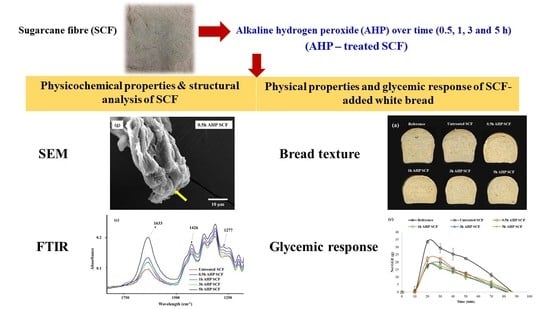
Effects of Incorporating Alkaline Hydrogen Peroxide Treated Sugarcane Fibre on The Physical Properties and Glycemic Potency of White Bread
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. AHP Modification of SCF
2.3. Characterization of SCF
2.3.1. Acid-Insoluble Lignin Content Determination
2.3.2. Sugar Binding Capacity (SBC)
2.3.3. Water Holding Capacity (WHC) and Oil Holding Capacity (OHC)
2.3.4. Particle Size Measurement
2.3.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.6. Scanning Electron Microscope (SEM)
2.4. Development of Dough and Bread
2.4.1. Hydration of Flour Using DoughLAB
2.4.2. Bread Making Process
2.5. Characterization of Dough and Bread
2.5.1. Dough Extensibility
2.5.2. Loaf Specific Volume and Maximum Height of Loaf
2.5.3. Texture Profile Analysis of Bread Crumb
2.5.4. Moisture Analysis and Water Activity of Bread Crumb
2.5.5. In Vitro Determination of Glycemic Potency
2.5.6. In Vitro Digestion of Bread Samples
2.5.7. Quantification of Glucose Release
2.6. Data Analysis for In Vitro Glycemic Potency Determination
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Sun, S.; Ai, L. Physical barrier effects of dietary fibres on lowering starch digestibility. Curr. Opin. Food Sci. 2022, 48, 100940. [Google Scholar] [CrossRef]
- Basu, S.; Yudkin, J.S.; Kehlenbrink, S.; Davies, J.I.; Wild, S.H.; Lipska, K.J.; Sussman, J.B.; Beran, D. Estimation of global insulin use for type 2 diabetes, 2018–30: A microsimulation analysis. Lancet. Diabetes Endocrinol. 2019, 7, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Hemdane, S.; Langenaeken, N.A.; Jacobs, P.J.; Verspreet, J.; Delcour, J.A.; Courtin, C.M. Study of the role of bran water binding and the steric hindrance by bran in straight dough bread making. Food Chem. 2018, 253, 262–268. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, B.Y.; Prudencio, S.H. Alkaline hydrogen peroxide improves physical, chemical, and techno-functional properties of okara. Food Chem. 2020, 323, 126776. [Google Scholar] [CrossRef]
- De Matos, M.; Santos, F.; Eichler, P. Sugarcane world scenario. In Sugarcane Biorefinery, Technology and Perspectives; Academic Press: Cambridge, MA, USA, 2020; pp. 1–19. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Anannya, F.R. Sugarcane bagasse—A source of cellulosic fibre for diverse applications. Heliyon 2021, 7, e07771. [Google Scholar] [CrossRef]
- Eggleston, G. Positive aspects of cane sugar and sugar cane derived products in food and nutrition. J. Agric. Food Chem. 2018, 66, 4007–4012. [Google Scholar] [CrossRef]
- Huang, G.; Guo, Q.; Wang, C.; Ding, H.H.; Cui, S.W. Fenugreek fibre in bread: Effects on dough development and bread quality. LWT 2016, 71, 274–280. [Google Scholar] [CrossRef]
- Sangnark, A.; Noomhorm, A. Effect of dietary fibre from sugarcane bagasse and sucrose ester on dough and bread properties. LWT Food Sci. Technol. 2004, 37, 697–704. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, S.; Wei, W.; Zhang, J.; Xie, J. Investigation of alkaline hydrogen peroxide pretreatment and Tween 80 to enhance enzymatic hydrolysis of sugarcane bagasse. Biotechnol. Biofuels 2019, 12, 107. [Google Scholar] [CrossRef]
- Dutra, E.D.; Santos, F.A.; Alencar, B.R.A.; Reis, A.L.S.; Souza, R.D.F.R.; Aquino, K.A.D.S.; Morais, M.A., Jr.; Menezes, R.S.C. Alkaline hydrogen peroxide pretreatment of lignocellulosic biomass: Status and perspectives. Biomass Convers. Biorefinery 2018, 8, 225–234. [Google Scholar] [CrossRef]
