolic acids, thus a good source of antioxidant compounds. | [48] |
| Opuntia ficus-indica (L.) Mill | Cladode powder samples were mixed with the enzymes Rapidase or Viscozyme in a mixture of ethanol /water (90:10). The vessel was subjected to pressure with CO2, and then a supercritical fluid extraction was performed. | 3-O-methyl quercetin 3-O-methyl kaempferol Luteolin Isorhamnetin tri- and diglycosides: Isorhamnetin-3-O-glucosyl-rhamnosyl-rhamnoside Isorhamnetin-3-O-glucosyl-rhamnosyl-pentoside Isorhamnetin-3-O-glucosyl-rhamnosyl-methylpentoside Isorhamnetin-3-O-glucosyl-pentoside Isorhamnetin-3-O-glucosyl-rhamnoside | The use of enzymes to assist the extraction with supercritical fluids improved the extraction of isorhamnetin conjugates. | [49] |
| Opuntia ficus-indica (L.) Mill | Cladodes powder was subjected to sequential extractions with different solvents. The resulting supernatants were mixed, and acid hydrolysis was performed under reflux using different concentrations of HCl and times. | Isorhamnetin Kaempferol Quercetin Total flavonoids Ferulic acid 4-Hydroxybenzoic acid | Acid hydrolysis was demonstrated to be advantageous for identifying and quantifying (poly)phenolic compounds through High Performance Liquid Chromatography with Diodde Array Detection (HPLC-DAD). The highest amount of total (poly)phenolic compounds was obtained from acid hydrolysis under the following conditions: 2 h, 90 °C, 1.5 M HCl. | [50] |
| Species/Variety | Extraction Procedure | Yield of Extraction (%) | Main Compounds Found | Conclusions | Ref. |
|---|---|---|---|---|---|
| Opuntia ficus-indica | Cladode powder was subjected to a sequential extraction to obtain water-soluble pectins (WSP), chelating-soluble pectins (CSP), and acid-soluble pectins (ASP). | WSP: 5.75% (w/w) of dry weight CSP: 0.21% (w/w) of dry weight ASP: 0.11% (w/w) of dry weight | Monosaccharide composition: Galactose Glucose Galacturonic acid Arabinose Xylose Rhamnose Mannose | All the pectin fractions obtained are mostly rich in galacturonic acid. Through the degree of methylation analysis, it was shown that all pectin fractions are lowly methylated (DM < 50%). | [60] |
| Opuntia ficus-indica | Mucilage extraction: Cladodes were washed and cut, and the interior was removed and pressed. Mucilage was precipitated in ethanol in a ratio of 2:3 (extract: ethanol). The precipitate was collected, washed with ethanol, dried (50 °C, 24 h), and then pulverized. | Not presented | Monosaccharide composition: Galactose Arabinose Xylose Rhamnose Glucose | The major sugars present in cactus mucilage are galactose and arabinose. | [61] |
| Opuntia monocantha | Mucilage extraction: Cladodes were washed, cut, and crushed. The resulting pulp was mixed with water at a ratio of 1:2 w/v (pulp: water), stirred, and heated (80 °C, 30 min). The mixture was filtered and centrifuged. The supernatant was precipitated in 95% ethanol and kept overnight at 4 °C. The mucilage was filtered under vacuum, dried, and sieved. | 12.0% (w/w) of dry weight | Monosaccharide composition: Arabinose Galactose Glucose Rhamnose Xylose Galacturonic acid Glucuronic acid | The extraction method shows promising results for obtaining mucilage powder, which is mainly composed of carbohydrates. | [54] |
