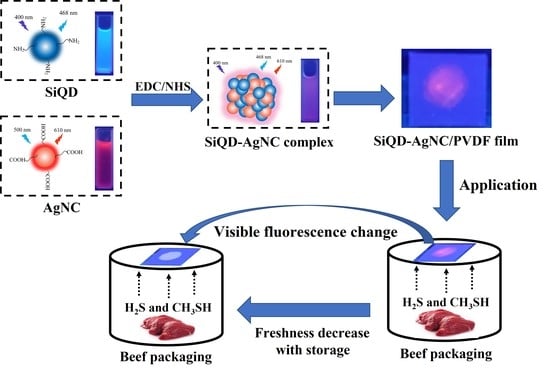
A Ratiometric Fluorescent Sensor Based on Silicon Quantum Dots and Silver Nanoclusters for Beef Freshness Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AgNC
2.3. Synthesis of SiQD
2.4. Synthesis of SiQD-AgNC Complex
2.5. Detection of H2S and CH3SH
2.6. Volatile Compounds Analysis of Beef Samples
2.7. Application of Sensor in Monitoring Beef Freshness
3. Results and Discussions
3.1. Optimization of SiQD and AgNC Synthesis
3.2. Fluorescence Characteristics of SiQD and AgNC
3.3. Principle of Detection
3.4. Optimization of pH and Ionic Strength
3.5. Sensitivity of SiQD-AgNC to H2S and CH3SH
3.6. Selectivity of SiQD-AgNC to H2S and CH3SH
3.7. Sensitivity of Fluorescent Film to H2S and CH3SH
3.8. Application of Fluorescent Film in Monitoring Beef Freshness
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dirpan, A.; Hidayat, S.H. Quality and shelf-life evaluation of fresh beef stored in smart packaging. Foods 2023, 12, 396. [Google Scholar] [CrossRef]
- Yam, K.L.; Takhistov, P.T.; Miltz, J. Intelligent packaging: Concepts and applications. J. Food Sci. 2005, 70, 0022–1147. [Google Scholar] [CrossRef]
- Yousefi, H.; Su, H.M.; Imani, S.M.; Alkhaldi, K.; Filipe, C.D.M.; Didar, T.F. Intelligent food packaging: A review of smart sensing technologies for monitoring food quality. ACS Sens. 2019, 4, 808–821. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Tech. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Zia, J.; Mancini, G.; Bustreo, M.; Zych, A.; Donno, R.; Athanassiou, A.; Fragouli, D. Porous pH natural indicators for acidic and basic vapor sensing. Chem. Eng. J. 2021, 403, 126373. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, P.; Cheng, W.; Yan, K.; Pan, L.; Shi, Y.; Yu, G. Highly Sensitive, printable nanostructured conductive polymer wireless sensor for food spoilage detection. Nano Lett. 2018, 18, 4570–4575. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, J.; Xing, J.; Xing, W.; Tang, C.; Rao, Z.; Zhang, J. Untargeted profiling and differentiation of volatiles in varieties of meat using GC orbitrap MS. Foods 2022, 11, 3997. [Google Scholar] [CrossRef]
- Shah, S.S.; Aziz, M.A.; Oyama, M.; Al-Betar, A.-R.F. Controlled-potential-based electrochemical sulfide sensors: A review. Chem. Rec. 2021, 21, 204–238. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Cao, S.; Jin, B.; Yuan, X.; Han, Y.; Wang, K. Construction of a novel fluorescent probe for on-site measuring hydrogen sulfide levels in food samples. Food Anal. Method. 2019, 12, 852–858. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Shi, J.; Huang, X.; Sun, Z.; Zhang, D.; Zou, X.; Sun, Y.; Zhang, J.; Holmes, M.; et al. A colorimetric hydrogen sulfide sensor based on gellan gum-silver nanoparticles bionanocomposite for monitoring of meat spoilage in intelligent packaging. Food Chem. 2019, 290, 135–143. [Google Scholar] [CrossRef]