- Mou, H.; Heikkilä, E.; Fardim, P. Topochemistry of environmentally friendly pretreatments to enhance enzymatic hydrolysis of sugar cane bagasse to fermentable sugar. J. Agric. Food Chem. 2014, 62, 3619–3625. [Google Scholar] [CrossRef]
- Ameram, N.; Muhammad, S.; Yusof, N.A.A.N.; Ishak, S.; Ali, A.; Shoparwe, N.F.; Ter, T.P. Chemical composition in sugarcane bagasse: Delignification with sodium hydroxide. Malays. J. Fundam. Appl. Sci. 2019, 15, 232–236. [Google Scholar] [CrossRef]
- Gould, J.M.; Jasberg, B.K.; Dexter, L.B.; Hsu, J.T.; Lewis, S.M.; Fahey, G.C.J. High-fibre, noncaloric flour substitute for baked foods. Properties of alkaline peroxide-treated lignocellulose. Cereal Chem. 1989, 66, 201–205. [Google Scholar]
- Meng, X.; Liu, F.; Xiao, Y.; Cao, J.; Wang, M.; Duan, X. Alterations in physicochemical and functional properties of buckwheat straw insoluble dietary fibre by alkaline hydrogen peroxide treatment. Food Chem. X 2019, 3, 100029. [Google Scholar] [CrossRef]
- Ho, M.C.; Ong, V.Z.; Wu, T.Y. Potential use of alkaline hydrogen peroxide in lignocellulosic biomass pretreatment and valorization—A review. Renew. Sustain. Energy Rev. 2019, 112, 75–86. [Google Scholar] [CrossRef]
- Sangnark, A.; Noomhorm, A. Effect of particle sizes on functional properties of dietary fibre prepared from sugarcane bagasse. Food Chem. 2003, 80, 221–229. [Google Scholar] [CrossRef]
- Fang, Z.; Li, B.; Liu, Y.; Zhu, J.; Li, G.; Hou, G.; Zhou, J.; Qiu, X. Critical Role of Degree of Polymerization of Cellulose in Super-Strong Nanocellulose Films. Matter 2020, 2, 1000–1014. [Google Scholar] [CrossRef]
- Dubey, R.; Toh, Y.R.; Yeh, A.I. Enhancing cellulose functionalities by size reduction using media-mill. Sci. Rep. 2018, 8, 11343. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Chen, M.; Bi, X.; Du, J. Physicochemical properties, structural characteristics and in vitro digestion of brown rice–pea protein isolate blend treated by microbial transglutaminase. Food Hydrocoll. 2023, 141, 108673. [Google Scholar] [CrossRef]
- Feng, X.; Dai, H.; Ma, L.; Fu, Y.; Yu, Y.; Zhou, H.; Guo, T.; Zhu, H.; Wang, H.; Zhang, Y. Properties of Pickering emulsion stabilized by food-grade gelatin nanoparticles: Influence of the nanoparticles concentration. Colloids Surf. B Biointerfaces 2020, 196, 111294. [Google Scholar] [CrossRef] [PubMed]
- AACC. Rheological Behavior of Flour by Farinograph: Constant Flour Weight Procedure. In AACC International Approved Methods 54-21.02; AACC: Washington, DC, USA, 2011; pp. 1–8. [Google Scholar]
- AACC. Basic Straight-Dough Bread-Baking Method—Long Fermentation. In AACC International Method 10-09.01; AACC: Washington, DC, USA, 2009; pp. 1–6. [Google Scholar] [CrossRef]
- Liu, W.; Brennan, M.; Serventi, L.; Brennan, C. Buckwheat flour inclusion in Chinese steamed bread: Potential reduction in glycemic response and effects on dough quality. Eur. Food Res. Technol. 2017, 243, 727–734. [Google Scholar] [CrossRef]
- AACC. Guidelines for Measurement of Volume by Rapeseed Displacement. In AACC International Method 10-05.01; AACC: Washington, DC, USA, 2001; pp. 3–6. [Google Scholar] [CrossRef]
- Akila, S.R.V.; Mishra, S.; Hardacre, A.; Matia-Merino, L.; Goh, K.; Warren, F.; Monro, J. Kernel structure in breads reduces in vitro starch digestion rate and estimated glycaemic potency only at high grain inclusion rates. Food Struct. 2019, 21, 100109. [Google Scholar] [CrossRef]