| Opuntia ficus-indica | Total pectic mucilage fraction extraction (TFC): Extraction is performed in a water bath using water as the solvent (adjusting the pH). The mixture was filtered and centrifuged, and the supernatant was concentrated. The solution was precipitated in isopropanol (4 °C, overnight). The resulting precipitate was centrifuged, washed with ethanol, and dried. Mucilage fraction extraction (MC): The extraction procedure was performed under agitation with water as the solvent and centrifuged. The supernatant was precipitated with isopropanol (4 °C, overnight), then washed with absolute ethanol and dried. Pectin fraction extraction (PC): obtained by the same method used for total pectic mucilage fraction; using the residues from the mucilage extraction as starting materials. | TFC: 13.1% (w/w) of dry weight MC: 10.2% (w/w) of dry weight PC: 6.13% (w/w) of dry weight | High content in carbohydrates | Extraction conditions may affect the extraction yield. PC was richer in uronic acid content than the other two fractions. Mucilage showed a lower content of uronic acid. | [57] |
| Opuntia ficus-indica | Pectin extraction: extraction from cladode’s powder was made with water, centrifuged, and the resulting residues were dried. The residues were mixed with water in an ultrasound water bath and centrifuged. The supernatant was precipitated in isopropanol and then dried. | 18.6% (w/w) of dry weight | High uronic acid content | The use of ultrasounds increased the pectin extraction and reduced the extraction time when compared with chemical extraction methods. | [62] |
| Opuntia ficus-indica | Mucilage was extracted from cladodes by performing a microwave-assisted extraction (different powers were applied). Then the samples were filtered, centrifuged, and precipitated in ethanol. The resulting precipitate was washed with ethanol and lyophilized. | 8.13% (w/w) of dry weight with 500 W for 7 min | Monosaccharide composition: Galactose Arabinose Xylose Rhamnose Glucose | Microwave irradiation promotes the interaction between the solvent and the material, enhancing the extraction. | [63] |
| Opuntia dillenii (Ker-Gawl) Haw | Mucilage extraction: fresh cladodes were extracted with water under stirring. The solution was filtered and concentrated. The concentrated solution was precipitated with and dialyzed against water (24 h, membrane of 12–14 kDa) and lyophilized. | 6.20% (w/w) of dry weight | Monosaccharide composition: Arabinose Galactose Rhamnose Xylose Glucose Uronic acid | The major neutral sugars found were arabinose and galactose. The uronic acid was found in a lower percentage (2.5%), demonstrating the neutrality of the mucilage. | [64] |
| Opuntia ficus-indica | Mucilage was previously extracted from cladodes, and the resulting residue was used for pectin enzyme-assisted extraction. Xylanase and cellulase were added under optimal conditions. After enzyme inactivation, the mixture was centrifuged, and the supernatant was precipitated with isopropanol. The precipitate was centrifuged, dried at 50 °C, and reduced to powder. | 16.7% (w/w) of dry weight | Arabinose Xylose Fructose Glutamic acid Galacturonic acid Glucose Galactose Mannose Rhamnose | Enzyme-assisted extraction proved effective for enhancing pectin extraction without using acid treatments. The resulting pectin was lowly methylated and showed a galacturonic acid content of 65%. | [65] |
| Opuntia ficus-indica | Pectin extraction was performed under three different extraction conditions: acidic (pH = 2), neutral (pH = 6), and basic (pH = 10). The samples were submitted to ultrasound and then placed under mechanical stirring, filtered, and centrifuged. The residues were submitted to the same extraction, and the supernatant was mixed at the end. The resulting supernatants were precipitated in ethanol and filtered. The residues were dialyzed (30 kDa membrane) against water, concentrated, and lyophilized. | Acidic (pH = 2): 8.80% (w/w) of dry weight Neutral (pH = 6): 24.5% (w/w) of dry weight Basic (pH = 10): 4.60% (w/w) of dry weight | Neutral sugars Galacturonic acid | Neutral extraction obtained the highest total yield, although acidic extraction presented a higher galacturonic acid content. | [66] |