- Sukhavattanakul, P.; Manuspiya, H. Influence of hydrogen sulfide gas concentrations on LOD and LOQ of thermal spray coated hybrid-bacterial cellulose film for intelligent meat label. Carbohydr. Polym. 2021, 254, 117442. [Google Scholar] [CrossRef] [PubMed]
- Teymouri, Z.; Shekarchizadeh, H. A colorimetric indicator based on copper nanoparticles for volatile sulfur compounds to monitor fish spoilage in intelligent packaging. Food Packag. Shelf 2022, 33, 100884. [Google Scholar] [CrossRef]
- Chow, C.F.; Ho, P.Y.; Sun, D.; Lu, Y.J.; Wong, W.L.; Tang, Q.; Gong, C.B. Development of sensitive and selective food sensors using new Re(I)-Pt(II) bimetallic complexes to detect volatile biogenic sulfides formed by meat spoilage. Food Chem. 2017, 216, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, M.; Chen, M.; Wang, Q.; Wu, C.; Sun, M.; Su, G.; Wang, X.; Wang, Y.; Zhou, X.; et al. Deep learning-assisted smartphone-based portable and visual ratiometric fluorescence device integrated intelligent gel label for agro-food freshness detection. Food Chem. 2023, 135640. [Google Scholar] [CrossRef]
- Jia, R.; Tian, W.; Bai, H.; Zhang, J.; Wang, S.; Zhang, J. Amine-responsive cellulose-based ratiometric fluorescent materials for real-time and visual detection of shrimp and crab freshness. Nat. Commun. 2019, 10, 795. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Lu, X.; Wang, J.; Zhang, Z.; Du, X.; Zhang, J.; Wang, J. Near-infrared fluorescent probe with a large stokes shift for detection of hydrogen sulfide in food spoilage, living cells, and zebrafish. J. Agric. Food Chem. 2022, 70, 3047–3055. [Google Scholar] [CrossRef]
- Hu, X.; Cao, H.; Dong, W.; Tang, J. Ratiometric fluorescent sensing of ethanol based on copper nanoclusters with tunable dual emission. Talanta 2021, 233, 122480. [Google Scholar] [CrossRef]
- Huang, X.; Sun, W.; Li, Z.; Shi, J.; Zhang, N.; Zhang, Y.; Zhai, X.; Hu, X.; Zou, X. Hydrogen sulfide gas sensing toward on-site monitoring of chilled meat spoilage based on ratio-type fluorescent probe. Food Chem. 2022, 396, 133654. [Google Scholar] [CrossRef]
- Shang, L.; Dong, S. Facile preparation of water-soluble fluorescent silver nanoclusters using a polyelectrolyte template. Chem. Commun. 2008, 9, 1088–1090. [Google Scholar] [CrossRef]
- Sharma, B.; Tanwar, S.; Sen, T. One pot green synthesis of Si quantum dots and catalytic Au nanoparticle-Si quantum dot nanocomposite. ACS Sustain. Chem. Eng. 2019, 7, 3309–3318. [Google Scholar] [CrossRef]
- Zhai, X.; Sun, Y.; Cen, S.; Wang, X.; Zhang, J.; Yang, Z.; Li, Y.; Wang, X.; Zhou, C.; Arslan, M.; et al. Anthocyanins-encapsulated 3D-printable bigels: A colorimetric and leaching-resistant volatile amines sensor for intelligent food packaging. Food Hydrocoll. 2022, 133, 107989. [Google Scholar] [CrossRef]