- Monro, J.A.; Mishra, S.; Venn, B. Baselines representing blood glucose clearance improve in vitro prediction of the glycaemic impact of customarily consumed food quantities. Br. J. Nutr. 2010, 103, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, G.; Bai, X.; Wu, Z.; Li, S.; Zhao, C.; Ramachandraiah, K. Modification of ginseng insoluble dietary fibre through alkaline hydrogen peroxide treatment and its impact on structure, physicochemical and functional properties. LWT 2021, 150, 111956. [Google Scholar] [CrossRef]
- Lee, Y.J.; Chung, C.H.; Day, D.F. Sugarcane bagasse oxidation using a combination of hypochlorite and peroxide. Bioresour. Technol. 2009, 100, 935–941. [Google Scholar] [CrossRef]
- Su, Y.; Du, R.; Guo, H.; Cao, M.; Wu, Q.; Su, R.; Qi, W.; He, Z. Fractional pretreatment of lignocellulose by alkaline hydrogen peroxide: Characterization of its major components. Food Bioprod. Process. 2015, 94, 322–330. [Google Scholar] [CrossRef]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; Esteban, R.M. Physicochemical properties and in vitro antidiabetic potential of fibre concentrates from onion by-products. J. Funct. Foods 2017, 36, 34–42. [Google Scholar] [CrossRef]
- Bhutkar, M.; Bhise, S. In vitro hypoglycemic effects of Albizzia lebbeck and Mucuna pruriens. Asian Pac. J. Trop. Biomed. 2013, 3, 866–870. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.; An, R.; Bao, J. Pretreatment refining leads to constant particle size distribution of lignocellulose biomass in enzymatic hydrolysis. Chem. Eng. J. 2018, 352, 198–205. [Google Scholar] [CrossRef]
- Tajvidi, M.; Azad, F. Effect of Particle Size, Fibre Content and Compatibilizer on the Long-term Water Absorption and Thickness Swelling Behavior of Reed Flour/Polypropylene Composites. J. Reinf. Plast. Compos. 2008, 28, 2341–2351. [Google Scholar] [CrossRef]
- Sompugdee, C.; Quan, V.M.; Sriroth, K.; Sukyai, P. Chemical composition of alkaline-pretreated sugarcane bagasse and its effects on the physicochemical characteristics of fat-replaced sausage. Int. J. Food Sci. Technol. 2021, 56, 5989–5999. [Google Scholar] [CrossRef]
- Kim, H.W.; Setyabrata, D.; Lee, Y.J.; Kim YH, B. Efficacy of Alkali-treated Sugarcane Fibre for Improving Physicochemical and Textural Properties of Meat Emulsions with Different Fat Levels. Korean J. Food Sci. Anim. Resour. 2018, 38, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qi, J.; Zeng, W.; Huang, Y.; Yang, X. Properties of dietary fibre from citrus obtained through alkaline hydrogen peroxide treatment and homogenization treatment. Food Chem. 2020, 311, 125873. [Google Scholar] [CrossRef] [PubMed]
- Bartos, A.; Anggono, J.; Farkas, Á.E.; Kun, D.; Soetaredjo, F.E.; Móczó, J.; Antoni; Purwaningsih, H.; Pukánszky, B. Alkali treatment of lignocellulosic fibres extracted from sugarcane bagasse: Composition, structure, properties. Polym. Test. 2019, 88, 106549. [Google Scholar] [CrossRef]
- Kininge, M.M.; Gogate, P.R. Intensification of alkaline delignification of sugarcane bagasse using ultrasound assisted approach. Ultrason. Sonochem. 2022, 82, 105870. [Google Scholar] [CrossRef]
- Jähn, A.; Schröder, M.W.; Füting, M.; Schenzel, K.; Diepenbrock, W. Characterization of alkali treated flax fibres by means of FT Raman spectroscopy and environmental scanning electron microscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002, 58, 2271–2279. [Google Scholar] [CrossRef]