| Opuntia ficus-indica | Mucilage extraction was performed by extrusion. The solution was centrifuged, and the supernatant was precipitated in ethanol and dried. | 1.50% (w/w) of dry weight | Not assessed | The yield of extraction was demonstrated to be dependent on mucilage synthesis, which depends on edaphoclimatic conditions. | [67] |
| Opuntia spinulifera Salm-Dyck | Mucilage extraction was accomplished by mixing cladodes with water under stirring and filtering at the end (process repeated four times). The filtrate obtained was precipitated with ethanol, dried, and reduced to powder. | Not assessed | Arabinose Rhamnose Xylose Galactose Uronic acid | Cladodes from Opuntia spinulifera are rich in carbohydrates. Furthermore, the presence of pectins was found through Fourier-Transform Infrared Spectroscopy (FTIR) analysis. This could be explained by the ability of pectins to also precipitate in ethanol. | [68] |
| Opuntia ficus-indica | Residue from cladode’s flour was obtained by depigmentation with ethanol and acetone and centrifugation. The extraction was performed by mixing EDTA and water, adjusting the pH, and stirring. Then, a centrifugation was performed, and the supernatant was precipitated. The precipitate was recovered through centrifugation and resuspended in water. The precipitate was filtered, and the solution was mixed with ethanol (5 °C, overnight), filtered again, and then dried. | 10.4% (w/w) of dry weight under conditions of: pH = 11; 20% EDTA; 80 °C | Galacturonic acid Arabinose Galactose Glucose Rhamnose | Pectin extractions under alkaline conditions demonstrated a higher yield than those performed under acidic conditions. The use of EDTA suggests that the chelation of calcium is an important factor in extraction. | [69] |
| Species/Variety | Provenance | Extraction Procedure | Main Bioactive Compounds | Main Conclusions | Ref. |
|---|---|---|---|---|---|
| Opuntia ficus-indica var. Gialla | The fruits were purchased from Sicily, Italy, and Portugal. They were separated according to color; i.e.,orange-red (Opuntia ficus-indica var. Gialla) and red-violet (Opuntia ficus-indica var. Sanguigna) | Hydro-alcoholic extraction (ethanol:water) was performed under agitation and followed by filtration. The resulting extracts were lyophilized and their content in total phenolic compounds, antioxidants, and betalain, was evaluated by using High Performance Liquid Chromatography (HPLC). | Piscidic acid: 1061 mg/g fruit Eucomic acid: 1.40 mg/g fruit Isorhamnetin- O—(di-deoxyhexosylhexoside): 0.22 mg/g fruit Isorhamentin- O—(deoxyhexosyl-pentosylhexoside): 0.77 mg/g fruit Isorhamnetin- O—(pentosylhexoside): 0.10 mg/g fruit Total phenolic compounds: 3.26 mg/g fruit Betaxanthines—Indicaxanthin I isomer: 25.3 mg/g fruit Indicaxanthin II isomer:64.5 mg/g fruit Betacyanins—Betanidin-5-O-β- glucoside (betanine): 1.25 mg/g fruit Total: 1.25 mg betacyanin compounds/g fruit | Opuntia ficus-indica var. Sanguigna has more phenolic compounds and betacyanin compounds than Opuntia ficus-indica var. Gialla | [77] |
| Opuntia ficus-indica var. Sanguigna | Eucomic acid: 2.2 mg/g fruit Isorhamnetin- O—(di-deoxyhexosylhexoside): 0.56 mg/g fruit Isorhamnetin- O—(di-deoxyhexosylhexoside): 0.21 mg/g fruit Isorhamentin- O—(deoxyhexosyl-pentosylhexoside): 1.3 mg/g fruit Isorhamentin- O—(pentosylhexoside): 0.67 mg/g fruit Isorhamentin- O—(deoxyhexosylhexoside): 1.7 mg/g fruit Total phenolic compounds: 3.7 mg/g fruit Indicaxanthin I isomer: 56.0 mg/g fruit Indicaxanthin II isomer: 2.8 mg/g fruit Betanidin-5-O -β-glucoside (betanine): 3.44 mg/g fruit Isobetanine: 0.54 mg/g fruit Total beta-cyanine compounds: 3.97 mg/g fruit | ||||