- Zhong, Y.; Peng, F.; Bao, F.; Wang, S.; Ji, X.; Yang, L.; Su, Y.; Lee, S.-T.; He, Y. Large-scale aqueous synthesis of fluorescent and biocompatible silicon nanoparticles and their use as highly photostable biological probes. J. Am. Chem. Soc. 2013, 135, 8350–8356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Qu, F.; Luo, H.Q.; Li, N.B. Sensitive and selective detection of biothiols based on target-induced agglomeration of silver nanoclusters. Biosens. Bioelectron. 2013, 42, 214–218. [Google Scholar] [CrossRef]
- Li, C.; Wei, C. DNA-functionlized silver nanoclusters as label-free fluorescent probe for the highly sensitive detection of biothiols and acetylcholinesterase activity. Sens. Actuators Chem. 2017, 240, 451–458. [Google Scholar] [CrossRef]
- Yan, L.; Gu, Q.-S.; Jiang, W.-L.; Tan, M.; Tan, Z.-K.; Mao, G.-J.; Xu, F.; Li, C.-Y. Near-infrared fluorescent probe with large stokes shift for imaging of hydrogen sulfide in tumor-bearing mice. Anal. Chem. 2022, 94, 5514–5520. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Yang, S.; Tian, H.; Liu, Y.; Sun, B. Highly selective and rapidly responsive fluorescent probe for hydrogen sulfide detection in wine. Food Chem. 2018, 257, 150–154. [Google Scholar] [CrossRef]
- Zhao, Q.; Huo, F.; Kang, J.; Zhang, Y.; Yin, C. A novel FRET-based fluorescent probe for the selective detection of hydrogen sulfide (H2S) and its application for bioimaging. J. Mater. Chem. B 2018, 6, 4903–4908. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cai, Q.; Luo, F.; Dong, N.; Guo, L.; Qiu, B.; Lin, Z. Sensitive fluorescent sensor for hydrogen sulfide in rat brain microdialysis via CsPbBr3 quantum dots. Anal. Chem. 2019, 91, 15915–15921. [Google Scholar] [CrossRef]
- Liu, G.; Ge, H.; Yin, R.; Yu, L.; Wang, S. Carbon dots tailored with a fluorophore for sensitive and selective detection of hydrogen sulfide based on a ratiometric fluorescence signal. Anal. Methods 2020, 12, 1617–1623. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Niu, Q.; Gao, P.; Zhang, G.; Dong, C.; Shuang, S. Gold nanoclusters as fluorescent sensors for selective and sensitive hydrogen sulfide detection. Talanta 2017, 171, 143–151. [Google Scholar] [CrossRef]
- Wen, Z.; Song, S.; Hu, T.; Wang, C.; Qu, F.; Wang, P.; Yang, M. A dual emission nanocomposite prepared from copper nanoclusters and carbon dots as a ratiometric fluorescent probe for sulfide and gaseous H2S. Microchim. Acta 2019, 186, 258. [Google Scholar] [CrossRef] [PubMed]