- Pavalaydon, K.; Ramasawmy, H.; Surroop, D. Comparative evaluation of cellulose nanocrystals from bagasse and coir agro-wastes for reinforcing PVA-based composites. Environ. Dev. Sustain. 2022, 24, 9963–9984. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- Guo, A.; Sun, Z.; Satyavolu, J. Impact of chemical treatment on the physiochemical and mechanical properties of kenaf fibres. Ind. Crop. Prod. 2019, 141, 111726. [Google Scholar] [CrossRef]
- Gao, Q.; Zhou, X.J.; Ma, R.; Lin, H.; Wu, J.L.; Peng, X.; Tanokura, M.; Xue, Y.L. Hydrogen peroxide modification affects the structure and physicochemical properties of dietary fibres from white turnip (Brassica Rapa L.). Sci. Rep. 2021, 11, 1024. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wu, L.; Zhang, F.; Kan, J.; Zheng, J. Comparison of different extraction methods on the physicochemical, structural properties, and in vitro hypoglycemic activity of bamboo shoot dietary fibres. Food Chem. 2022, 386, 132642. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Z.; Liu, N.; Zhou, P.; Bao, Q.; Wang, X. Effect of wheat bran dietary fibre on the rheological properties of dough during fermentation and Chinese steamed bread quality. Int. J. Food Sci. Technol. 2021, 56, 1623–1630. [Google Scholar] [CrossRef]
- Gómez, M.; Ronda, F.; Blanco, C.A.; Caballero, P.A.; Apesteguía, A. Effect of dietary fibre on dough rheology and bread quality. Eur. Food Res. Technol. 2003, 216, 51–56. [Google Scholar] [CrossRef]
- Wang, C.C.; Yang, Z.; Guo, X.N.; Zhu, K.X. Effects of insoluble dietary fibre and ferulic acid on the quality of steamed bread and gluten aggregation properties. Food Chem. 2021, 364, 130444. [Google Scholar] [CrossRef]
- Anil, M. Using of hazelnut testa as a source of dietary fiber in breadmaking. J. Food Eng. 2007, 80, 61–67. [Google Scholar] [CrossRef]
- Kiumarsi, M.; Shahbazi, M.; Yeganehzad, S.; Majchrzak, D.; Lieleg, O.; Winkeljann, B. Relation between structural, mechanical and sensory properties of gluten-free bread as affected by modified dietary fibres. Food Chem. 2019, 277, 664–673. [Google Scholar] [CrossRef]
- Nawrocka, A.; Miś, A.; Niewiadomski, Z. Dehydration of gluten matrix as a result of dietary fibre addition—A study on model flour with application of FT-IR spectroscopy. J. Cereal Sci. 2017, 74, 86–94. [Google Scholar] [CrossRef]
- Zhu, F. Glycemic control in Chinese steamed bread: Strategies and opportunities. Trends Food Sci. Technol. 2019, 86, 252–259. [Google Scholar] [CrossRef]
- Dhital, S.; Gidley, M.J.; Warren, F.J. Inhibition of α-amylase activity by cellulose: Kinetic analysis and nutritional implications. Carbohydr. Polym. 2015, 123, 305–312. [Google Scholar] [CrossRef]
- Seki, T.; Nagase, R.; Torimitsu, M.; Yanagi, M.; Ito, Y.; Kise, M.; Mizukuchi, A.; Fujimura, N.; Hayamizu, K.; Ariga, T. Insoluble fibre is a major constituent responsible for lowering the post-prandial blood glucose concentration in the pre-germinated brown rice. Biol. Pharm. Bull. 2005, 28, 1539–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nsor-Atindana, J.; Goff, H.D.; Saqib, M.N.; Chen, M.; Liu, W.; Ma, J.; Zhong, F. Inhibition of α-amylase and amyloglucosidase by nanocrystalline cellulose and spectroscopic analysis of their binding interaction mechanism. Food Hydrocoll. 2019, 90, 341–352. [Google Scholar] [CrossRef]