| Opuntia ficus-indica (green peel) | Fruits were harvested from Texas A&M University, Kingsville orchard. Following that, the separation of the peels and pulp was performed and the material was weighed and stored at −80 °C. | Flavonoid extraction: skin and pulp were homogenized with 25 mL of methanol. Acid hydrolysis was performed with the flavonoids aglycones. Quantification was performed by using HPLC. Ascorbic acid extraction: frozen tissues were sprayed with a dismembrator, centrifuged, and the supernatant was filtered. Quantification was performed by using HPLC. Carotenoids extraction: skin and pulp were mixed with a mixture of hexane/acetone/methanol, followed by centrifugation, and then filtration. Total carotenoids determination was performed by using a UV-Vis spectrophotometer. | Flavonol content Kaempferol: 2.2 µg/g fruit Quercetin: 43.2 µg/g fruit Isorhamnetin: 24.1 µg/g fruit Ascorbic acid: 458 µg/g fruit Total carotenoids: 2.9 µg/g fruit Total flavonoids: 69.5 µg/g fruit | Opuntia stricta var. stricta has presented more Kaempferol; Opuntia streptacantha has more Quercetin; and the Opuntia ficus-indica has more Isorhamnetin. In addition to that, Opuntia stricta var. stricta has more ascorbic acid; Opuntia lindheimeri has more carotenoids; and Opuntia streptacantha has more flavonoids. | [78] |
| Opuntia streptacantha (red peel) | Flavonol content Kaempferol: 1.1 µg/g fruit Quercetin: 90.5 µg/g fruit Isorhamnetin: 1.9 µg/g fruit Ascorbic acid: 121 µg/g fruit Total carotenoids: 6.7 µg/g fruit Total flavonoids: 93.5 µg/g fruit | ||||
| Opuntia stricta var. stricta (yellow peel) | Flavonol content Kaempferol: 3.8 µg/g fruit Quercetin: 51.0 µg/g fruit Isorhamnetin: not detected Ascorbic acid: 815 µg/g fruit Total carotenoids: 14.6 µg/g fruit Total flavonoids: 54.8 µg/g fruit | ||||
| Opuntia lindheimeri (purple peel) | Flavonol content Kaempferol: not detected Quercetin: 9.8 µg/g fruit Isorhamnetin: not detected Ascorbic acid: 437 µg/g fruit Total carotenoids: 23.7 µg/g fruit Total flavonoids: 9.8 µg/g fruit | ||||
| Opuntia ficus-indica (Mexico, purple peel) | The fruits used in this study were obtained from Mexico and Spain. They were chosen based on their similar characteristics in terms of ripeness, uniformity, and quality of the material. | Extraction was performed on freeze-dried fruits using methanol and water. The mixture passed through an ultrasonic bath and was centrifuged. After removing the supernatant, the process was repeated twice. The supernatant was analyzed by HPLC. | Whole fruit Betaxanthins: 246.2 µg/g fruit Betacyanins: 2176 µg/g fruit Betalains: 2423 µg/g fruit Phenolic acids: 27,556 µg/g fruit Flavonoids: 643.4 µg/g fruit Phenolics: 28,199 µg/g fruit Pulp Betaxanthins: 206.8 µg/g fruit Betacyanins: 2070 µg/g fruit Betalains: 2274 µg/g fruit Phenolic acids: 18,498 µg/g fruit Flavonoids: 319.6 µg/g fruit Phenolics: 18,818 µg/g fruit Peel Betaxanthins: 69.1 µg/g fruit Betacyanins: 630 µg/g fruit Betalains: 699 µg/g fruit Phenolic acids: 45,587 µg/g fruit Flavonoids: 3426 µg/g fruit Phenolics: 49,012 µg/g fruit | The whole fruits of the purple-colored species from Mexico and Spain were the richest in betacyanin, and those of red coloration from Spain and yellow from Mexico have a higher content of betaxanthin. Regarding the peel, a large amount of phenolic compounds was observed, mainly in the purple variety from Spain. | [79] |