- Olivares, A.; Dryahina, K.; Španěl, P.; Flores, M. Rapid detection of lipid oxidation in beef muscle packed under modified atmosphere by measuring volatile organic compounds using SIFT-MS. Food Chem. 2012, 135, 1801–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhu, L.; Han, Y.; Xu, L.; Jin, J.; Cai, Y.; Wang, H. Analysis of volatile compounds between raw and cooked beef by HS-SPME–GC–MS. J. Food Process. Pres. 2018, 42, 13503. [Google Scholar] [CrossRef]








| Sensor Materials | Detection Target | Linear Range (μM) | LOD (nM) | References |
|---|---|---|---|---|
| Isophorone-xanthene dye | H2S | 1.0–40.0 | 250 | [25] |
| 6-(2, 4-dinitrophenoxy)-2-naphthonitrile | H2S | 0–70 | 76 | [26] |
| 7-amino-4-methylcoumarin and fluorescein | H2S | 0–140 | 31 | [27] |
| CsPbBr3 quantum dots | H2S | 0–100 | 180 | [28] |
| Carbon quantum dots | S2− | 0–3 | 62.7 | [18] |
| Carbon quantum dots -PNBD | H2S | 0–35 | 57 | [29] |
| Gold nanoclusters | H2S | 0.002–120 | 1.8 | [30] |
| Carbon dots-copper nanoclusters | H2S | 26–128 | 4.3 | [31] |
| Silicon quantum dots- silver nanoclusters | H2S | 1.125–17 | 53.6 | This work |
| Category | Name | Relative Contents (w/w%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 d | 1 d | 2 d | 3 d | 4 d | 5 d | 6 d | ||
| Sulfides | Dimethyl disulfide | - | - | 0.45 ± 0.22 | - | 0.58 ± 0.22 | 0.64 ± 0.35 | - |
| Hydrogen sulfide | - | - | - | - | 0.23 ± 0.10 | 1.17 ± 0.61 | 1.68 ± 0.24 | |
| Methanethiol | - | - | - | 0.35 ± 0.23 | 2.48 ± 0.43 | 4.46 ± 1.29 | 3.59 ± 2.37 | |
| Dimethyl trisulfide | - | - | - | - | - | 0.74 ± 0.32 | 0.81 ± 0.39 | |
| Hydrocarbon | Pentane | 4.17 ± 1.85 | 6.33 ± 2.42 | 3.28 ± 2.06 | 2.30 ± 0.15 | - | 4.3 0 ± 2.74 | - |
| Hexane | 2.66 ± 0.56 | 5.54 ± 1.69 | 3.51 ± 1.78 | 1.57 ± 0.56 | - | 0.34 ± 0.24 | 3.16 ± 0.88 | |
| Pentadecane | 3.39 ± 0.45 | 1.22 ± 0.62 | - | 3.53 ± 0.75 | 1.01 ± 0.14 | - | - | |
| 2,6,10-trimethyl-dodecane | 4.87 ± 0.85 | - | 2.10 ± 0.43 | - | - | 0.77 ± 0.17 | - | |
| Heptadecane | 2.40 ± 0.33 | 5.04 ± 0.54 | 4.21 ± 2.16 | - | - | 1.20 ± 0.53 | ||
| Alcohols | 1-pentanol | 3.34 ± 1.84 | 5.08 ± 2.42 | 3.14 ± 1.92 | 2.12 ± 0.67 | 1.56 ± 0.33 | 0.81 ± 0.81 | 4.21 ± 0.47 |
| 1-hexen-3-ol | 2.89 ± 0.15 | - | 4.22 ± 0.43 | 3.78 ± 0.85 | - | 5.21 ± 1.64 | - | |
| Ethanol | 0.63 ± 0.35 | 1.26 ± 0.27 | - | 0.85 ± 0.11 | - | 1.53 ± 0.38 | 1.69 ± 0.56 | |
| 1-octene-3-ol | - | 2.2 ± 0.75 | 2.31 ± 0.64 | 1.89 ± 0.76 | 1.45 ± 0.63 | 0.80 ± 0.24 | - | |
| 4-methyl-1-amyl alcohol | - | 1.88 ± 0.53 | - | - | - | 3.22 ± 0.68 | - | |
| Hexyl alcohol | - | - | 1.57 ± 0.45 | 1.56 ± 0.16 | 1.91 ± 0.37 | 1.81 ± 0.55 | 3.56 ± 0.49 | |
| Butanol | - | - | 0.38 ± 0.12 | - | 3.49 ± 0.26 | - | ||
| Aldehydes/ketones | Hexanal | 30.59 ± 7.12 | 23.40 ± 7.33 | 20.76 ± 5.68 | 21.72 ± 8.24 | 11.88 ± 5.15 | 7.01 ± 2.19 | 24.43 ± 8.32 |
| Valeraldehyde | - | 0.47 ± 0.14 | - | 0.94 ± 0.18 | 1.89 ± 0.15 | - | - | |
| 3-methylbutyral | - | 0.56 ± 0.19 | - | - | - | 1.47 ± 0.13 | - | |