- Alencar, B.R.A.; Reis, A.L.S.; de Souza, R.D.F.R.; Morais, M.A., Jr.; Menezes, R.S.C.; Dutra, E.D. Recycling the liquid fraction of alkaline hydrogen peroxide in the pretreatment of corn stover. Bioresour. Technol. 2017, 241, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Monteith, E.R.; Mampuys, P.; Summerton, L.; Clark, J.H.; Maes, B.U.; McElroy, C.R. Why we might be misusing process mass intensity (PMI) and a methodology to apply it effectively as a discovery level metric. Green Chem. 2020, 22, 123–135. [Google Scholar] [CrossRef]
- Sultanova, N.; Kasarova, S.; Nikolov, I. Dispersion properties of optical polymers. Acta Phys. Pol. A 2009, 116, 585–587. [Google Scholar] [CrossRef]






| Reference | SCF | |||
|---|---|---|---|---|
| Ingredients | Mass (g) | w/w (%) | Mass (g) | w/w (%) |
| Wheat Flour | 300.0 | 59.6 | 300.0 | 56.2 |
| SCF | - | - | 15.0 | 2.8 |
| Water | 193.5 | 38.5 | 208.8 | 39.2 |
| Dry Yeast | 3.60 | 0.7 | 3.60 | 0.7 |
| Salt | 6.00 | 1.2 | 6.00 | 1.1 |
| Total | 503.1 | 100.0 | 533.4 | 100.0 |
| Sample | Acid-Insoluble Lignin (%) | SBC (g/g SCF) | Particle Size (µm) | |
|---|---|---|---|---|
| D(4,3) | D(3,2) | |||
| Untreated SCF | 88.7 ± 0.15 a | 3.8 × 10−5 ± 2.3 × 10−6 b | 46.1 ± 0.47 d | 27.8 ± 0.48 c |
| 0.5 h AHP SCF | 55.6 ± 0.29 c | 4.2 × 10−5 ± 3.0 × 10−7 a | 49.3 ± 0.62 c | 28.0 ± 0.18 c |
| 1 h AHP SCF | 52.8 ± 0.32 c | 4.3 × 10−5 ± 1.7 × 10−6 a | 50.9 ± 0.50 a | 29.9 ± 0.12 a |
| 3 h AHP SCF | 65.0 ± 0.70 b | 4.1 × 10−5 ± 3.7 × 10−8 ab | 50.1 ± 0.72 b | 29.9 ± 0.11 a |
| 5 h AHP SCF | 65.7 ± 4.03 b | 4.2 × 10−7 ± 2.5 × 10−7 a | 49.7 ± 0.37 bc | 29.4 ± 0.25 b |
| Samples | Resistance to Extension (g) | Maximum Height (mm) | Specific Volume (cm3/g) |
|---|---|---|---|
| Reference | 20.08 ± 0.45 c | 61.7 ± 1.9 a | 2.24 ± 0.05 a |
| Untreated SCF | 26.62 ± 1.87 b | 61.6 ± 1.5 a | 2.20 ± 0.05 ab |
| 0.5 h AHP SCF | 30.41 ± 1.04 a | 57.6 ± 3.2 b | 2.13 ± 0.04 ab |
| 1 h AHP SCF | 13.10 ± 1.05 d | 54.1 ± 0.7 b | 2.00 ± 0.15 b |
| 3 h AHP SCF | 20.56 ± 2.71 c | 54.2 ± 0.4 b | 2.14 ±0.09 ab |
| 5 h AHP SCF | 11.72 ± 0.88 d | 54.7 ± 0.9 b | 2.05 ± 0.18 ab |
| Samples | Estimated GI | ||
|---|---|---|---|
| iAUC | RGP | RDS | |
| Reference | 100 | 100 | 100 |
| Untreated SCF | 56 | 60 | 65 |
| 0.5 h AHP SCF | 63 | 58 | 70 |
| 1 h AHP SCF | 53 | 50 | 56 |
| 3 h AHP SCF | 49 | 50 | 54 |
| 5 h AHP SCF | 51 | 52 | 54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binte Abdul Halim, F.N.; Taheri, A.; Abdol Rahim Yassin, Z.; Chia, K.F.; Goh, K.K.T.; Goh, S.M.; Du, J. Effects of Incorporating Alkaline Hydrogen Peroxide Treated Sugarcane Fibre on The Physical Properties and Glycemic Potency of White Bread. Foods 2023, 12, 1460. https://doi.org/10.3390/foods12071460
Binte Abdul Halim FN, Taheri A, Abdol Rahim Yassin Z, Chia KF, Goh KKT, Goh SM, Du J. Effects of Incorporating Alkaline Hydrogen Peroxide Treated Sugarcane Fibre on The Physical Properties and Glycemic Potency of White Bread. Foods. 2023; 12(7):1460. https://doi.org/10.3390/foods12071460
Chicago/Turabian StyleBinte Abdul Halim, Fatin Natasha, Afsaneh Taheri, Zawanah Abdol Rahim Yassin, Kai Feng Chia, Kelvin Kim Tha Goh, Suk Meng Goh, and Juan Du. 2023. "Effects of Incorporating Alkaline Hydrogen Peroxide Treated Sugarcane Fibre on The Physical Properties and Glycemic Potency of White Bread" Foods 12, no. 7: 1460. https://doi.org/10.3390/foods12071460