| Opuntia ficus-indica (Mexico, red peel) | Whole fruit Betaxanthins: 177.9 µg/g fruit Betacyanins: 404.7 µg/g fruit Betalains: 582.6 µg/g fruit Phenolic acids: 23,169 µg/g fruit Flavonoids: 798 µg/g fruit Phenolics: 23,967 µg/g fruit Pulp Betaxanthins: 246.8 µg/g fruit Betacyanins: 525 µg/g fruit Betalains: 771.8 µg/g fruit Phenolic acids: 13,030 µg/g fruit Flavonoids: 171.7 µg/g fruit Phenolics: 13,202 µg/g fruit Peel Betaxanthins: 118.3 µg/g fruit Betacyanins: 389.4 µg/g fruit Betalains:507.6 µg/g fruit Phenolic acids: 42,106 µg/g fruit Flavonoids: 2564 µg/g fruit Phenolics: 44,669 µg/g fruit | ||||
| Opuntia ficus-indica (Mexico, yellow peel) | Whole fruit Betaxanthins: 487.9 µg/g fruit Betacyanins: 100.1 µg/g fruit Betalains: 587.9 µg/g fruit Phenolic acids: 19,990 µg/g fruit Flavonoids: 1073 µg/g fruit Phenolics: 21,063 µg/g fruit Pulp Betaxanthins: 390.8 µg/g fruit Betacyanins: 89.2 µg/g fruit Betalains: 479.9 µg/g fruit Phenolic acids: 8902 µg/g fruit Flavonoids: 139.5 µg/g fruit Phenolics: 9042 µg/g fruit Peel Betaxanthins: 252.3 µg/g fruit Betacyanins: 96.1 µg/g fruit Betalains: 384.4 µg/g fruit Phenolic acids: 28,074 µg/g fruit Flavonoids: 3218 µg/g fruit Phenolics: 31,292 µg/g fruit | ||||
| Opuntia ficus-indica (Spain, purple peel) | Whole fruit Betaxanthins: 278.7 µg/g fruit Betacyanins: 1372 µg/g fruit Betalains: 1651 µg/g fruit Phenolic acids: 27,252 µg/g fruit Flavonoids: 615.3 µg/g fruit Phenolics: 27,867 µg/g fruit Pulp Betaxanthins: 209.8 µg/g fruit Betacyanins: 1084 µg/g fruit Betalains: 1293 µg/g fruit Phenolic acids: 23,131 µg/g fruit Flavonoids: 204.0 µg/g fruit Phenolics: 23,335 µg/g fruit Peel Betaxanthins: 175.7 µg/g fruit Betacyanins: 1022 µg/g fruit Betalains: 1197 µg/g fruit Phenolic acids: 35,163 µg/g fruit Flavonoids: 3268 µg/g fruit Phenolics: 38,430 µg/g fruit | ||||
| Opuntia ficus-indica (Spain, red peel) | Whole fruit Betaxanthins: 435.4 µg/g fruit Betacyanins: 384.4 µg/g fruit Betalains: 819.8 µg/g fruit Phenolic acids: 17,022 µg/g fruit Flavonoids: 550.9 µg/g fruit Phenolics: 17,573 µg/g fruit Pulp Betaxanthins: 405.1 µg/g fruit Betacyanins: 343.0 µg/g fruit Betalains: 1293 µg/g fruit Phenolic acids: 14,340 µg/g fruit Flavonoids: 161.5 µg/g fruit Phenolics: 14,502 µg/g fruit Peel Betaxanthins: 210.3 µg/g fruit Betacyanins: 379.3 µg/g fruit Betalains: 589.6 µg/g fruit Phenolic acids: 36,183 µg/g fruit Flavonoids: 3571 µg/g fruit Phenolics: 39,754 µg/g fruit | ||||
| Opuntia ficus-indica (Spain, yellow peel) | Whole fruit Betaxanthins: 134.8 µg/g fruit Betacyanins: 21.4 µg/g fruit Betalains: 156.2 µg/g fruit Phenolic acids: 18,165 µg/g fruit Flavonoids: 554.9 µg/g fruit Phenolics: 18,720 µg/g fruit Pulp Betaxanthins: 214.2 µg/g fruit Betacyanins: 26.2 µg/g fruit Betalains: 240.5 µg/g fruit Phenolic acids: 10,437 µg/g fruit Flavonoids: 117.2 µg/g fruit Phenolics: 10,554 µg/g fruit Peel Betaxanthins: 98.0 µg/g fruit Betacyanins: 27.8 µg/g fruit Betalains: 125.7 µg/g fruit Phenolic acids: 38,820 µg/g fruit Flavonoids: 2860 µg/g fruit Phenolics: 41,680 µg/g fruit | ||||
| Opuntia ficus-indica (green peel) | Fruits were harvested in Bejaia, Argelia, in August 2008. They were selected based on spines, colors, and shapes. This study compared the seed oil composition extracted from Opuntia ficus-indica of different peel colors (green, yellow, orange, and red). | Seed oil was obtained by Soxhlet extraction, using hexane. For the extraction of phenolic compounds from the oil, a solid-liquid extraction method was applied using ethanol. The content in phenolic compounds was assessed by the Velioglu method and also analyzed by High Performance Liquid Chromatography -Mass Spectrometry (HPLC-MS). | Total phenolic compounds: 61 mg GAE */100 g seed Flavonoids: 1.5 mg/100 g seed Tannins: 4.5 mg/100 g seed | The orange variety has more phenolic compounds. The study concludes that the seeds from Opuntia ficus-indica have a good number of phenolic compounds that can be used by the industry. | [80] |