| Heptanal | - | - | 1.88 ± 0.37 | - | - | 1.08 ± 0.36 | 2.37 ± 0.54 | |
| Acetone | 18.44 ± 6.52 | 10.69 ± 5.17 | 5.53 ± 2.63 | 3.15 ± 0.35 | 3.25 ±1.72 | 2.62 ± 0.87 | 1.90 ± 0.66 | |
| Hypnone | - | - | - | 2.32 ± 0.52 | - | - | - | |
| Acids/esters | Acetic acid | 0.31 ± 0.16 | 0.52 ± 0.24 | 1.42 ± 0.12 | 2.53 ± 0.73 | 1.34 ± 0.41 | 2.88 ± 0.59 | 2.13 ± 0.81 |
| Propionic acid | - | - | 0.79 ± 0.42 | 1.33 ± 0.39 | 3.31 ± 0.37 | 2.78 ± 0.56 | 3.54 ± 1.17 | |
| Ethyl oenanthate | 3.87 ± 0.82 | 3.52 ± 1.15 | - | 1.73 ± 0.21 | ||||
| Ethyl caprylate | - | - | 0.91 ± 0.27 | - | - | 0.48 ± 0.19 | - | |
| Ethyl acetate | - | 2.75 ± 0.22 | - | 0.71 ± 0.12 | 0.85 ± 0.34 | 0.41 ± 0.15 | 0.66 ± 0.26 | |
| Ethyl valerate | - | - | - | - | 0.71 ± 0.23 | 0.96 ± 0.41 | 1.48 ± 0.36 | |
| Nitrogenous compounds | Hexylamine | 0.89 ± 0.31 | - | 0.46 ± 0.18 | - | - | - | - |
| Ethanediamine | 1.43 ± 0.35 | - | - | 1.09 ± 0.12 | 1.92 ± 0.45 | - | - | |
| Trimethylamine | - | 0.77 ± 0.26 | 2.80 ± 0.70 | 3.32 ± 1.02 | 3.67 ± 0.89 | 6.23 ± 2.40 | 7.59 ± 2.37 | |
| Dimethylamine | - | - | - | 1.26 ± 0.39 | - | 2.32 ± 0.94 | - | |
| Heptylamine | - | - | - | - | 0.80 ± 0.28 | 0.29 ± 0.09 | - | |
| Others | Carbon dioxide | 11.20 ± 3.34 | 5.11 ± 1.72 | 2.32 ± 0.25 | 1.58 ± 0.31 | 3.28 ± 0.78 | 8.33 ± 2.04 | 15.23 ± 2.11 |
| Butylated hydroxytoluene | - | - | - | - | - | 1.31 ± 0.22 | 3.45 ± 0.67 | |
| Storage Time (d) | TVC of Beef (lg(CFU/g)) | Color Parameters of SiQD-AgNC/PVDF Film | |||
|---|---|---|---|---|---|
| R | G | B | ∆C | ||
| 0 | 3.21 ± 0.33 | 156 ± 2.6 | 76 ± 1.3 | 220 ± 1.3 | 0 |
| 1 | 3.43 ± 0.24 | 150 ± 4.4 | 79 ± 2.5 | 221 ± 3.5 | 46 |
| 2 | 3.81 ± 0.35 | 140 ± 5.1 | 85 ± 3.3 | 225 ± 4.4 | 362 |
| 3 | 4.09 ± 0.31 | 128 ± 2.2 | 94 ± 4.7 | 229 ± 2.0 | 1189 |
| 4 | 5.41 ± 0.28 | 110 ± 3.3 | 109 ± 2.8 | 243 ± 0.9 | 3734 |
| 5 | 6.81 ± 0.26 | 96 ± 4.5 | 130 ± 3.1 | 255 ± 2.8 | 7741 |
| 6 | 7.65 ± 0.31 | 87 ± 3.5 | 150 ± 2.6 | 270 ± 1.9 | 12,737 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhai, X.; Zou, X.; Shi, J.; Huang, X.; Li, Z. A Ratiometric Fluorescent Sensor Based on Silicon Quantum Dots and Silver Nanoclusters for Beef Freshness Monitoring. Foods 2023, 12, 1464. https://doi.org/10.3390/foods12071464
Sun Y, Zhai X, Zou X, Shi J, Huang X, Li Z. A Ratiometric Fluorescent Sensor Based on Silicon Quantum Dots and Silver Nanoclusters for Beef Freshness Monitoring. Foods. 2023; 12(7):1464. https://doi.org/10.3390/foods12071464
Chicago/Turabian StyleSun, Yue, Xiaodong Zhai, Xiaobo Zou, Jiyong Shi, Xiaowei Huang, and Zhihua Li. 2023. "A Ratiometric Fluorescent Sensor Based on Silicon Quantum Dots and Silver Nanoclusters for Beef Freshness Monitoring" Foods 12, no. 7: 1464. https://doi.org/10.3390/foods12071464