| Opuntia ficus-indica (yellow peel) | Total phenolic compounds: 74 mg GAE */100 g seed Flavonoids: 1.9 mg/100 g seed Tannins: 4.8 mg/100 g seed | ||||
| Opuntia ficus-indica orange peel) | Total phenolic compounds: 89 mg GAE */100 g seed Flavonoids: 2.6 mg/100 g seed Tannins: 6.6 mg/100 g seed | ||||
| Opuntia ficus-indica (red peel) | Total phenolic compounds: 48 mg GAE */100 g seed Flavonoids: 1.5 mg/100 g seed Tannins: 4.1 mg/100 g seed |
| Species/Variety | Provenance | Extraction Procedure | Main Bioactive Compounds and Levels Found | Main Conclusion | Ref. |
|---|---|---|---|---|---|
| Opuntia ficus-indica | Flowers were collected during the post-flowering stage in June 2013, from wild populations located in Tunisia (latitude 34°46′29″ N, longitude 10°39′73″ E; elevation: 41 m), where the climate is semi-arid, characterized by a mean rainfall of 200 mm/year. | Extraction procedure was performed by using different solvents: water, methanol, acetonitrile, acetone, ethyl acetate, dichloromethane, and hexane. Two different extraction processes were tested: Soxhlet extraction and maceration. | Total phenolic content (mg GAE */g extract) Soxhlet extraction Water: 58.7 Methanol: 270.9 Acetonitrile: 132.4 Acetone: 67.5 Ethyl acetate: 122.3 Dichloromethane: 10.1 Hexane: 7.5 Maceration extraction Water: 44.2 Methanol: 227.8 Acetonitrile: 17.6 Acetone: 70 Ethyl acetate: 70.8 Dichloromethane: 14.7 Hexane: 14.8 Total flavonoid content (mg RE*/g extract) Soxhlet extraction Water: 9.7 Methanol: 60.81 Acetonitrile: 19.4 Acetone: 15.29 Ethyl acetate: 17.65 Dichloromethane: 1.73 Hexane: Not detected Maceration extraction Water: 22.47 Methanol: 27.47 Acetonitrile: 3.25 Acetone: 4.6 Ethyl acetate: 15.75 Dichloromethane: 8.11 Hexane: Not detected | In terms of extract yield, maceration is more efficient than Soxhlet, and the two best solvents are water and methanol. Regarding the bioactive compounds and antioxidant activity, the solvent’s polarity had a significant effect on antioxidant activity. Aqueous and methanolic extracts have proved to be best in terms of high extraction of phenolic compounds and antioxidant activity. It was proven that the polyphenols are thermostable, and once they are responsible for the antioxidant activity of the flower extracts, the Soxhlet method was the most interesting to preserve the antioxidant activity. | [51] |
| Opuntia ficus-indica and Opuntia stricta | From May to June 2009, the flowers were collected from 4 different flowering stages (vegetative, initial flowering, full flowering, and post-flowering) from wild populations located in Tunisia (latitude 34°46′29″ N, longitude 10°39′73″ E; elevation: 41 m), where the climate is semi-arid and is characterized by a mean rainfall of 200 mm/year. | Maceration was used for extraction using hexane. After 7 days, extracts were filtered and concentrated and finally characterized by Gas Chromatography with flame-ionization detection (GC FID). | Fifty different components were detected, representing around 85–99.7% of the total integrated peak area. No remarkable differences were observed between the flower compositions. Phytochemical compounds present in the Opuntia flower extract essentially belong to carboxylic acids (28–97%), terpenes (0.2–57%), esters (0.2–27%), and alcohol classes (<1.8%). Aromatic compounds, mostly phenolic compounds ((2-methoxy-4-vinylphenol and phenol) represented only 9.3% of the identified molecules. Monoterpenes d1-limonene and linalool were found in small quantities in the early stages of O. ficus-indica flowering, and only at post-flowering for O. stricta. Camphor represented 32.4% of the compounds found in the vegetative stage for O. ficus-indica. In terms of total phenolic content: Opuntia ficus-indica: Vegetative: 16.3 mg GAE */g extract Initial flowering: 13.4 mg GAE */g extract Full flowering: 13.3 mg GAE */g extract Post-flowering: 49.0 mg GAE */g extract Opuntia stricta: Vegetative: 16.7 mg GAE */g extract Initial flowering: 15.3 mg GAE */g extract Full flowering: 13.1 mg GAE */g extract Post-flowering: 80.9 mg GAE */g extract | The extraction yield increased with the evolution of the flowering stage, with the highest yield found at the post-flowering stage (O. ficus-indica) and for O. stricta at full flowering. Overall, O. stricta presented more phenolic compounds than O. ficus-indica, which corroborates the superior antioxidant activity of its extract. However, the antioxidant power was not related to the number of phenolic compounds; once at the post-flowering stages, it presented the highest content but the lowest activity. This property was attributed to the presence of terpenes and aromatic compounds. The extracts exhibited good antimicrobial activity against Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli, with O. stricta extracts being more active than O. ficus-indica. | [92] |
| Opuntia ficus-indica (L) Miller | Flowers were collected during the post-flowering stage in June 2014 from wild populations located in Tunisia (latitude 34°46′29″ N, longitude 10°39′73″ E; elevation: 41 m), where the climate is semi-arid, characterized by a mean rainfall of 200 mm/year. | Two flower extracts were evaluated: an aqueous extract (mucilage) and a methanolic extract (Soxhlet extraction). Mucilage extract was characterized in terms of monosaccharide composition by GC. Furthermore, antimicrobial activity was evaluated against Gram-positive and Gram-negative foodborne bacteria. | Mucilage composition found: Glucose: 32.41 mg/g extract Mannose: 11.46 mg/g extract Arabinose: 7.98 mg/g extract Xylose: 4.97 mg/g extract Galactose: 2.61 mg/g extract Rhamnose: 1.99 mg/g extract Total sugar: 61.4% | The mucilage yield of th extraction found was 18.3%, while 14.8% was reported for the Soxhlet extract. Glucose was the major component of the mucilage and originated from the plant flower’s hemicellulose and cellulose. Both mucilage and methanolic extracts exhibited antimicrobial activity against both Gram-positive and Gram-negative foodborne bacteria, with a superior effect against the former. The methanolic extract was more efficient against the bacteria tested than mucilage, and the highest inhibition was found against Listeria monocytogenes. In terms of antioxidant properties, the mucilage and methanol extracts exhibited significant anti-radical activity, although the mucilage extract showed lower radical-scavenging activity than the methanolic extract. | [93] |
| Opuntia ficus-indica (L) Miller | Flowers of O. ficus-indica were collected in Monasterace, Italy, in May 2007. | Dried flowers were subjected to a maceration extraction process using methanol. Purification was performed by using Solid-Phase Extraction. The dried residue was analyzed by High Performance Liquid Chromatography coupled with Photo Diode Array and Electrospray Ionization Gas Chromatography (HPLC-PDA-ESI-MS) for qualitative investigation and by HPLC-PDA for quantitative analysis. Volatiles were analyzed by GC and Gas Chromatography with Electron Impact Mass Spectrometry (GC-EIMS) system. | O. ficus-indica flower methanol extract composition (mg/g): Quercetin 3-O-rutinoside: 7.09 Kaempferol 3-O-rutinoside: 4.00 Quercetin 3-O-glucoside: 4.47 Isorhamnetin 3-O-robinobioside: 42.69 Isorhamnetin 3-O-galactoside: 9.79 Isorhamnetin 3-O-glucoside: 7.24 Kaempferol 3-O-arabinoside: 3.24 Composition of O. ficus-indica flower volatiles (%): (E)-3-Hexen-1-ol: 3.7 1-Hexanol: 12.3 Nonanal: 2.5 2-Ethyl hexyl acetate: 1.2 Tetrahydrolavandulol: 5.5 1-Nonanol: 1.2 Pinocampheol: 1.7 Dihydrocitronellol: 2.6 Decanal: 8.2 Tridecane: 5.9 cis-2,3-Pinanediol: 1.9 η-Tetradecane: 9.1 β-Ylangene: 1.5 (E)-Geranyl acetone: 4.8 Allo-aromadendrene: 3.9 Germacrene D: 12.6 η –Pentadecane: 4.0 η –Hexadecane: 1.6 | Secondary metabolites belonging to the flavonol glycoside class were found in the cactus flowers. Total flavonoids of the flowers were 81.75 mg/g of fresh plant material, and Isorhamnetin 3-O-robinobioside was the major component, representing 52.22%, followed by isorhamnetin 3-O-galactoside (11.98%). The flower extract has a pharmaceutical interest as it can be used for the treatment of depression, and its major component is associated with a testosterone 5α-reductase inhibitor. This was the first report on the volatile composition of O. ficus-indica. There were no monoterpene hydrocarbons found, but oxygenated monoterpenes were at 16.5% and sesquiterpene hydrocarbons were at 18%, with Germacrene D as the major component. | [94] |
| Opuntia ficus-indica f. inermis | The roots were collected from municipal areas of Gafsa, Tunisia. | Extraction from dried roots was performed by using methanol under stirring. The solution was then centrifuged, and the supernatant was dried. Total phenolic compounds and flavonoid content were determined using UV/Vis spectroscopy. | Total phenolic: 57.56 mg GAE */g extract Total flavonoids: 23.5 mg RE*/g extract | The yield of extraction found was 26%. Root methanolic extract exhibited remarkable content in phenolic and flavonoid compounds. In comparison to swallow root (Decalepis hamiltonii), Opuntia root extract presented almost double the phenolic compounds. Regarding the flavonoid content, Opuntia root extracts reported concentrations superior to those reported for Opuntia fruit extracts, which are known for their antioxidant power. Extracts presented antiulcerogenic activities tested in vivo in rats. Phenolic and flavonoid wealth, radical scavenging activity, and reducing power have been implicated in the extract’s antiulcer properties. | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, C.; Paula, C.D.d.; Lahbouki, S.; Meddich, A.; Outzourhit, A.; Rashad, M.; Pari, L.; Coelhoso, I.; Fernando, A.L.; Souza, V.G.L. Opuntia spp.: An Overview of the Bioactive Profile and Food Applications of This Versatile Crop Adapted to Arid Lands. Foods 2023, 12, 1465. https://doi.org/10.3390/foods12071465
Rodrigues C, Paula CDd, Lahbouki S, Meddich A, Outzourhit A, Rashad M, Pari L, Coelhoso I, Fernando AL, Souza VGL. Opuntia spp.: An Overview of the Bioactive Profile and Food Applications of This Versatile Crop Adapted to Arid Lands. Foods. 2023; 12(7):1465. https://doi.org/10.3390/foods12071465
Chicago/Turabian StyleRodrigues, Carolina, Camila Damásio de Paula, Soufiane Lahbouki, Abdelilah Meddich, Abdelkader Outzourhit, Mohamed Rashad, Luigi Pari, Isabel Coelhoso, Ana Luísa Fernando, and Victor G. L. Souza. 2023. "Opuntia spp.: An Overview of the Bioactive Profile and Food Applications of This Versatile Crop Adapted to Arid Lands" Foods 12, no. 7: 1465. https://doi.org/10.3390/foods12071465